Beyond Fischer and Schrock carbenes: non-heteroatom-stabilized group 6 metal carbene complexes – a general overview†
Javier
Santamaría
* and
Enrique
Aguilar
 *
*
Instituto Universitario de Química Organometálica “Enrique Moles”, Departamento de Química Orgánica e Inorgánica, Universidad de Oviedo, C/Julián Clavería, 8. 33006, Oviedo, Spain. E-mail: jsv@uniovi.es; eah@uniovi.es; Fax: +34-985103 446
First published on 9th August 2016
Abstract
Non-heteroatom-stabilized group 6 metal carbene complexes are a particular type of Fischer carbene complex that lacks stabilizing heteroatoms. The aim of this review is to provide a comprehensive overview of the usefulness of these organometallic reagents in organic synthesis. Thus, the diverse methodologies that have been developed for their synthesis are initially disclosed, followed by their reactivities. In this regard, the decisive role of these complexes in early studies of alkene metathesis and cyclopropanation and enyne metathesis is highlighted, as well as their participation in insertion reactions. However, the major part of the review highlights recent achievements in both stoichiometric and catalytic processes, which have led to the synthesis of structurally diverse carbo- and heterocycles, acyclic compounds, and even metal carbene complexes of other groups by transmetallation reactions.
1. Introduction
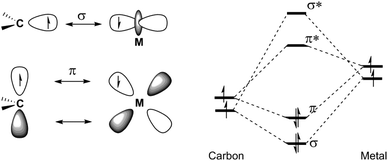
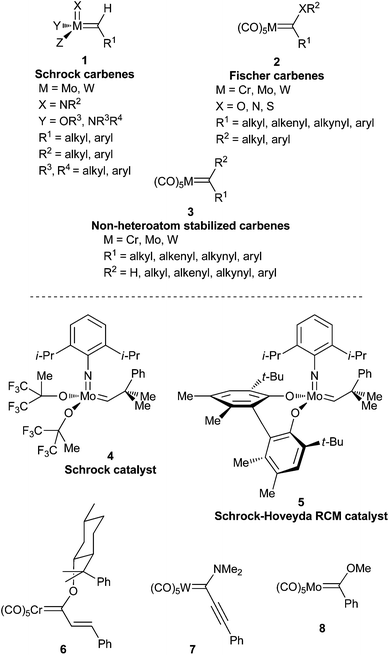
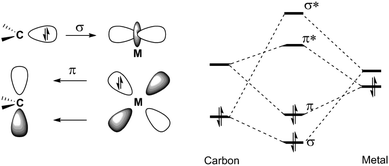
A carbene complex can be defined as an organometallic species composed of the formal combination of a carbene and a metallic fragment. Some carbene complexes of metals of several groups in the periodic table are stable at room temperature and have been synthesized and employed as reagents or catalysts in many synthetic transformations. Contrastingly, some other, less stable carbene complexes have been recently proposed, detected or, in some cases, isolated as synthetic intermediates. Although alkylidene complexes of group 6 metals in several oxidation states (0, +2, +4, +6) are known, carbene complexes are typically divided into two major categories:(a) Schrock-type carbene complexes or Schrock carbenes. In these carbenes, the metal is in a high oxidation state; they are nucleophilic in nature (the carbon atom of the carbene possesses a negative charge) and have short M–C-carbene bonds.1 The nucleophilicity of these compounds derives from the dominant orbital interactions between the metal and the carbene in the triplet state (Fig. 1).2 The most relevant carbene complexes of this type are Mo(VI)- or W(VI)-based carbenes 1 (Fig. 2). Indeed, despite their sensitivity to air and moisture, which requires their handling under strictly inert conditions (glove box or Schlenk techniques), molybdenum alkylidenes are amongst the most powerful olefin metathesis catalysts known to date. They are usually referred to as Schrock catalysts or Schrock alkylidenes; for example, Schrock catalyst 4 and Schrock–Hoveyda catalyst 5 (Fig. 2) are widely employed and commercially available.
(b) Fischer-type carbene complexes or Fischer carbenes. In contrast, for this type of carbene complex, the metal is in a low oxidation state (usually, they are metal(0) complexes); they have much longer M–C-carbene bonds than the high-valent complexes, and the pair of electrons in the π-bonding MO resides mainly on the metal. In this case, the bond is explained as an interaction between a carbene carbon in a singlet state with σ-donation from the carbene ligand to the metal and π-back donation from the metal to the ligand (Fig. 3).2,3 This has the effect of generating a positive charge on the carbon atom and, as a result, these carbenes are (1) electrophilic in nature and (2) stabilized due to the presence of an electron-donating heteroatom linked to the carbene carbon. They are highly valuable reagents in synthetic organic chemistry. Indeed, de Meijere coined the expression “chemical multitalents” to label these versatile complexes,4 as they display several patterns of reactivity which have led to the construction of a large variety of highly functionalized structures in a regio- and stereoselective manner.5 Usually, these complexes are employed as stoichiometric reagents, although they have occasionally been proposed as reactive intermediates in catalytic cycles.6 Because of their balance of reactivity and stability combined with easy accessibility, chromium complexes have found, by far, the broadest application of group 6 Fischer carbenes. A possible reason lies in the fact that they are more prone to carbonyl insertion than their tungsten or molybdenum analogues, mainly due to the differences in metal–CO strength through backbonding. The general formula for Fischer carbenes 2 and several representative examples are depicted in Fig. 2, including: (1) an alkoxy alkenylcarbene chromium complex bearing a chiral auxiliary 6, (2) an amino alkynylcarbene tungsten complex 7, and (3) an alkoxy arylcarbene molybdenum complex 8.
Non-heteroatom-stabilized carbene complexes 3 (Fig. 2) are a particular type of Fischer carbene complex that lacks stabilizing hetereoatoms. Therefore, they are low valent metal carbenes and are electrophilic in nature; however, they have received much less attention, mainly due to their low stability, particularly those with hydrogens at the carbene carbon. Although they have been frequently used as stoichiometric reagents, seminal contributions to the development of organic chemistry (i.e. alkene metathesis, enyne metathesis) as well as more recent transformations have arisen either from their employment as catalysts or by adopting roles as intermediates in catalytic processes.
The chemistry of non-heteroatom-stabilized carbene complexes has been partially reviewed;5a,7 however, a comprehensive manuscript covering all aspects of their rich chemistry has not been published to date. In this manuscript, even though a chronological line will not be strictly followed, an historical perspective will be used as a starting point to outline the developed methods of synthesis for these carbene complexes. Then, their different synthetic applications will be disclosed, ranging from their useful participation and their tremendous impact in early studies of alkene metathesis to the state of the art, which includes the synthesis of structurally complex compounds under stoichiometric or catalytic conditions and their role in multicomponent reactions or as precursors to carbene complexes of other metals by transmetallation reactions.
Usually, the stabilizing heteroatom in Fischer carbene complexes is directly linked to the carbene carbon. However, vinylogous stabilization occurs when a double bond is placed between the heteroatom and the carbene.8 Remote N-heterocyclic carbene complexes (rNHCs) can also be included in this family.9 Thus, although the carbene carbon is linked to two carbon moieties, they behave as stabilized carbenes; therefore, vinylogous stabilization and rNHCs will not be treated in this review.
On the other hand, in a similar manner, arylogous stabilization occurs when an arene is placed between the heteroatom and the carbene carbon; these cases are scarcer than vinylogous stabilization cases and have indeed been included in this review, mainly for the sake of comparison (for example, to point out the striking differences between carbenes with two phenyl or two p-anisyl substituents).
2. Synthesis and stability
The synthetic methods for non-heteroatom-stabilized carbene complexes fall into four strategies, as follows:2.1 Reaction of a gem-dichlorocompound with sodium pentacarbonylchromate
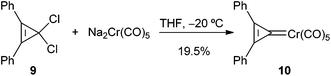
This approach was employed by Öfele to synthesize pentacarbonyl(2,3-diphenylcyclopropenylidene)chromium(0) 10, which was the first group 6 metal non-heteroatom-stabilized carbene complex prepared; it was isolated in 19.5% yield by treating 1,1-dichloro-2,3-diphenylcyclopropene 9 with sodium pentacarbonylchromate in THF at −20 °C (Scheme 1). Carbene complex 10 was described as astonishingly stable, due to the aromaticity of the cyclopropenyl carbene moiety: thus, it is a solid that is stable to air, decomposes above 192 °C in an inert atmosphere, and can be sublimed at 110 °C in vacuum without decomposition.102.2 Nucleophilic substitution of Fischer alkoxy carbene complexes
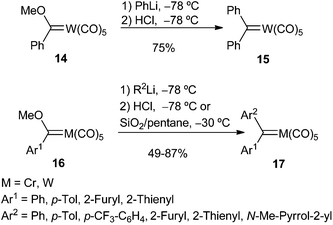
Nucleophilic substitution is a well known reaction in the chemistry of heteroatom-stabilized Fischer carbene complexes; it is particularly useful as a procedure to synthesize a new stabilized carbene complex from a pre-existing one by addition of an heteroatom nucleophile (alcohol, amine, thiol). This approach has been by far the most employed strategy for the synthesis of group 6 non-heteroatom-stabilized carbene complexes. Organolithium reagents, metal hydride complexes or enamines have been the nucleophiles employed. The reactions presumably proceed via nucleophilic addition to starting Fischer alkoxy carbene complex 11 leading to tetrahedral intermediate 12, which upon addition of the electrophile evolves with elimination of the E-OR2 moiety to generate non-heteroatom-stabilized carbene complex 13 (Scheme 2).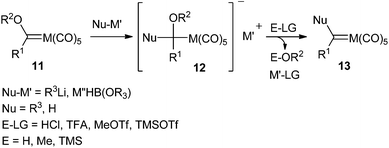 | ||
| Scheme 2 General strategy for the synthesis of non-heteroatom-stabilized carbene complexes via nucleophilic addition to Fischer alkoxy carbene complexes. | ||
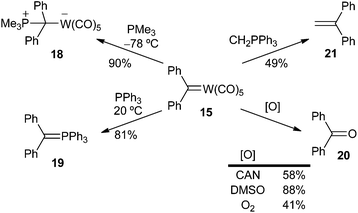
On the one hand, carbenes 17 are moderately air-stable solids, although they are more thermally labile than their methoxy aryl (or heteroaryl) carbene complex precursors 16. For instance, thermal decomposition of pentacarbonyl(diphenylmethylidene) tungsten (0) 15 in heptane completes in less than 2 hours at 100 °C, leading to W(CO)6, diphenylmethane and tetraphenylethylene.11a On the other hand, the metal plays an important role in the stability of the carbene complex; thus, chromium carbene complexes are more labile than their tungsten analogues.14
Evidence for the formation of carbene complexes was also obtained by several methods, as shown in Scheme 4 for carbene complex 15: (i) by trapping phosphine adducts 18, 19 formed by the reaction of 15 with phosphines,11b,15 (ii) by oxidation to the corresponding carbonyl compounds, such as 20, either with cerium ammonium nitrate (CAN), dimethyl sulfoxide (DMSO) or oxygen,11b (iii) by methylenation with methylenetrimethylphosporane, leading to 1,1-diphenylethylene 21,11b or (iv) by thermolysis (vide supra).11b
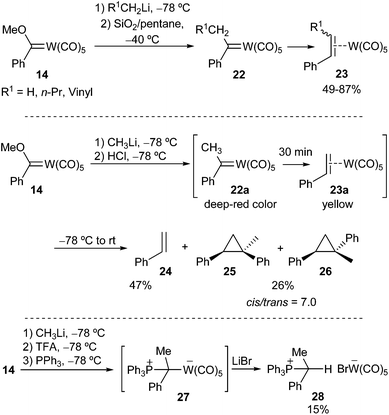
The formation of aryl alkyl carbene metal(0) complexes is more problematic, as they are highly unstable and can be prepared but not isolated. After addition of alkyllithium to solutions of methoxy phenyl(methylidene)tungsten(0) 14 at −78 °C, followed by treatment with silica gel, a brown (or deep red) colour was observed that was attributed to the formation of the desired aryl alkyl carbene metal(0) complexes 22 (Scheme 5). However, the colour lightened to yellow under low temperature reaction conditions, leading to the formation of pentacarbonyl π-olefin tungsten(0) complexes 23, which can be isolated (Scheme 5; top).16
Casey was also able to generate phenylethylidenecarbene complex 22a by successive addition of methyllithium and HCl to methoxy phenyl(methylene)tungsten(0) 14 at −78 °C; however, the deep red colour attributable to 22a faded within 30 min at that temperature. Warming the reaction mixture to room temperature led to the formation of styrene 24 (47%) and cis- and trans-1-methyl-1,2-diphenylcyclopropane 25, 26 (26%, in a cis/trans ratio of 7.0) (Scheme 5; middle). It was also demonstrated that the cyclopropane mixture did not result from direct cyclopropanation of styrene but involved two different tungsten organometallic species.17 Chemical evidence for the formation of carbene complex 22a (R1 = H) was achieved by reaction with PPh3 to generate its phosphine adduct 27, which was transformed into isolable tungsten complex 28 (Scheme 5; bottom).
