Metal-free synthesis of 3-methylthiofurans from homopropargylic alcohols and DMSO via a tandem sulfenylation/cyclization reaction in a one-pot manner†
Zhenyu
An
,
Yue
She
,
Xiaodong
Yang
,
Xiaobo
Pang
and
Rulong
Yan
*
State Key Laboratory of Applied Organic Chemistry, Key laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, Department of Chemistry, Lanzhou University, Lanzhou, Gansu 730000, China. E-mail: yanrl@lzu.edu.cn; Fax: +0931-8912596
First published on 28th September 2016
Abstract
A tandem sulfenylation/cyclization reaction for the synthesis of 3-methylthiofurans from homopropargylic alcohols and DMSO in a one-pot manner has been developed. DMSO as a methylthiolation agent has been used for C–S bond formation in this process. Homopropargylic alcohols with various substituted groups proceed smoothly and the desired 3-methylthiofurans are obtained in moderate to good yields.
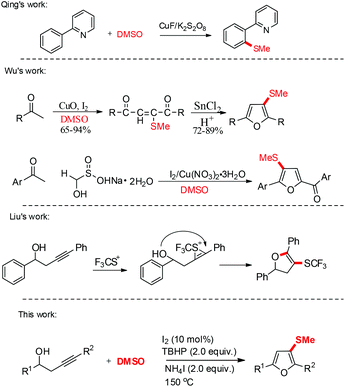
Substituted furans, as key heterocyclic compounds, play increasingly important roles in organic chemistry, drug discovery, and materials science.1 Moreover, alkylthiofurans have drawn more attention for their utilization in bioactive and pharmaceutical synthesis.2 The increasing importance of substituted furans has spurred vigorous research for the development of new synthetic methods. The oxidative tandem reaction has emerged as a powerful and environmentally friendly approach for the synthesis of heterocyclic compounds with simple starting materials.3 In particular, metal-free oxidative tandem reaction systems, such as iodine and acid catalyzed systems, have drawn more attention due to their advantages.4 On the other hand, the oxidative tandem reaction has been rarely exploited for the synthesis of alkylthiofurans. Last year, the Liu group reported the synthesis of SCF3-substituted dihydrofurans from homopropargylic alcohols and trifluoromethanesulfenamide.5 Thus, it is still attractive and challenging to synthesize S-substituted furans with simple substrates via an iodine-catalyzed tandem sulfenylation/cyclization reaction.
DMSO has been widely used as a common, cheap and aprotic polar solvent in organic synthesis. Furthermore, DMSO can also be utilized as a methylthiolation agent in C–S bond formation. In 2010, the Qing group reported Cu(II)-mediated methylthiolation of aryl C–H bonds with DMSO under oxidative conditions.6 Since then, a series of studies about DMSO as a methylthiolation source have been reported successively.7 Some excellent and significant studies of methylthiolation and cyclization to synthesize S-substituted heterocyclic compounds with DMSO have been reported by the group of Wu.8 In 2007, the Wu group reported that methylthiofurans were generated from ketones through a multistep reaction of homocoupling and cyclization in the presence of DMSO.9 Recently, the Wu group also reported an I2/Cu(NO3)2·3H2O-mediated C(sp3)–H functionalization reaction for the synthesis of methylthiofurans from ketones, rongalite and DMSO.10 However, there are very few examples of the synthesis of methylthiofurans with DMSO through an intermolecular oxidative tandem sulfenylation/cyclization reaction. Herein, we developed a direct method to form substituted methylthiofurans from homopropargylic alcohols and DMSO under metal free reaction conditions (Scheme 1).
With our continuing interest in the construction of structurally important heterocyclic scaffolds and C–S bond formation,11 this new reaction was accidentally found based on our hypothesis for the synthesis of disubstituted pyrroles with homopropargylic alcohols and NH4OAc in DMSO under oxidative conditions. Our hypothesis was tested with homopropargylic alcohol (1a), I2 (1.2 equiv.), and TBHP (2.0 equiv.) in DMSO at 150 °C. To our surprise, an unexpected product, 3-(methylthio)-2,5-diphenylfuran 3a, was generated in 70% yield.
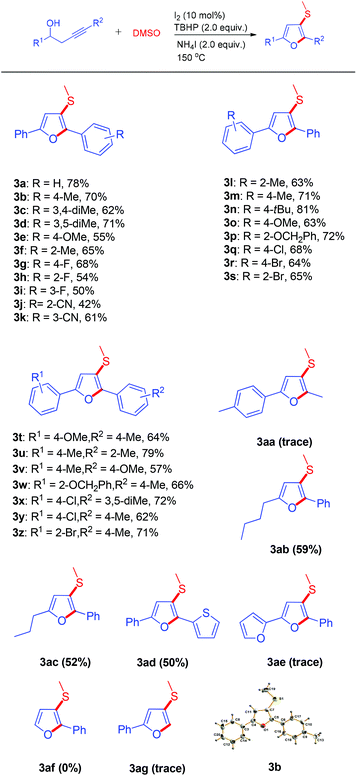
The result was a significant discovery because the method for synthesizing methylthiofurans was very limited and an improved method was attractive. In order to improve the yield of the methylthiofuran, we decided to optimize the reaction conditions and 1,4-diphenylbut-3-yn-1-ol 1a was chosen as a model substrate for this process. Firstly, various oxidants were tested; TBHP was proved to be the best oxidant for this process. Then various ammonium salts were also evaluated for this reaction, and NH4I exhibited the highest activity and gave the corresponding product in 78% yield (Table 1, entry 9). Without I2 or NH4I, this reaction could not give better results. The temperature and the equivalents of iodine were also screened, but no improvement was obtained. So the optimized reaction system was established as shown in Table 1, entry 9.
| Entry | Catalysis | Oxidant | Additive | Yieldb |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), I2 (0.03 mmol), oxidant (0.6 mmol), additive (0.6 mmol), DMSO (2 mL), 150 °C. b Yields of isolated products. c I2 (0.36 mmol). d 120 °C. Entry in bold highlights optimized reaction conditions, and the reaction time was monitored by TLC. | ||||
| 1 | I2 | O2 | NH4OAc | 53 |
| 2 | I2 | TBP | NH4OAc | 70 |
| 3 | I2 | K2S2O8 | NH4OAc | 68 |
| 4 | I2 | DDQ | NH4OAc | 70 |
| 5 | I2 | BPO | NH4OAc | 66 |
| 6 | I2 | PIDA | NH4OAc | 27 |
| 7 | I2 | TBHP | NH4OAc | 73 |
| 8 | I2 | mCPBA | NH4OAc | 43 |
| 9 | I 2 | TBHP | NH 4 I | 78 |
| 10c | I2 | TBHP | NH4I | 63 |
| 11 | I2 | TBHP | NH4HCO3 | Trace |
| 12 | I2 | TBHP | (NH4)2SO4 | 55 |
| 13 | I2 | TBHP | NH4Cl | 71 |
| 14 | — | TBHP | NH4I | 46 |
| 15 | I2 | TBHP | — | 34 |
| 16d | I2 | TBHP | NH4I | 61 |
With the optimized conditions in hand, we explored the scope of synthesizing 3-methylthiofurans. As shown in Scheme 2, the scope of this transformation is very broad and both electron-withdrawing and electron-donating groups on the aromatic ring of the homopropargylic alcohols had good compatibility and gave the desired products in moderate to good yields. To our delight, different substituents on the homopropargylic alcohols did not significantly affect the efficiency of the reaction under the optimized conditions. Similarly, sterically hindered substrates also showed good reactivity and the target products were afforded in quite high yields except for 3j. The reaction scope was also investigated with alkyl substituted homopropargylic alcohols; the substrates 1ab and 1ac with alkyl groups reacted smoothly and gave the corresponding furans in 59% and 52% yields while substrate 1aa did not perform well in the process. The homopropargylic alcohol 1ad with a thienyl group displayed good compatibility and produced the desired furan 3ad in 50% yield. Unfortunately, the substrates 1ae, 1af, and 1ag could not tolerate this transformation and no desired products were isolated.