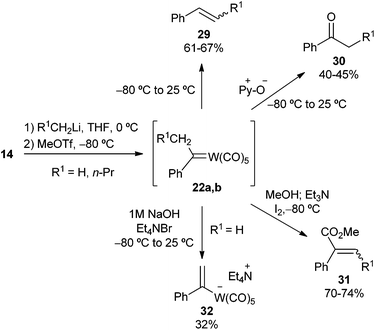
More recently, Barluenga generated non-heteroatom-stabilized tungsten carbene complexes 22a,b by adding methyl triflate at −80 °C to the lithium tungstate formed after treating 14 with alkyllithium species (R1 = H, n-Pr, Scheme 6).18 As expected, non-stabilized carbene complexes 22a,b could not be isolated nor characterized; however, they evolve to (i) furnish alkenes 29 when allowed to reach room temperature (61 to 67% yield, E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 1
Z = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) through 1,2-hydrogen migration and β-elimination reactions, (ii) afford phenones 30 (40 to 45% yield) when oxidized with pyridine oxide at −80 °C, (iii) produce methyl 2-phenylalkenoates 31 (70 to 74% yield, E
1) through 1,2-hydrogen migration and β-elimination reactions, (ii) afford phenones 30 (40 to 45% yield) when oxidized with pyridine oxide at −80 °C, (iii) produce methyl 2-phenylalkenoates 31 (70 to 74% yield, E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 1
Z = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) when treated under Iwasawa methoxycarbonylation conditions,19 and (iv) give the stable tetraethylammonium alkenyl tungstate 32 (R1 = H, 32% yield), which was fully characterized, when allowed to reach room temperature in the presence of aqueous 1 M NaOH/Et4NBr.
5) when treated under Iwasawa methoxycarbonylation conditions,19 and (iv) give the stable tetraethylammonium alkenyl tungstate 32 (R1 = H, 32% yield), which was fully characterized, when allowed to reach room temperature in the presence of aqueous 1 M NaOH/Et4NBr.
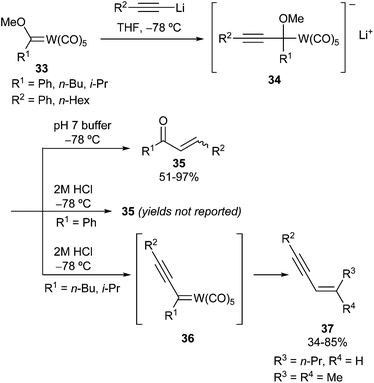
Iwasawa has pioneered the reaction of alkynyllithium species with Fischer tungsten carbene complexes 33. Smooth addition took place at −78 °C, presumably leading to tetrahedral intermediate 34, whose evolution depended on both the reaction conditions and the nature of the R1 carbene substituent20 (Scheme 7). On the one hand, neutral aqueous workup followed by mild acid treatment led to enones 35; on the other hand, acidic workup with 2 M HCl or TFA produced enynes 37 in moderate to good yields, presumably via non-heteroatom-stabilized carbene complexes 36, when R1 bears acidic hydrogen atoms (R1 = i-Pr, n-Bu). Alternatively, enones 35 are the major products, even under acidic conditions, when the β-elimination reaction cannot take place (R1 = Ph).
Therefore, alkynyllithium addition is preferred to deprotonation even in cases of primary alkyl-substituted carbene complexes. Moreover, from these results, it seems obvious that the use of alkynyllithium reagents is essential for the success of the addition reaction: carbene complexes 33 were recovered, and no addition products formed when phenyllithium or n-octyllithium was employed.
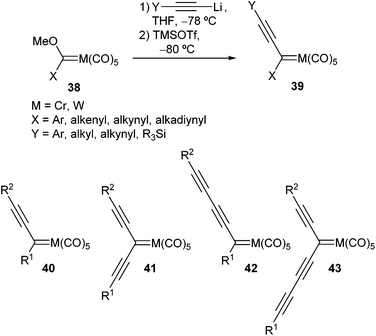
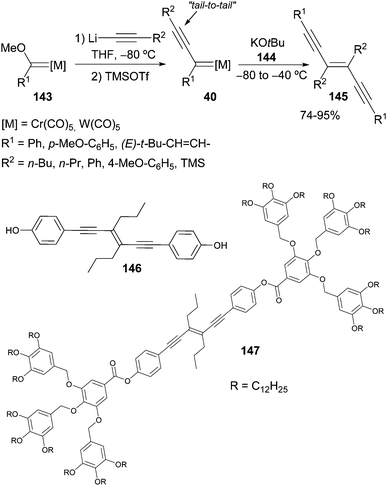
Following this pioneering work, Barluenga generated a plethora of chromium or tungsten non-heteroatom-stabilized alkynyl carbene complexes 39 at low temperature in THF by sequential treatment of alkoxycarbene complexes 38 with various lithium acetylides and trimethylsilyl triflate (TMSOTf) at −80 °C (Scheme 8).21 In these transformations, a change of colour is usually a good indicator of how the reaction is proceeding. For example, once the addition of lithium phenylacetylide was completed, the colour of the solution changed from orange or red to yellow. Then, after addition of TMSOTf, the solution underwent an instantaneous colour change, usually to deep blue. The presumed formation of alkynyl carbene complex by elimination of methyl trimethylsilyl ether from the tetrahedral addition species should account for the observed colour change.
Metal carbene scaffolds prepared by this method are outlined in Scheme 8; they have been classified according to the number of ethyne units in the dimer precursors: (i) alkynyl carbene complexes 40, (ii) cross-conjugated diynyl carbene complexes 41, (iii) linear-conjugated diynyl carbene complexes 42, and (iv) cross-conjugated triynyl carbene complexes 43.22 The structure of these non-heteroatom-stabilized carbene complexes was unambiguously established by X-ray analysis of a chromium alkynyl and a tungsten cross-conjugated diynyl carbene complex.
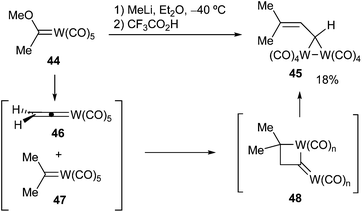
The formation of group 6 metal dialkyl carbene complexes remains rather elusive due to their high instability. Thus, the addition of methyllithium to 1-methoxyethylidene(pentacarbonyl)tungsten 44 resulted in the isolation of three-membered cyclic bimetallic compound 45.23 Its formation is proposed to proceed by [2 + 2]-cycloaddition between two intermediate tungsten complexes (ethynylidene 46 and pentacarbonyl dimethyl carbene complex 47), leading to a four-membered tungstenacycle 48, which should evolve into the final product (Scheme 9). For the purpose of this review, dimethyl carbene tungsten complex 47 has been proposed as an intermediate; however, it could not be isolated or detected.
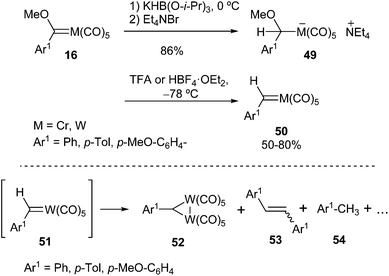 | ||
| Scheme 10 Synthesis, characterization and decomposition of chromium and tungsten benzylidene complexes. | ||
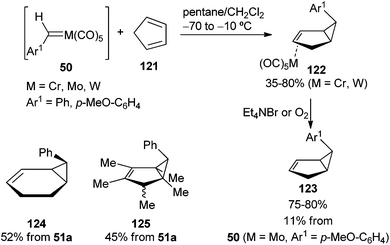
The thermal stabilities of complexes 50 are strongly influenced by the nature of the substituent R1 and the metal. Thus, for tungsten carbenes 51, the p-anisyl derivative (51b, Ar1 = p-MeO-C6H4) can be handled at room temperature for short periods of time; for the phenyl derivative (51a, Ar1 = Ph), the colour in solution is retained for several hours at −78 °C, fading to light orange upon warming to 0 °C, or it decomposes at −56 °C or 20 °C in solution with a half-life of ca. 24 min (−56 °C) or 2 min (20 °C), trans-stilbene being the major organic product observed by GC. Therefore, as expected, benzylidene complex 51a is more stable than methylphenyl carbene tungsten complex 22a. In addition to the cis and trans-stilbenes 53, other compounds detected in the thermolysis of carbene complexes 51 include the red-brown μ-benzylidenebis(pentacarbonyl)tungsten complexes 52 and the corresponding p-substituted toluene derivatives 54 (Scheme 10; bottom).
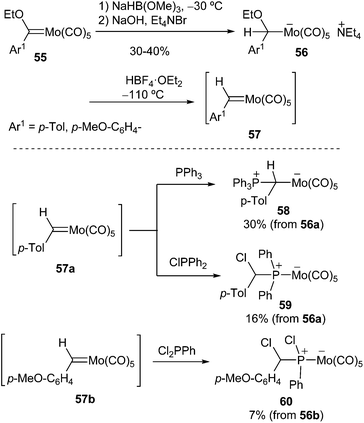
In a similar manner, benzylidene complexes of molybdenum 57 were prepared by abstraction of the ethoxy group of pentacarbonyl(α-ethoxybenzyl)molybdates 56 with HBF4·OEt2. Carbene complexes 57 are thermally very labile and react rapidly with nucleophiles even at −100 °C (Scheme 11). For example, they can be trapped with phosphines to form zwitterionic adducts, such as 58–60.26
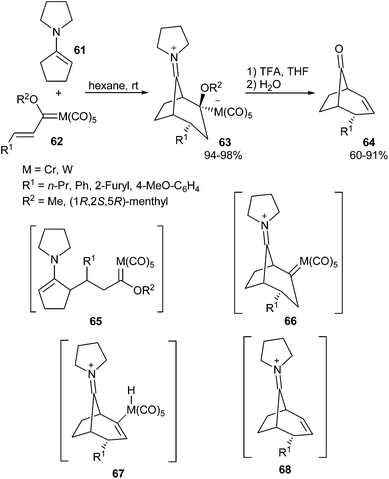 | ||
| Scheme 12 Non-heteroatom-stabilized metal carbenes from the reaction between alkenyl carbene complexes and enamines. | ||
Compounds 66 are the only examples of group 6 dialkyl carbene complexes reported to date. Although not isolated, tungsten carbene complexes 66a,b (M = W, R1 = Ph, 2-furyl) were generated from the corresponding zwitterionic metallate complexes 63a,b and were characterized by 13C NMR spectroscopy. Complexes 66a,b are fairly stable at −80 °C; however, they decompose above −70 °C to imonium salts 68 in a quantitative manner, via a carbon-to-metal hydrogen shift to species 67 followed by metal elimination. Imonium salts 68 have not been isolated but hydrolyse to 8-bicyclo[3.2.1]octanones 64.