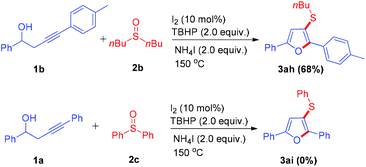
The methodology also can be extended to substituted sulfoxides (Scheme 3), and the sulfenylation/cyclization reaction of these substrates under standard conditions resulted in the formation of 1ag in 68% yield. However, the substrate sulfinyldibenzene 2c cannot generate the product through this transformation.
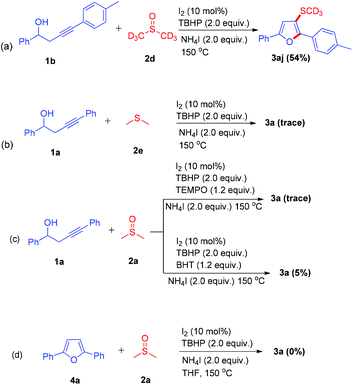
To gain insight into the reaction mechanism, several mechanistic experiments were carried out. First, a deuterium labeling experiment with DMSO-d6 was operated to explore the potential role of DMSO in the methylthiolation process (Scheme 4a). The desired deuterated product 3aj was generated from DMSO-d6 and this result disclosed that DMSO acts as a methylthiolation source. When dimethylsulfane 2e was tested, only trace amounts of the desired product were detected. (Scheme 4b) The radical scavengers TEMPO (2,2,6,6-tetramethylpiperidinooxy) and BHT (butylated hydroxytoluene) were also employed for this transformation and the yield of 3a was sharply decreased (Scheme 4c). This result suggested that this reaction underwent a radical pathway. When 2,5-diphenylfuran 4a was subjected to standard conditions, no desired furan was observed (Scheme 4d).
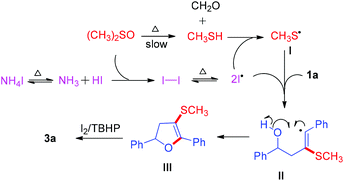
Based on these experimental results and previous mechanistic studies, a plausible mechanism is proposed in Scheme 5. Firstly, the NH4I decomposes to form NH3 and HI.
Then the iodine radical is generated from iodine.12 At the same time, the MeSH is afforded from DMSO under optimized conditions.13 The iodine radical attacks MeSH to yield methylthiyl radical I.14 Subsequently, the radical intermediate II, which is formed by the addition of radical I to the homopropargylic alcohol, is deprived of an electron by the iodine radical to generate dihydrofuran III through intramolecular radical addition.15 Finally, III is quickly transformed to the desired furan 3a under the I2/TBHP reaction system.
In conclusion, we have developed a simple and direct protocol to synthesize 3-alkylthiofurans from homopropargylic alcohols and DMSO via a tandem sulfenylation/cyclization reaction. In this transformation, homopropargylic alcohols with various substituted groups proceed smoothly with DMSO and the desired 3-methylthiofurans are obtained in moderate to good yields.
This work was supported by National Natural Science Foundation of China (21672086, 21202067), Gansu Province Science Foundation for Youths (1606RJYA260) and the Fundamental Research Funds for the Central Universities (lzujbky-2016-39).
Notes and references
- (a) Y. Jiang, A. J. J. Woortman, G. O. R. A. V. Ekenstein, D. M. Petrovic and K. Loos, Biomacromolecules, 2014, 15, 2482 CrossRef CAS PubMed; (b) M. B. Phillips, M. M. Sullivan, P. W. Villalta and L. A. Peterson, Chem. Res. Toxicol., 2014, 27, 129 CrossRef CAS PubMed; (c) A. P. Riley, B. M. Kivell and T. E. Prisinzano, J. Med. Chem., 2014, 57, 10464 CrossRef CAS PubMed.
- (a) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596 CrossRef CAS PubMed; (b) M. I. Konaklieva and B. J. Plotkin, Recent Pat. Anti-Infect. Drug Discovery, 2006, 1, 177 CrossRef CAS; (c) K. Kobayashi, R. Shimaoka, M. Kawahata, M. Yamanaka and K. Yamagu-chi, Org. Lett., 2006, 8, 2385 CrossRef CAS PubMed; (d) M. O. Awaleh, A. Badia and F. Brisse, Inorg. Chem., 2007, 46, 3185 CrossRef CAS PubMed; (e) U. A. Huber and D. Bergamin, Helv. Chim. Acta, 1993, 76, 2528 CrossRef CAS.
- (a) L.-N. Guo, X.-H. Duan and Y.-M. Liang, Acc. Chem. Res., 2011, 44, 111 CrossRef CAS PubMed; (b) Z.-X. Jia, Y.-C. Luo and P.-F. Xu, Org. Lett., 2011, 13, 832 CrossRef CAS PubMed; (c) B. Eftekharisis, M. Zirak and A. Akbari, Chem. Rev., 2013, 113, 2958 CrossRef CAS PubMed; (d) A. V. Gulevich, A. S. Dudnik, N. Chernyak and V. Gevorgyan, Chem. Rev., 2013, 113, 3084 CrossRef CAS PubMed; (e) A. Padwa, Chem. Soc. Rev., 2009, 38, 3072 RSC; (f) Z.-J. Zhang, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2013, 15, 4822 CrossRef CAS PubMed.
- (a) H. Pellissier, Chem. Rev., 2013, 113, 442 CrossRef CAS PubMed; (b) J. Yu, F. Shi and L.-Z. Gong, Acc. Chem. Res., 2011, 44, 1156 CrossRef CAS PubMed; (c) M. M. Hussain and P. J. Walsh, Acc. Chem. Res., 2008, 41, 883 CrossRef CAS PubMed; (d) J.-C. Wasilke, S. J. Obrey, R. T. Baker and G. C. Bazan, Chem. Rev., 2005, 105, 1001 CrossRef CAS PubMed.
- D.-Q. Chen, P. Gao, P.-X. Zhou, X.-R. Song, Y.-F. Qiu, X.-Y. Liu and Y.-M. Liang, Chem. Commun., 2015, 51, 6637 RSC.
- L.-L. Chu, X.-Y. Yue and F.-L. Qing, Org. Lett., 2010, 12, 1644 CrossRef CAS PubMed.
- (a) P. Sharma, S. Rohilla and N. Jain, J. Org. Chem., 2015, 80, 4116 CrossRef CAS PubMed; (b) W.-N. Zhao, P. Xie, Z.-G. Bian, A.-H. Zhou, H.-B. Ge, M. Zhang, Y.-C. Ding and L. Zheng, J. Org. Chem., 2015, 80, 9167 CrossRef CAS PubMed; (c) X.-F. Gao, X.-J. Pan, J. Gao, H.-F. Jiang, G.-Q. Yuan and Y.-W. Li, Org. Lett., 2015, 17, 1038 CrossRef CAS PubMed; (d) T. Shen, X.-Q. Huang, Y.-F. Liang and N. Jiao, Org. Lett., 2015, 17, 6186 CrossRef CAS PubMed; (e) F. Luo, C.-D. Pan, L.-P. Li, F. Chen and J. Cheng, Chem. Commun., 2011, 47, 5304 RSC; (f) X.-F. Gao, X.-J. Pan, J. Gao, H.-W. Huang, G.-Q. Yuan and Y.-W. Li, Chem. Commun., 2015, 51, 210 RSC; (g) H. Cao, S. Lei, N.-Y. Li, L.-B. Chen, J.-Y. Liu, H.-Y. Cai, S.-X. Qiu and J.-W. Tan, Chem. Commun., 2015, 51, 1823 RSC; (h) X.-J. Pan, Q. Liu, L.-M. Chang and G.-Q. Yuan, RSC Adv., 2015, 5, 51183 RSC; (i) A. A. Vieira, J. B. Azeredo, M. Godoi, C. Santi, E. N. S. Júnior and A. L. Braga, J. Org. Chem., 2015, 80, 2120 CrossRef CAS PubMed; (j) F.-L. Liu, J.-R. Chen, Y.