2.3. Synthesis from diazocompounds with pentacarbonyl(η2-cis-cyclooctene)chromium(0)
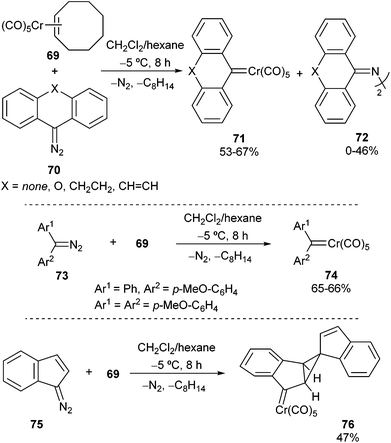
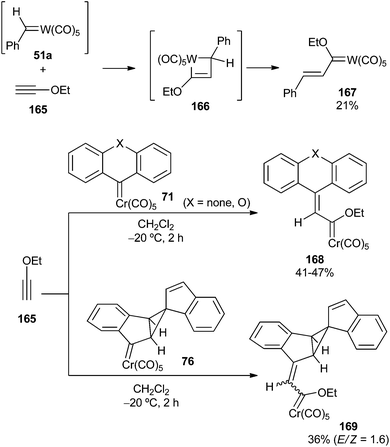
Diaryl carbene complexes of chromium can be synthesized by the reaction of diaryl diazo compounds 70 with pentacarbonyl(η2-cis-cyclooctene)chromium(0) 69 in mixtures of dichloromethane/hexane at −5 °C for 8 h. Crystallization at −78 °C followed by chromatographic work-up led to the desired carbene complexes 71 in moderate to good yields, along with the corresponding azines 72 (Scheme 13; top). Interestingly, carbene complex 71a (X = O) is stable under inert gas at room temperature due to vinylogous resonance stabilization, while other derivatives decompose above −20 °C.27Unbridged diaryl diazo compounds 73 also provided good yields of non-heteroatom-stabilized diaryl chromium carbene complexes 74 under the optimized reaction conditions (Scheme 13; middle). However, the presence of at least a methoxy group seems to be required to provide further carbene stabilization. Additionally, reaction of 1-diazo-1(1H)-indene 75 with pentacarbonyl(η2-cis-cyclooctene)chromium(0) 69 led to pentacyclic non-heteroatom-stabilized carbene complex 76, which incorporates two indene moieties; its formation may be explained by the high reactivity of the expected and initially formed carbene complex, which evolves through cyclopropane formation by reaction with the double bond of another molecule of carbene complex (Scheme 13; bottom).28
Two possible reaction pathways can be used to rationalize the formation of non-heteroatom-stabilized carbene complexes 71 by this methodology: (i) a direct electrophilic attack of the Lewis acid Cr(CO)5 at the diazo carbon to form 79, followed by loss of molecular nitrogen, or (ii) an end-on (77) or side-on (78) coordination of the diazo compound to the Cr(CO)5 fragment (which may behave either as a Lewis acid or as a π-acid) followed by metal migration to the diazo carbon and N2 elimination (Scheme 14).
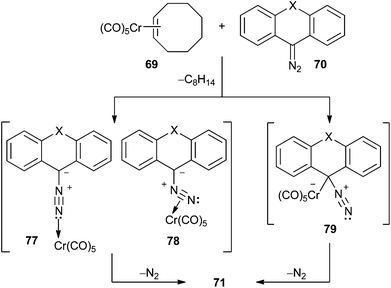 | ||
| Scheme 14 Alternative reaction pathways for the synthesis of non-heteroatom-stabilized metal carbene complexes 71 from diazo compounds. | ||
2.4 Synthesis from M(CO)5L and alkynes, conjugated dienynes or heterodienynes
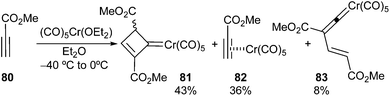
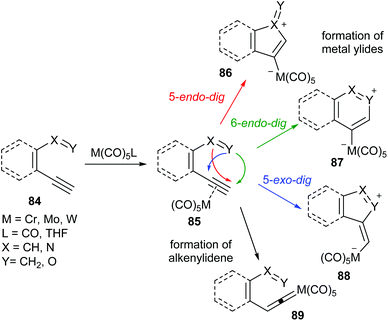
Group 6 metal carbonyl complexes can coordinate to C–C triple bonds. The formed π-complex may evolve differently depending on the reaction conditions and on the structure of the alkyne. Thus, 2,4-bis(methoxycarbonyl)-cyclobut-2-en-1-yliden-1-pentacarbonylchromium(0) 81 has been obtained as the major reaction product of the reaction of methyl propiolate with (CO)5Cr(OEt2) (Scheme 15).29 The synthesis of 81 involves the cyclocondensation of two units of methyl propiolate with the chromium carbonyl species.30 Two other minor reaction products, 82 and 83, which may be reaction intermediates, have also been isolated.Dienynes or heterodienynes of the general formula 84 have been shown to be suitable starting materials for the formation of non-heteroatom-stabilized group 6 carbene complexes by reaction with a suitable carbonyl metal complex (Scheme 16). In these transformations, the carbonyl metal complex acts as a π-acid, activating the triple bond, such as that in 85, for the ulterior cyclization. This cyclization may occur in 5-endo-dig, 6-endo-dig or 5-exo-dig modes depending on the nature of the starting material and the reaction conditions. In some cases, resulting metal-containing ylides (86–88) are resonant structures of non-heteroatom-stabilized carbene complexes, which may be isolated (see below), or, in other cases, may react with dipolarophiles to provide new non-heteroatom-stabilized carbene complexes, some of which have also been isolated. At this point, it is worth mentioning that complexes 86–88 could also be considered, depending on the nature of X and Y, to be double-vinylogous-stabilized Fischer carbenes.
An additional reaction pathway may take place by the formation of alkenylidene complex 89, which then may evolve by electrocyclization and lead to the reaction products, supposedly through non-heteroatom-stabilized carbene complexes. However, in these cases, these carbene complexes have been proposed as reaction intermediates but have neither been detected nor isolated.
All the situations where non-heteroatom-stabilized carbene complexes have been proposed as reaction intermediates but neither detected nor isolated will not be discussed here but in section 3.10.
Most of these reactions may occur in a catalytic manner, although stoichiometric conditions are sometimes required or, on other occasions, have been employed to isolate intermediate carbene complexes, as shown in the following two cases.
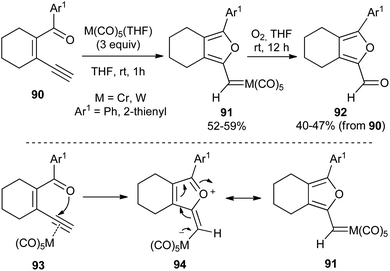
The synthesis of carbene complexes 91 is explained by initial coordination of the metal carbonyl to the triple bond, followed by 5-exo-dig cyclization by nucleophilic attack of the oxygen of the ketone carbonyl to the activated triple bond of 93 (Scheme 17). The formed metal carbonyl ylide 94 is indeed a resonant form of carbene complex 91 (Scheme 17; bottom).
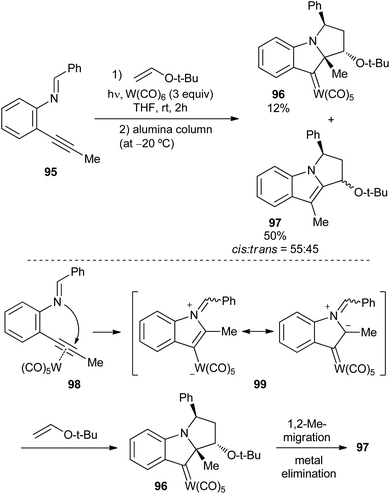 | ||
| Scheme 18 Synthesis of an (indolin-3-ylidene)pentacarbonyl tungsten complex from a tungsten-containing azomethine ylide. | ||
After the initial coordination of tungsten carbonyl to the triple bond, a 5-endo-dig cyclization would form tungsten azomethine ylide 99 by nucleophilic attack of the nitrogen to the activated triple bond of 98. Then, a [3 + 2]-cycloaddition should take place, leading to non-heteroatom-stabilized carbene complex 96, which may be isolated. In a later evolution, upon standing, compound 96 is transformed into indole derivative 97 through 1,2-methyl migration and metal elimination (Scheme 18; bottom).
3. Reactivity
3.1. Cyclopropanation and metathesis
For example, in early studies of alkene metathesis, prior to the development of Grubbs or Schrock carbene catalysts, Casey and co-workers studied the role of pentacarbonyl(diphenylmethylene) tungsten(0) 15 in reactions with alkenes to synthesize cyclopropanes.33 They found that both new alkenes originating from metathesis and cyclopropanes were formed in variable amounts, depending on the nature of the olefin. Thus, cyclopropane 101 was the major product for the reaction with ethyl vinyl ether 100, while 1,1-diphenylethylene 102 was mainly obtained when carbene 15 was reacted with isobutylene 103 (Scheme 19; top). Remarkably, only trace amounts of cyclopropane were observed for the reaction with α-methoxystyrene 105; 1,1-diphenylethylene and tungsten methoxy carbene complex 14 (both metathesis compounds) were the main products.
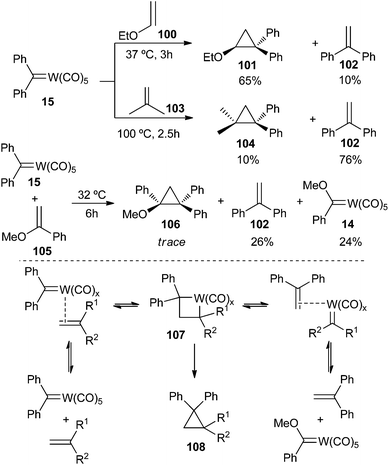 | ||
| Scheme 19 Cyclopropanation and metathesis reactions of pentacarbonyl(diphenylmethylene)tungsten(0) 15. | ||
This result gave a boost to the proposal of Chauvin for the mechanism involving the non-pairwise exchange of alkylidene units;34 indeed, metallacyclobutane 107 is a key intermediate in all these transformations. It should be generated by a rearrangement of the metal complex containing both an alkene and a carbene ligand, formed by the complexation of the alkene by the metal carbene. Metallacyclobutane 107 can evolve by reductive elimination to form cyclopropane 108 or by a retro-[2 + 2]-reaction to finally give 1,1-diphenylacetylene 102 and Fischer carbene complex 14 (Scheme 19; bottom).
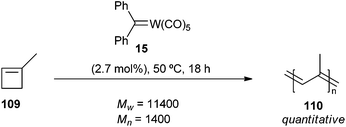
On the other hand, Katz showed that tungsten diphenylcarbene 15 was an effective initiator for cross-metathesis of terminal olefins (1-hexene, 1-octene), 1,1-disubstituted olefins (2-methyl-1-heptene and 2-methyl-1-pentene) or 1,2-disubstituted olefins (2-hexene) without requiring a Lewis acid co-catalyst.35 Carbene complex 15 can also catalyse the formation of polyisoprene 110 from 1-methylcyclobutene 109 as expected from a ring-opening metathesis polymerization (ROMP). The obtained polymer had a broad molecular weight distribution, with Mw = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 and Mn = 1400. These results also provided evidence of metal carbene complexes as intermediates in olefin metathesis reactions (Scheme 20).36
400 and Mn = 1400. These results also provided evidence of metal carbene complexes as intermediates in olefin metathesis reactions (Scheme 20).36
Moreover, in a study by Casey and co-workers, pentacarbonyl[di(p-tolyl)methylidene]tungsten(0) 17 (Ar1 = Ar2 = p-Tol; M = W) was a useful tool to provide information for the metathesis reaction; indeed, research with this carbene complex evidenced (i) the transfer of the less substituted alkylidene unit on an alkene to the initial carbene ligand and (ii) the higher reactivity of 17 (Ar1 = Ar2 = p-Tol; M = W) towards less substituted alkenes. Formation of cyclopropanes was observed as a side reaction in these experiments.13
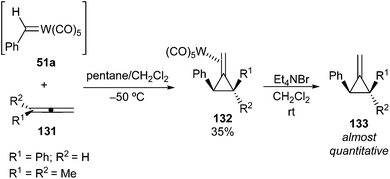
Asymmetrically substituted tungsten carbene complexes 22a and 51a were also tested as mechanistic probes for cyclopropanation and metathesis reactions (Scheme 21). Most of the research was performed with 51a due to the rapid decomposition of 22avia β-hydride elimination from the methyl group. Interestingly, only cyclopropanes were obtained in the reaction of 51a with alkenes, without formation of metathesis products. This fact emerges as the main difference in reactivity between carbenes 51a and 15. Additionally, cyclopropanation of alkenes with 51a takes place rapidly at −78 °C, while 15 requires 40 °C for reaction of the alkene to give cyclopropanes and metathesis products. Phenyl carbene complex 51a is a more electrophilic reagent and is more reactive toward the most substituted alkene, while diphenyl carbene complex 15 [and 17 (Ar1 = Ar2 = p-Tol; M = W)] displays the opposite behaviour.24 Some conclusions regarding the reactivity of alkenes with tungsten phenyl carbene 51a were also established: (i) cis and trans-2-butene form cyclopropanes with retention of the stereochemistry of the alkene precursor; (ii) the number of alkyl groups attached to the more substituted end of the carbon–carbon double bond determines the relative reactivity of the alkenes; (iii) the relative reactivity of monosubstituted alkenes is sterically controlled: it decreases as the bulk of the substituent increases (Scheme 21). Doyle also concluded that neither differential olefin coordination to a coordinatively unsaturated carbene system nor carbene exchange occur in the reactions of carbene complex 51a with alkenes.37
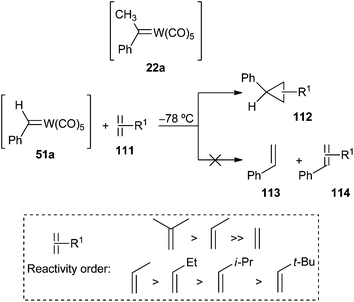 | ||
| Scheme 21 Cyclopropanation of alkenes with non-heteroatom-stabilized benzylidene tungsten complex 51a. | ||
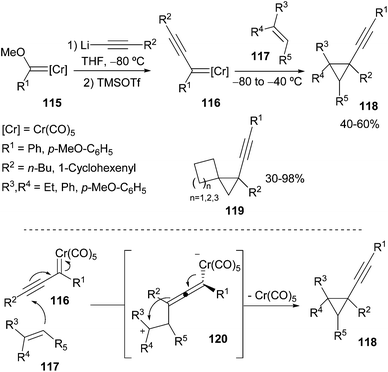
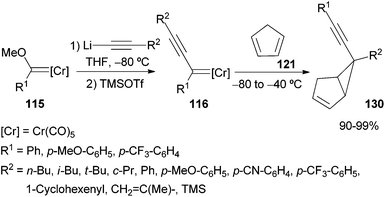
Finally, non-heteroatom-stabilized alkynyl chromium carbene complexes 116, synthesized in situ by Barluenga and co-workers from the corresponding alkoxycarbenes 115, can undergo cyclopropanation of non-activated olefins 117.38 The formation of the corresponding alkynylcyclopropanes 118 takes place in good yields and in an almost totally diastereoselective manner (Scheme 22; top). Alkynylspiro[2.3]hexanes, spiro[2.4]heptanes and spiro[2.5]octanes 119 are also accessible by this methodology. Cyclopropane formation occurs through an initial nucleophilic conjugate attack of the alkynylcarbene 116 to generate a zwitterionic intermediate 120 that evolves to the final product 118 by cyclopropane formation and metal elimination (Scheme 22; bottom).
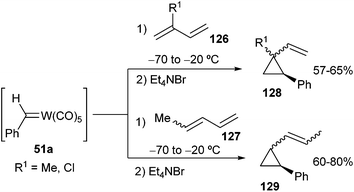
Scheme 24 describes the cyclopropanation of acyclic dienes, such as cis- and trans-pentadiene, isoprene and chloroprene, with 51a. This cyclopropanation is also regioselective and provides E and Z alkenylarylcyclopropanes 128, 129 with moderate diastereoselectivities (E/Z ratio = 0.22 to 0.52).
Similarly to simple non-activated olefins 117, non-heteroatom-stabilized alkynyl chromium carbene complexes 116 can also furnish cyclopentadiene cyclopropanation (Scheme 25). A large family of [3.1.0]bicyclic products 130 is obtained with total regio- and diastereoselectivity in almost quantitative yield.38 Thus, the endo-adducts are formed as single products through a nucleophilic attack of the cyclopentadiene to the conjugate carbon of the in situ synthesized non-heteroatom-stabilized chromium carbene complex 116. The gold catalysed isomerization of the resulting compounds 130 has been recently reported.40
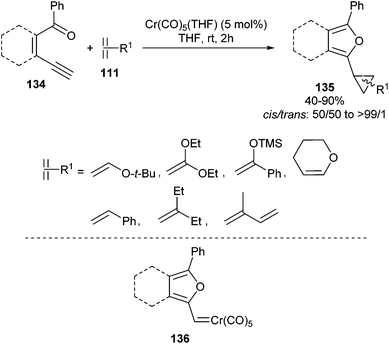
The most plausible reaction pathway should involve a 5-exo-dig cyclization of ene–yne–ketone 134 by nucleophilic attack of the carbonyl oxygen on the internal carbon of the π-acid activated triple bond, as depicted in Scheme 17. Furyl carbene complexes 136 are thus generated and can undergo cyclopropanation of the abovementioned double bonds, leading to 2-furylcyclopropanes 135 in moderate to excellent yields.
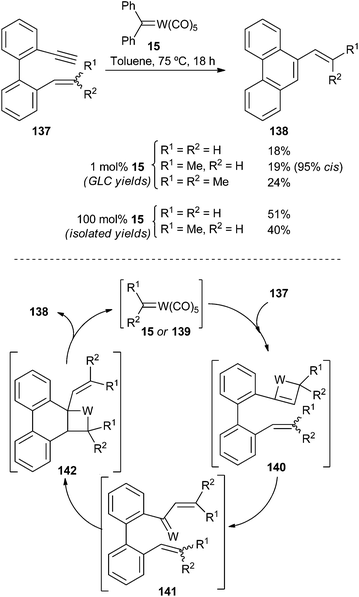 | ||
| Scheme 28 Non-heteroatom-stabilized carbene complex 15-catalysed ring-closing metathesis of enynes 137. | ||
The catalytic cycle should follow an yne-then-ene pathway, as usually occurs for Mo and W alkylidene complexes (Schrock type catalysts).43 Therefore, [2 + 2]-cycloaddition between the carbene catalyst species 139 and the triple bond should lead to tungstenacyclobutene 140. Next, a retro-[2 + 2]-cycloaddition would generate carbene complex 141, which should evolve by an intramolecular [2 + 2]-cycloaddition with the remaining double bond to form tungstenacyclobutane 142. A final retro-[2 + 2]-cycloaddition reaction will liberate 9-alkenylphenanthrenes 138 and regenerate the catalyst species 139.
It should be noted that non-heteroatom-stabilized carbene complexes are active intermediates in this catalytic cycle. In addition to 141, and regardless of the nature of the initiator (heteroatom stabilized or non-stabilized) carbene complex, the true catalytic species for the enyne metathesis cycle (Scheme 28) is indeed a non-stabilized carbene complex 15 or 139.
3.2. Dimerization
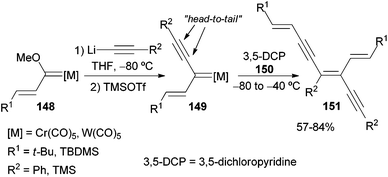
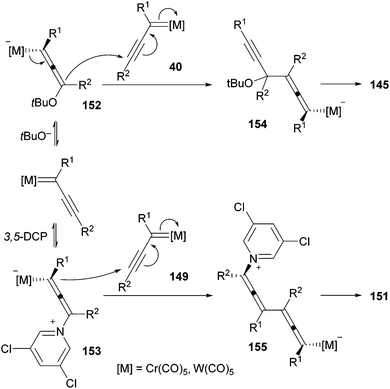
A large family of diethynylethenes is accessible through a dimerization reaction of non-heteroatom-stabilized group 6 alkynyl carbenes. Thus, carbene complexes 40 dimerize at low temperature, with complete chemo-, regio-, and steroselectivity in a nucleophile-induced reaction.22 The selectivity of the process, and consequently the structure of the endiyne adduct obtained, is highly dependent on the nature of the nucleophile used. Thus, the use of potassium tert-butoxide 144 as a nucleophile results in tail-to-tail dimerization of carbene complexes 40 in high yields. Following this methodology, different di-, tri- and tetraynylethenes 145 can be accessed (Scheme 29). This approach has also been applied to the synthesis of molecules such as 1,6-bis(4-hydroxyphenyl)-3-hexen-1,5-diyne 146, a precursor of compound 147 that self-assembles towards a columnar liquid-crystalline phase and organogels.45In addition to tail-to-tail dimerization, several dimers resulting from head-to-tail processes can also be accessed. The synthesis of 3-alkenylocta-3,7-dien-1,5-diynes 151 with excellent selectivity from the corresponding alkenyl-substituted carbene complexes 149 (generated in situ from Fischer carbene complexes 148) is described in Scheme 30. This reaction has been performed in the presence of 3,5-dichloropyridine (DCP) 150 as the nucleophile.22 Although small amounts of the head-to-head regioisomer have also been observed (head-to-tail/head-to-head ratio: 10/1 to >20/1), the major component can be isolated in pure form by flash chromatography.