-Q. Zou, Q. Wei and W.-J. Xiao, Org. Lett., 2014, 16, 3768 CrossRef CAS PubMed; (k) X.-F. Wu and K. Natte, Adv. Synth. Catal., 2016, 358, 336 CrossRef CAS; (l) Y. Xu, T. Cong, P. Liu and P. Sun, Org. Biomol. Chem., 2015, 13, 9742 RSC; (m) C. Ravi, D. C. Mohan and S. Adimurthy, Org. Biomol. Chem., 2016, 14, 2282 RSC; (n) J.-F. Zou, W.-S. Huang, L. Li, Z. Xu, Z.-J. Zheng, K.-F. Yang and L.-W. Xu, RSC Adv., 2015, 5, 30389 RSC; (o) C. Moussallem, F. Gohier and P. Frère, Tetrahedron Lett., 2015, 56, 5119 CrossRef; (p) Z. Fu, Z. Li, Q. Xiong and H. Cai, Eur. J. Org. Chem., 2014, 7798 CrossRef CAS; (q) P. J. Amal Joseph, S. Priyadarshini, M. Lakshmi Kantam and B. Sreedhar, Tetrahedron, 2013, 69, 8276 CrossRef; (r) J.-M. Ebenezer, K. Megha and M. Jakob, Synthesis, 2016, 1412 Search PubMed.
- (a) S. Liu, H.-L. Xi, J.-J. Zhang, X. Wu, Q.-H. Gao and A.-X. Wu, Org. Biomol. Chem., 2015, 13, 8807 RSC; (b) Q.-H. Gao, X. Wu, Y.-H. Li, S. Liu, X.-G. Meng and A.-X. Wu, Adv. Synth. Catal., 2014, 356, 2924 CrossRef CAS.
- G.-D. Yin, Z.-H. Wang, A.-H. Chen, M. Gao, A.-X. Wu and Y.-J. Pan, J. Org. Chem., 2008, 73, 3377 CrossRef CAS PubMed.
- M. Wang, J.-C. Xiang, Y. Cheng, Y.-D. Wu and A.-X. Wu, Org. Lett., 2016, 18, 524 CrossRef CAS PubMed.
- (a) X. Kang, R. L. Yan, G.-Q. Yu, X.-B. Pang, X.-X. Liu, X.-N. Li, L.-K. Xiang and G.-S. Huang, J. Org. Chem., 2014, 79, 10605 CrossRef CAS PubMed; (b) X.-B. Pang, L.-K. Xiang, X.-D. Yang and R.-L. Yan, Adv. Synth. Catal., 2016, 358, 321 CrossRef CAS; (c) R. Yan, X. Liu, C. Pan, X. Zhou, X. Li, X. Kang and G. Huang, Org. Lett., 2013, 15, 4876 CrossRef CAS PubMed; (d) R. Yan, X. Kang, X. Zhou, X. Li, X. Liu, L. Xiang, Y. Li and G. Huang, J. Org. Chem., 2014, 79, 465 CrossRef CAS PubMed; (e) L. Xiang, Y. Niu, X. Pang, X. Yang and R. Yan, Chem. Commun., 2015, 51, 6598 RSC; (f) R. Yan, X. Li, X. Yang, X. Kang, L. Xiang and G. Huang, Chem. Commun., 2015, 51, 2573 RSC.
- J. Gromada and K. Matyjaszewski, Macromolecules, 2001, 34, 7664 CrossRef CAS.
- (a) A. Smith and R. P. Calvert, J. Am. Chem. Soc., 1914, 36, 1363 CrossRef CAS; (b) H. Gilman and J. Eisch, J. Am. Chem. Soc., 1955, 77, 3862 CrossRef CAS; (c) V. J. Traynelis and W. L. Hergenrother, J. Org. Chem., 1964, 29, 221 CrossRef CAS.
- A. Fava, G. Reichenbach and U. Peron, J. Am. Chem. Soc., 1967, 89, 6696 CrossRef CAS.
- R. Walsh and S. W. Benson, J. Am. Chem. Soc., 1966, 88, 3480 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, and spectroscopic, analytical and X-ray data. CCDC 1495452. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00462h |
| This journal is © the Partner Organisations 2016 |