A mechanistic proposal for the formation of the two different families of dimeric structures 145 and 151 is shown in Scheme 31. In both cases, the reaction should begin through a conjugate addition of the nucleophile [t-BuO− or 3,5-dichloropyridine] to the electrophilic β-carbon of non-heteroatom-stabilized carbene complexes 40, 149, leading to allenylmetallate species 152, 153. At this point, the reaction continues following two different pathways depending on the different nature of these two allenyl intermediates: anionic 152versus zwitterionic 153. Thus, intermediate 152 evolves with a conjugate attack through its propargylmetallate structure on the second equivalent of non-stabilized carbene complexes 40 to form intermediate 154. Finally, intermediate 154 would afford tail-to-tail dimer 145 upon elimination of tert-butoxide and the metal fragment. Contrastingly, zwitterionic intermediate 153 should undergo conjugate addition through its allenylmetallate structure to carbene complexes 149, leading to intermediate 155. Elimination of 3,5-dichloropyridine and the metal fragment from intermediate 155 would lead to head-to-tail dimer 151.
 | ||
| Scheme 31 Proposed mechanisms for the dimerization of non-heteroatom-stabilized alkynylcarbene complexes. | ||
3.3. C–H insertion
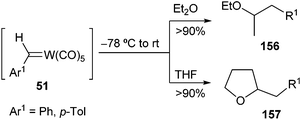
Tungsten benzylidene complexes 51 are able to regiospecifically insert the benzylidene ligand into the α-C–H bond of diethyl ether or THF in very high yield (>90%) (Scheme 32).46 The kinetics of the reaction in THF indicated that the insertion follows an associative mechanism initiated by nucleophilic attack of THF on the strongly electrophilic carbene carbon.3.4. Insertion of S or Se
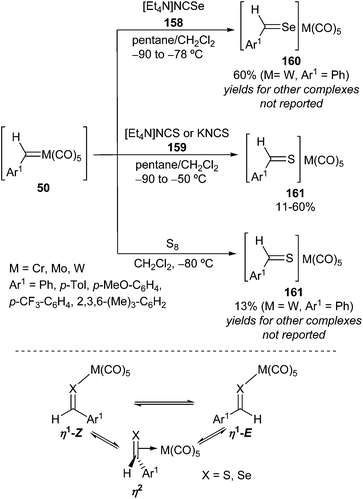
Insertion of selenium or sulphur into the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of a carbene complex has been observed by the reaction of benzylidene(pentacarbonyl) complexes of group 6 metals 50 with selenocyanates 158
C bond of a carbene complex has been observed by the reaction of benzylidene(pentacarbonyl) complexes of group 6 metals 50 with selenocyanates 158![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26,47 or thiocyanates 159 at low temperature to form chalcogenobenzaldehyde ligand complexes 160 and 161, respectively (Scheme 33).26,48
26,47 or thiocyanates 159 at low temperature to form chalcogenobenzaldehyde ligand complexes 160 and 161, respectively (Scheme 33).26,48
Thiobenzaldehyde complexes 161 may also be formed by the reaction of benzylidene(pentacarbonyl) complexes with elemental sulphur (Scheme 33).48
In solution, the chalcogenobenzaldehyde ligand may be bonded to the metal either in complexes 160 and 161 in a η1-fashion or in a dynamic equilibrium between two η1-forms and one η2-isomeric form (Scheme 33; bottom).47,48 The coordination mode depends on the metal, the chalcogen, the aryl group, the solvent and the temperature.
3.5. Dötz reaction
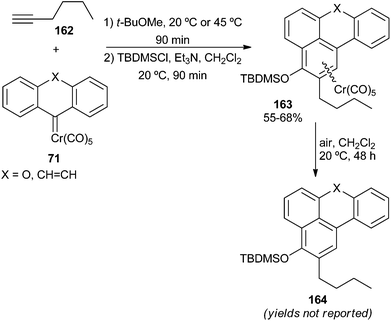
The Dötz benzannulation reaction is one of the most useful reactions in the chemistry of Fischer carbene complexes.49 It involves the formation of a benzene ring and the creation of three carbon–carbon bonds by the coupling of an internal or terminal alkyne and two ligands (the carbene and a carbonyl) from a Fischer alkenyl or aryl carbene complex. The alkyne partner is regioselectively inserted into the metal–carbene bond, with the smaller alkyne substituent placed closer to the carbene carbon. The subsequent insertion of a carbonyl ligand allows the synthesis of phenols (from alkenylcarbenes) or naphthols (from arylcarbenes). For non-heteroatom-stabilized carbene complexes, the only case reported to date corresponds to the reaction of symmetric chromium carbene complexes 71 with 1-hexyne.27 The resulting naphthol derivative is protected in situ with TBDMS chloride in moderate yields. Air oxidation to remove the coordinated chromium fragment of 163 allows the regiochemically controlled synthesis of oxygenated benzo[k,l]xanthenes (X = O) or cyclohepta[1,2,3-de]naphthalenes (X = CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) 164 (Scheme 34).
CH) 164 (Scheme 34).
 | ||
| Scheme 34 Dötz benzannulation reaction between non-heteroatom-stabilized chromium carbene complexes 71 and 1-hexyne. | ||
Interestingly, no benzannulation reaction took place with fluorenylidene complex 71 (X = none); instead, carbene dimerization and oxidation were observed. This result was attributed to hampering of the electrocyclic ring closure mechanistic step due to smaller bond angles in the central five-membered ring.27
3.6. Reaction with ynol ethers and ynamines
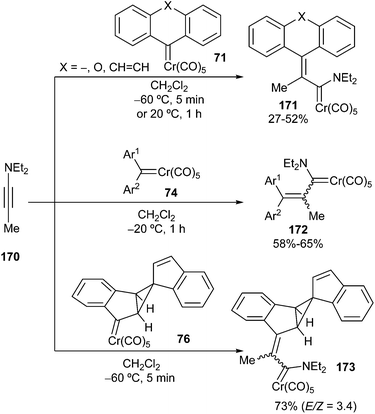
Electron-rich alkynes, such as ynol ethers or ynamines, undergo insertion into the metal–carbene bond, leading to new stabilized Fischer carbene complexes. The insertion follows a [2 + 2]-/retro-[2 + 2] cycloaddition sequence with the formation of metallacyclobutene intermediate 166. In contrast to the Dötz benzannulation reaction, once the electron-rich alkyne is inserted, the reaction does not proceed further due to the high stability of the newly formed carbene complex. Alkoxy alkenyl carbene complexes 167–169 were synthesized by this procedure when ethoxyacetylene 165 was employed as an alkyne partner (Scheme 35).24b,28Ynamine 170 displays similar behaviour, and its reactions with non-heteroatom-stabilized carbene complexes 71, 74 or 76 lead to amino alkenyl carbenes 171–173 in moderate yields and diastereoselectivities (for unsymmetrical starting carbenes 74 or 76)28 (Scheme 36).
3.7. Reaction with 2-methoxyfuran
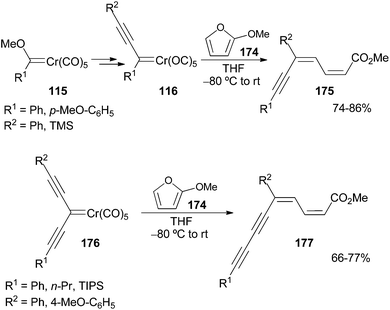
Non-heteroatom-stabilized alkynyl chromium carbene complexes 116, synthesized in situ by the previously reported methodology (section 2.2.1), react with 2-methoxyfuran 174 to form linear dienyne adducts 175 in good yields (Scheme 37). The reaction occurs upon warming the reaction mixture from low to room temperature.50 The reaction is initiated by a conjugate attack of the 2-metoxyfuran 174 on the alkynylcarbene 116, involving formal 1,2-migration of the triple bond and opening of the furan ring. The overall procedure represents a regioselective olefination at Cβ of the alkynyl carbene complexes 116. This approach has been applied to form linear dienediyne adducts 177 from cross-conjugated diyne carbene complexes 176.3.8. Reaction with heterocumulenes
Benzylidene tungsten complex 51a quickly reacts with triphenylketenimine 178 at −70 °C to form a red zwitterionic tungsten complex 179 in 80% yield (Scheme 38; top). A nucleophilic addition of the ketenimine 178 to the carbene 51a, leading to zwitterionic species 180, followed by 1,3-tungsten migration would account for the observed results.51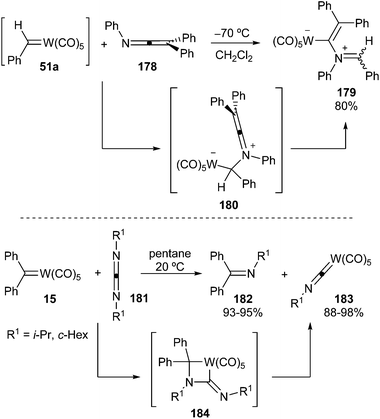 | ||
| Scheme 38 Reactivity of non-heteroatom-stabilized tungsten carbene complexes against heterocumulenes. | ||
On the other hand, non-heteroatom-stabilized carbene complex 15 undergoes metathesis at room temperature with symmetric dialkylcarbodiimides 181 to form imines 182 and alkylisocyanide(pentacarbonyl)tungsten complexes 183 in excellent yields (Scheme 38; bottom). The reaction course can be readily explained through azatungstacyclobutane intermediate 184.52
3.9. Reactions with imines
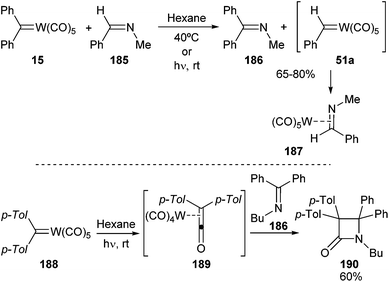 | ||
| Scheme 39 Metathesis and lactonization of non-heteroatom-stabilized tungsten carbene complexes with imines. | ||
However, a similar reaction performed with keteneimine 186 progresses only under photochemical conditions and provides β-lactam 190 by a formal [2 + 2]-cycloaddition of imine 186 with tungsten ketene complex 189 (Scheme 39; bottom).
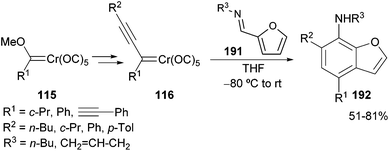
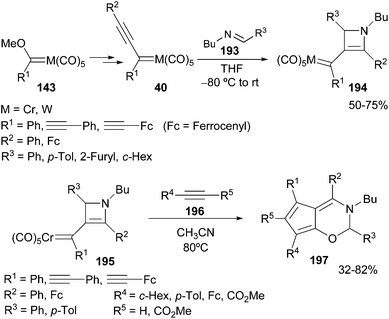
The formation of azetinyl carbenes 194 is also possible with furfural imines. In this case, the synthesis of the azetinyl derivative 194 competes with the formation of the corresponding benzofuran 192 (vide supra). However, the reaction can be driven to one product or the other depending on the electronic nature of the substitution pattern of the non-heteroatom-stabilized carbene complexes 40.55
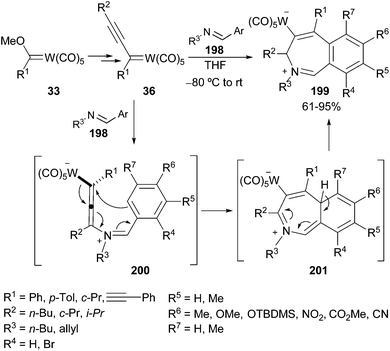 | ||
| Scheme 42 Regioselective synthesis of benzo[c]azepinyl derivatives 199 from alkynyl carbenes 36 and imines. | ||
Finally, taking into account the electrophilic and also the nucleophilic nature of the zwitterionic benzoazepinum tungstates 201, several reactions have been accomplished with this compound, allowing access to a high number of benzo- and dihydrobenzo[c]azepines with an important degree of substitution.
3.10. Stoichiometric or catalytic transformations involving non-stabilized carbene complexes as intermediates
In this section, several stoichiometric or catalytic transformations involving non-heteroatom-stabilized carbene complexes as presumed intermediates are covered. A general feature of all of these transformations is the employment of group 6 metal carbonyls as π-acids to activate triple bonds and their further evolution through several reaction pathways depending on the nature of the intramolecular nucleophile (see Scheme 16).When the internal alkyne substituent bears a conjugate double bond, as in 208, seven-membered heterocycles 210 may be formed as a result of a formal [5 + 2]-dipolar cycloaddition (Scheme 44).60 The reaction proceeds smoothly, even with a 10 mol% W(CO)6 loading, through tungsten-containing azomethine ylide 211. Then, a [5 + 2]-cycloaddition should take place to form non-heteroatom-stabilized tungsten carbene complex intermediate 212, which evolves into the final azepino[1,2-a]indole derivative 210 (Scheme 44; top). Tricyclic indoles formed by [3 + 2]-cycloaddition are sometimes obtained as by-products or even as major products, especially with sterically less demanding acetals and high tungsten hexacarbonyl loadings.
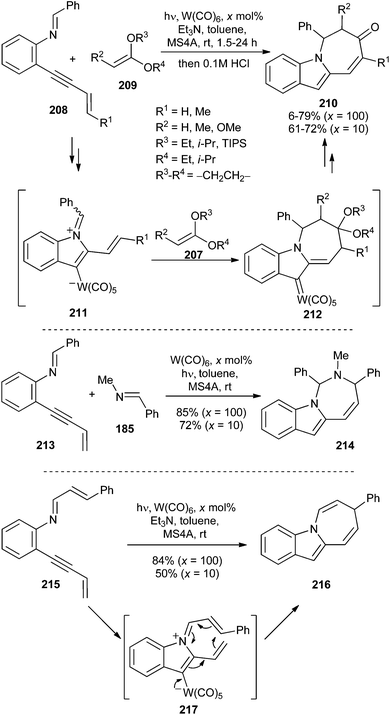 | ||
| Scheme 44 Synthesis of seven-membered heterocycles by tungsten carbonyl-catalysed reactions of N-(o-alkynylphenyl)imine derivatives. | ||
This transformation could be extended to the employment of imines 185 as dipolarophiles, as illustrated by the synthesis of diazepine derivative 214 in good yield, as a mixture of diastereomers (Scheme 44; middle).
An intramolecular example has also been reported: good yields of azepino[1,2-a]indole derivative 216 were obtained from 215 through [1,7]-electrocyclization of tungsten-containing azomethine ylide 217 (Scheme 44; bottom).
More recently, it was found that other metal complexes are able to catalyze this transformation efficiently, particularly ReBr(CO)5, AuBr3 or PtCl2. In this regard, PtCl2 was the catalyst choice for a study toward mitomycin C synthesis from imidate derivatives.61
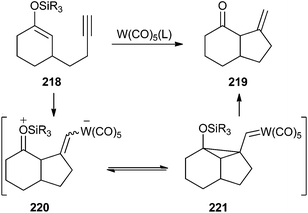
Iwasawa also reported the W(CO)5(L)-catalysed 5-exo-cyclization of ω-allenyl or ω-acetylenic silyl enol ethers, such as 218, to form enones, such as 219.62,63 In a similar manner, ω-iodoacetylenic silyl enol ethers undergo cyclization to give endo-cyclized products in good yield, although stoichiometric amounts of W(CO)5(THF) are required.64 Their mechanistic proposal for the non-iodinated acetylenes 218 suggests the participation of zwitterionic addition species 220 as intermediates for this transformation. However, theoretical studies performed on the reaction found cyclopropyl carbene structure 221 to be the favourable species in the gas phase (Scheme 45).65
The previous result prompted a search for reactions with low activation energies which could be feasible through cyclopropyl carbene intermediates, such as 221.66 Indeed, according to this approach, bicyclo[5.3.0]decane derivatives 223 can be readily prepared in good yields by the intramolecular W(CO)5(THF)-catalysed reaction of substrates 222, which contain 2-silyloxydiene and conjugate enyne moieties in their structures (Scheme 46; top). The reaction tolerates a wide range of substituents on the dienol silyl ether skeleton. Indeed, this reaction has been applied to the synthesis of tricyclic compounds such as 224 and 225 using the appropriate starting materials: dienol silyl ethers bearing a cyclohexenyl group as part of the diene moiety or at the alkyne terminus (Scheme 46; middle).
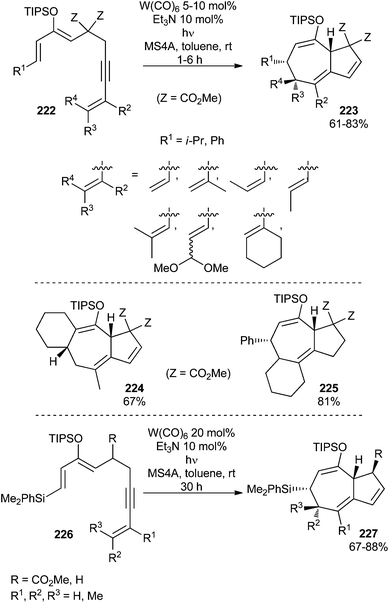 | ||
| Scheme 46 Tungsten carbonyl complex-catalysed stereoselective synthesis of bicyclo[5.3.0]decanes 227. | ||
The synthetic strategy has also been successfully extended to mono-ester substituted substrates (226, R = CO2Me) and to dienynes having no substituent at the 5-position (226, R = H) to provide bicyclo[5.3.0]decanes 227 (Scheme 46; bottom).67
The overall sequence is stereospecific and highly diastereoselective. Regarding the two contiguous stereocenters generated at the seven-membered ring, all four stereoisomers may be prepared by choosing the appropriate combination of the configurations of the silyl enol ether and enyne double bonds. Indeed, important mechanistic considerations can be inferred from stereochemical analysis. Thus, after the initial 5-endo-dig cyclization, two mechanisms seem to be operating depending on the silyl enol ether double bond configuration: (a) for (Z)-enol silyl ether 222, a Cope rearrangement of cis-divinylcyclopropane carbene complex intermediate 229 would explain the configuration observed in the final product 223 (Scheme 47; top); (b) for (E)-enol silyl ether 231, nucleophilic addition of the dienyl tungsten moiety to the α,β-unsaturated silyloxonium moiety in the zwitterionic intermediate 232, would lead to the bicyclo[5.3.0]decane skeleton 234 (Scheme 47; bottom). This alternative mechanism overcomes the limitation caused by the impossibility of a Cope rearrangement in a trans-divinylcyclopropane carbene complex.
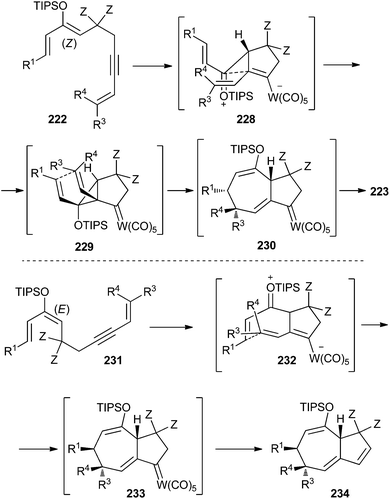 | ||
| Scheme 47 Proposed mechanisms for tungsten carbonyl complexes-catalysed stereoselective synthesis of bicyclo[5.3.0]decane frameworks. | ||
In a similar manner, 1-azabicyclo[5.3.0]decane derivatives 236 have been formed by treating starting thioimidates 235 with catalytic amounts of chromium hexacarbonyl (as low as 2 mol%).68 A varied substitution pattern on the starting thioimidates is tolerated for this transformation. The stereochemical results obtained under photochemical or thermal conditions were identical. This fact, together with the relative stereochemistry of the products, suggests that the reaction sequence occurs through conrotatory 1,7-electrocyclization of zwitterionic intermediates 237, the opposite way to that of the carbon analogues, to form non-heteroatom-stabilized chromium carbene complexes 238, which evolve into the final 1-azabicyclo[5.3.0]decane derivatives 236 (Scheme 48).
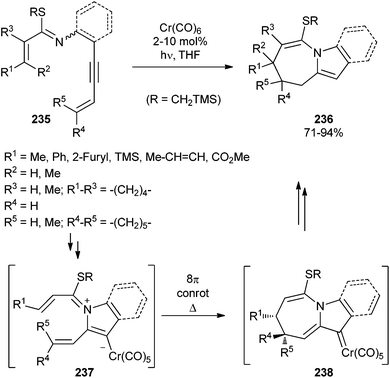 | ||
| Scheme 48 Chromium hexacarbonyl-catalysed stereoselective synthesis of 1-azabicyclo[5.3.0]decane frameworks. | ||
Additionally, an external component can be incorporated when the reaction is performed in the presence of a nucleophile, leading to tricyclic indoles 240 bearing up to three consecutive stereocenters (Scheme 49).68 Methanol or a silyl ketene ketal have been employed as nucleophiles. In most cases, only single isomers are obtained due to stereoselective 1,4-addition of the nucleophile to the less hindered face of the α,β-unsaturated non-heteroatom-stabilized carbene complex intermediate 241.
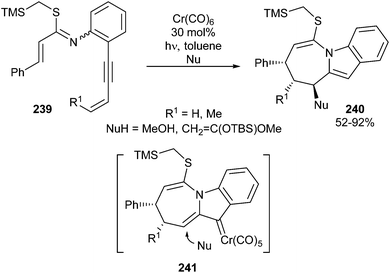 | ||
| Scheme 49 Reaction sequence of chromium hexacarbonyl-catalysed cyclization/1,4-addition of nucleophiles. | ||
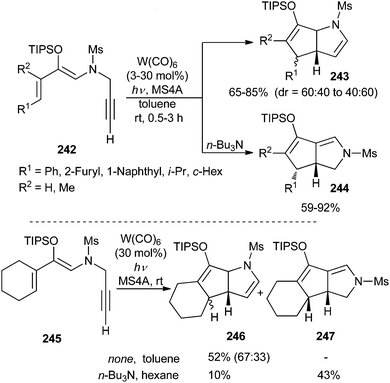
Terminal acetylenic dienol silyl enol ethers 242 also undergo W(CO)6 catalysed cyclization to form different types of nitrogen-containing bicyclic compounds.69 Thus, 2-azabicyclo[3.3.0]octane derivatives 243 are obtained as mixtures of diastereomers when the reaction is carried out in the presence of MS4A under photoirradiation. On the other hand, isomeric 3-azabicyclo[3.3.0]octanes 244 are formed when the reaction is performed under identical conditions but with tri-n-butylamine as an additive (Scheme 50; top). Both reactions are completely selective for substrates having an aryl group at the diene terminus, although small amounts of 2-azabicyclo[3.3.0]octanes are formed as by-products in the amine promoted reaction for substrates having an alkyl group at that position.
Additionally, tricyclic products 246 and 247 have been obtained in reasonable yields from dienol silyl ether 245, having a cyclohexenyl group at the diene moiety (Scheme 50; bottom).
These results can be explained by considering an equilibrium between π-alkyne complex 248 and alkenylidene complex 249 (Scheme 51). In the absence of tri-n-butylamine, the reaction could evolve by an initial 5-endo-dig nucleophilic cyclization to form zwitterionic tungstate 250, followed by a Michael-type addition to produce bicyclic non-heteroatom-stabilized carbene complex 251, which finally should lead to 2-azabicyclo[3.3.0]octane 243.
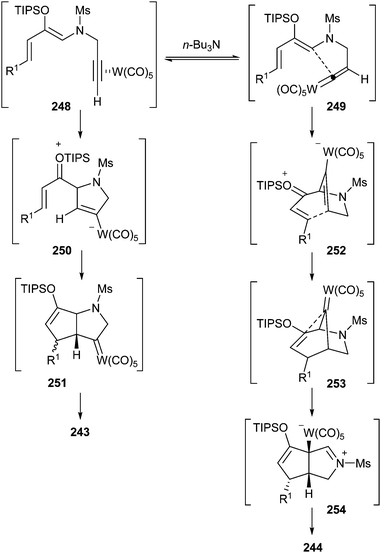 | ||
| Scheme 51 Proposed mechanism for the tungsten hexacarbonyl-catalysed synthesis of 2- or 3-azabicyclo[3.3.0]octane frameworks. | ||
On the other hand, the addition of the tertiary amine facilitates the tautomerization to tungsten alkenylidene 249 (for other transformations initiated by formation of an alkenylidene, see section 3.10.3). Then, nucleophilic attack of the enol silyl ether at the alkenylidene carbon would generate zwitterionic tungstate 252, and its evolution by Michael-type addition to the α,β-unsaturated silyloxonium moiety would produce non-heteroatom-stabilized bridged-carbene complex 253. Then, facilitated by electron donation from the nitrogen atom, a 1,2-alkyl migration should take place to form zwitterionic species 254. Finally, a metal elimination from intermediate 254 would form 3-azabicyclo[3.3.0]octane 244 (Scheme 51). Deuterium- and 13C-labelling experiments support the proposed mechanism.
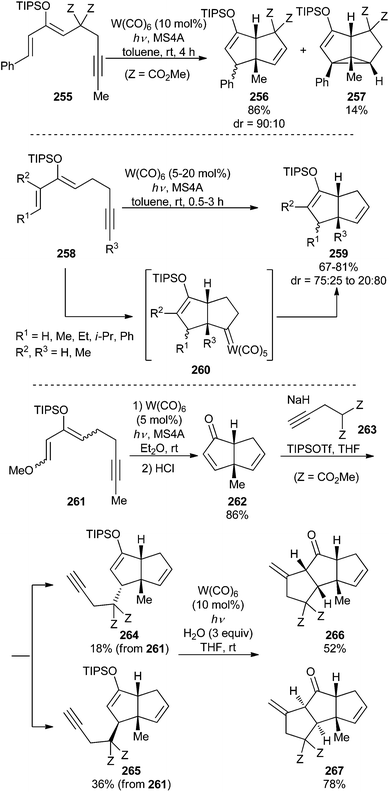
In a similar manner, bicyclo[3.3.0]octane derivative 256 is obtained as mixture of isomers when the analogous all-carbon skeleton acetylenic dienol silyl ether 255 is treated with a catalytic amount of W(CO)6 (Scheme 52). The isolation of tricyclic compound 257 as a by-product indicates the presence of a carbene complex intermediate (Scheme 52; top).70
The scope of the reaction indicates that presence of gem-diester groups is not necessary for the reaction to proceed. Thus, a variety of bicyclo[3.3.0]octane derivatives 259 are formed with moderate yields and stereoselectivities from acetylenic dienol silyl ethers 258. A mechanism analogous to the one depicted in Scheme 51 would explain the formation of the reaction products through non-heteroatom-stabilized carbene complex intermediate 260 (Scheme 52; middle). A rhenium complex, [ReCl(CO)5], also catalyzes this triple-bond geminal carbo-functionalization reaction very efficiently (68 to 92% yield) with lower catalyst loading.
This tandem cyclization protocol has been applied to a concise synthesis of the basic carbon skeleton of triquinanes (Scheme 52; bottom). Thus, tungsten carbonyl (5 mol%) treatment of dienol silyl ether 261, containing a methoxy group at the diene terminus, followed by hydrolysis, led to bicyclic ketone 262 in very good yield. Conjugate addition of dimethyl propargylmalonate sodium salt 263 in the presence of TIPSOTf provided distereomeric ω-acetylenic silyl enol ethers 264 and 265 in moderate yields. A new photoirradiation, in the presence of catalytic amounts of tungsten carbonyl and water, gave tricyclic ketones 266 and 267 bearing a triquinane skeleton.
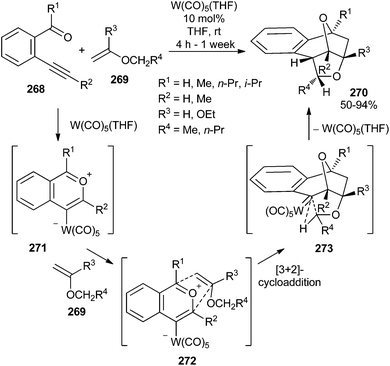 | ||
| Scheme 53 Synthesis of polycyclic compounds 270 by tungsten carbonyl-catalysed rearrangement of o-ethynylphenylketones. | ||
Interestingly, the absence of the ketene acetal leads to the formation of a benzopyranylidene complex.
Therefore, three different possible reaction pathways have been reported for the tungsten carbonyl mediated cyclization of o-ethynylphenylketone derivatives 274 once generation of activated alkyne complex 275 has been accomplished (Scheme 54):
(a) 5-exo-dig cyclization, leading to the formation of tungsten-containing carbonyl ylide 277, which is a resonance form of furyl carbene complex 276. As previously mentioned in Schemes 17 and 27, this pathway has been observed also for β-ethynyl α,β-unsaturated ketones. Additionally, 1,2-bis (acetyl)benzene 279 (R1 = Me) was isolated in approximately 50% yield when the reaction was performed in the presence of 5 equiv. of H2O.71b Compound 279 is presumably produced by hydrolysis of NMR-observed methyleneisobenzofuran 278, which originates from 277 by deprotonation from the R1 group and protonation of the tungsten–carbon bond.
(b) 6-endo-dig cyclization, to generate tungsten carbonyl ylide 280, which will explain the synthesis of bicyclic compounds 281 and the results described in Scheme 55 (vide infra).
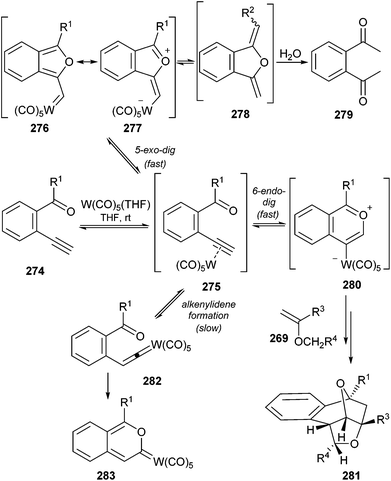 | ||
| Scheme 54 Alternative reaction pathways for tungsten hexacarbonyl-catalysed reactions of o-ethynylphenylketones. | ||
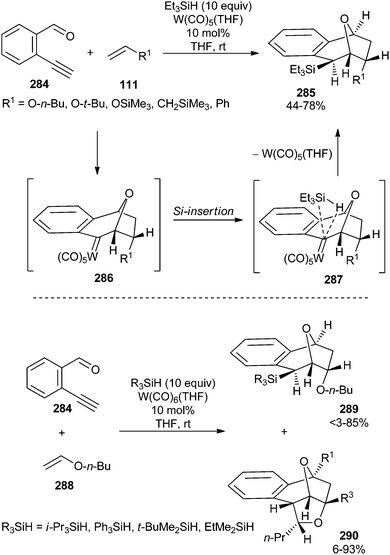 | ||
| Scheme 55 Trapping of non-stabilized carbene complex intermediate 286 with silanes. Synthesis of polycyclic tetralkylsilanes. | ||
(c) Formation of alkenylidene282, by a 1,2-hydrogen shift, which leads to benzopyranylidene tungsten Fischer carbene complex 283.72
The obtained results pointed out that, presumably, pathways (a) and (b) are faster than (c), which occurs in the absence of trapping agent (H2O, alkene). Additionally, (a) and (b) should be reversible and in equilibrium through metal-complexed species 277, while the formation of tungsten carbene complex 283 forces pathway (c) to be irreversible.
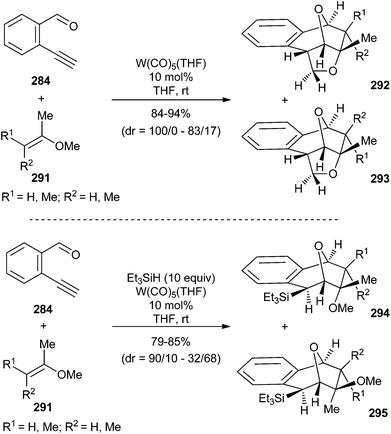
Intermediate polycyclic carbene complexes in these transformations (273, Scheme 53; 286, Scheme 55) could not be isolated. However, their intermolecular trapping was achieved by carrying out the reaction with monosubstituted olefins in the presence of triethylsilane to form polycyclic tetraalkylsilanes 285 in moderate to good yields and with complete regio- and diastereoselectivity (Scheme 55).71b Carbene insertion into the silicon–hydrogen bond of silanes, described here for a catalytic multicomponent reaction involving non-heteroatom-stabilized carbene complexes, is a well-established reaction of free carbenes or carbene–metal complexes. Its scope was also extended to other trialkylsilanes; however, in these cases, in addition to the corresponding tetraalkylsilane polycyclic products 289, polycyclic compounds 290 with no insertion in the Si–H bond were also obtained in variable amounts, depending on the nature (mainly, the bulkiness) of the trialkylsilane (Scheme 55; bottom).
The extension of these transformations (in the presence or absence of triethylsilane) to di- and tri-substituted olefins provided useful mechanistic information (Scheme 56).71b For example, the reaction with 2-methoxypropene 291 (R1 = R2 = H) provides a single isomer 292 in the absence of trapping agent, while a mixture of two diastereomers, 294 and 295, were obtained in the presence of triethylsilane; this suggests that the facial selectivity of these two reactions strongly depends on the presence of trapping agent. Therefore, the combination of all these findings suggests that the [3 + 2]-cycloaddition reaction step in these catalytic transformations is concerted and reversible.
Indeed, a theoretical study of the cycloaddition reactions of tungsten-containing carbonyl ylides, depicted in Schemes 53, 55 and 56, indicates that the [3 + 2]-cycloaddition proceeds in a reversible and concerted manner and that the endo-mode cycloaddition is kinetically favoured.73
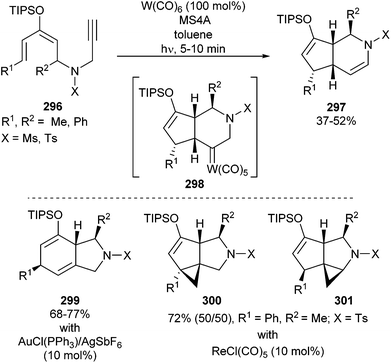
In a similar manner, terminal acetylenic dienol silyl enol ethers 296, containing N-Ts or N-Ms components in their tethers, undergo geminal carbo-functionalization to produce 3-azabicyclo[4.3.0]nonane derivatives 297 in moderate yields when photoirradiated in the presence of equimolar amounts of W(CO)6 (Scheme 57; top).70,74 A tandem cyclization sequence consisting of an initial 6-endo-dig cyclization on the tungsten carbonyl activated triple bond followed by a Michael-type addition would lead to tungsten carbene complex 298, which should evolve into the final products. The overall sequence takes place in a diastereoselective manner.
Remarkably, the reaction outcome can be controlled by the selection of appropriate π-acid catalysts. Thus, 8-azabicyclo[4.3.0]nonane derivatives 299 or tricyclic compounds 300 and 301 are selectively formed by employing either cationic gold or rhenium catalysts (Scheme 57; bottom). In a different behaviour than for the tungsten catalyst, all these products originate from 5-exo-dig cyclizations.
For example, cis-1-alkenyl-2-ethynylcyclopropanes 302 undergo rearrangement in the presence of stoichiometric amounts of Cr(CO)5(THF) to provide moderate yields of mixtures of isomeric 1,3,5-cycloheptatrienes 303 and 304 (Scheme 58).75 The reaction should proceed via formation of alkenylidenechromium complex 305, which should evolve by [3,3]-sigmatropic rearrangement to non-heteroatom-stabilized carbene complex 306. Subsequent [1,5]- or [1,3]-hydrogen shifts in 306 to generate metallated cycloheptatrienes 307 and 308, followed by reductive elimination, would readily explain the formation of regioisomeric reaction products.
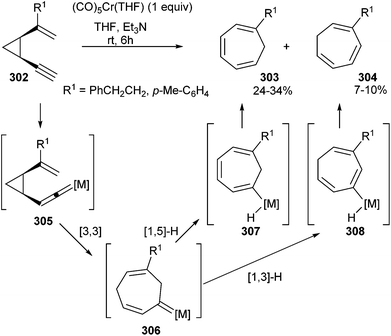 | ||
| Scheme 58 Cr(CO)5(THF)-catalysed rearrangement of cis-1-alkenyl-2-ethynylcyclopropanes to 1,3,5-cycloheptatrienes. | ||
Naphthalene derivatives 310 and heteropolyaromatic compounds 314 have been synthesized from benzenes substituted at the ortho-position with ethynyl and alkenyl (309) or heteroaromatic groups (313), usually in good to excellent yields (Scheme 59).76 Catalytic or, in some cases, stoichiometric amounts of (CO)5W(THF) are required, depending on the nature of the substrate. The reaction seems to proceed via alkenyldiene intermediates 311, which undergo a 6π electrocyclization to non-heteroatom-stabilized carbene complexes 312. Isomerization, followed by reductive elimination, should lead to the reaction products. Deuterium-labelling experiments support the proposed mechanism.
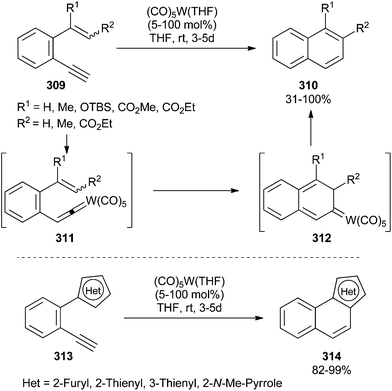 | ||
| Scheme 59 Synthesis of polyaromatic compounds 310 and heteropolyaromatic compounds 314 by the tungsten carbonyl-catalysed cyclization of aromatic enynes. | ||
Transformation of 1-iodoalkynes 315 can be also catalysed by tungsten carbonyl complexes to form iodo-substituted benzene or naphthalene derivatives 316 (Scheme 60). The reaction proceeds satisfactorily not only for o-(iodoethynyl)styrenes but also for non-aromatic dienyne derivatives. Good yields are usually reached when a stoichiometric amount of W(CO)5(THF) is used; however, the yield of the catalytic reaction depends on the structure and the substituents of the substrate.64,77
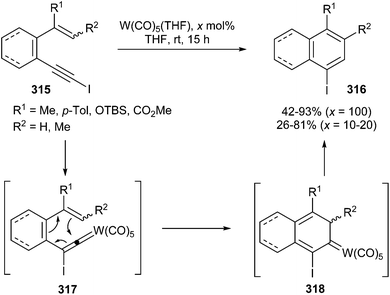 | ||
| Scheme 60 Synthesis of iodobenzene or -naphthalene derivatives by the tungsten carbonyl-catalysed rearrangement of 1-iodoalkynes. | ||
Regarding the reaction mechanism, after the initial π-activation of the alkyne (see also Scheme 16), an isomerization via 1,2-migration of the iodo group occurs to form tungsten iodoalkenylidene 317. The evolution of tungsten alkenylidene 317 by a 6π-electron electrocyclization leads to non-heteroatom-stabilized carbene complex intermediate 318, which yields the final products by 1,2-hydrogen migration and regeneration of W(CO)5(THF).
3.14. Transmetallation reactions
Mainly due to the low stability of non-heteroatom carbene complexes, very few examples related to their transmetallation reactions can be found in the literature.78 However, diphenyl tungsten carbene complex 15 can be transformed into manganese carbene complex 320 (Scheme 61).79 The transmetallation reported here occurs through thermolysis of the metal carbene in the presence of dicarbonyl(cyclopentadienyl) manganese complex 319.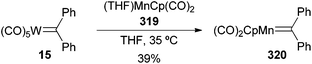 | ||
| Scheme 61 Transmetallation of a non-heteroatom-stabilized carbene complex from tungsten to manganese. | ||
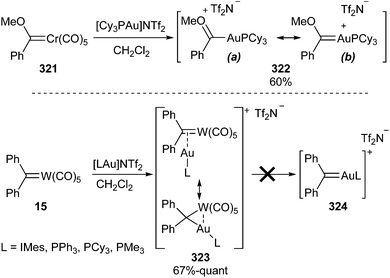
On the other hand, gold species have emerged over the last two decades as powerful carbophilic catalysts which can activate double and triple C–C bonds (alkenes, allenes, alkynes) toward nucleophilic addition. In many of these transformations, gold carbene complexes appear to be plausible reaction intermediates.80 Indeed, gold carbene complexes can be readily prepared by transmetallation from group 6 stabilized Fischer carbene complexes. For instance, Fürstner has described the synthesis of carbene complex 322 by treating chromium carbene complex 321 with [Cy3PAu]NTf2; in this complex, the bond-distances in its X-ray structure indicate that oxocarbenium resonance form 322-a is dominant (Scheme 62; top).81
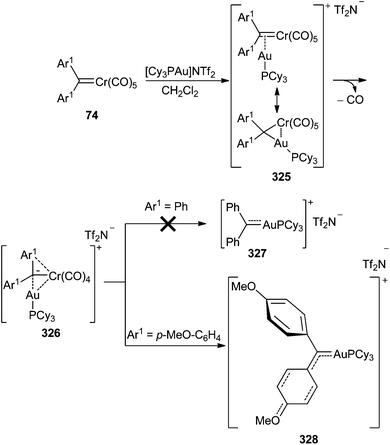
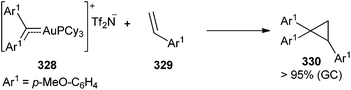
However, the employment of the same transmetallation strategy for the preparation of non-heteroatom-stabilized gold carbene complex 324 was unsuccessful, either from tungsten diphenyl carbene complex 15 or from chromium diphenyl carbene complex 74 (Ar1 = Ph). Instead, hetero-bimetallic complexes 323 and 325 (Ar1 = Ph) were obtained in good to excellent yields (Scheme 62; bottom and Scheme 63).
A heteroatom stabilizing effect is necessary to achieve an effective gold-for-chromium exchange.82 Thus, the treatment of chromium carbene complex 74 (Ar1 = p-MeO-C6H4) with [Cy3PAu]NTf2 at low temperature provides the heterobimetallic 325 (Ar1 = p-MeO-C6H4) species, which upon warming to −50 °C is transformed into a mixture of hetero-bimetallic compound 326 (ca. 40%) and gold carbene complex 328 (ca. 60%) (Scheme 63).
Addition of p-methoxystyrene 329 to an intensely pink solution of carbene complex 328 at −78 °C, followed by warming, resulted in full conversion into cyclopropane 330 when room temperature was reached (Scheme 64). This result demonstrates that gold carbene complex 328 is also a reactive species.
4. Conclusions
The work compiled in this review highlights the significant contribution of non-heteroatom-stabilized group 6 metal carbene complexes to organic synthesis. Thus, diverse strategies for their preparation have been developed, ranging from those limited to the synthesis of specific carbene complexes to others presenting a broader scope.Regarding their reactivity, early contributions mainly focused on cyclopropanations, olefin and enyne metathesis, and insertion reactions, and highly relevant findings in these fields were achieved by pioneers, such as E. O Fischer, Casey, Katz, H. Fischer, Dötz, etc. They paved the way for a better understanding of the mechanisms involved in these reactions.
Thus, Barluenga pointed out that non-heteroatom-stabilized alkynyl carbene complexes, easily formed by sequential treatment of Fischer carbenes with lithium acetylides and TMSOTf, are highly valuable stoichiometric reagents for the efficient and selective synthesis of diverse conjugate acyclic, carbocyclic and/or heterocyclic products through reactions with unsaturated reagents (alkenes, dienes, imines, etc.).
On the other hand, seminal approaches for the synthesis of a variety of carbo- and heterocycles have been developed, mainly by Iwasawa, based on the ability of group 6 metal carbonyl complexes to coordinate to C–C triple bonds as π-acids to form π-complexes and on their further evolution involving non-heteroatom-stabilized carbene complexes as stoichiometric or catalytic intermediates. Thus, taking advantage of this behavior, transformations such as high-yielding and atom-economic catalytic cycloisomerizations, cycloadditions with olefins or imines, or even multi-component reactions have provided straightforward access to structurally complex molecules.
Although less explored, transmetallation from non-heteroatom-stabilized carbene complexes has emerged as an attractive option to generate reactive carbene complexes of other metals.
As shown, either as stoichiometric reagents or as catalytic intermediates, non-heteroatom-stabilized group 6 metal carbene complexes offer direct and highly valuable approaches for the synthesis of organic skeletons that are difficult to access by other routes. Therefore, although the chemistry of non-heteroatom-stabilized carbene complexes is a rather mature area, relevant contributions in the field are still to come.
Acknowledgements
The authors would like to kindly acknowledge financial support from the Spanish MINECO (Grants CTQ2013-41336-P and CTQ2013-41511-P) and the Principality of Asturias (GRUPIN14-013).Notes and references
- For selected references and reviews in the field, see: (a) J. Heppekausen and A. Fürstner, Angew. Chem., Int. Ed., 2011, 50, 7829–7832 CrossRef CAS PubMed; (b) E. S. Sattely, S. J. Mee, S. J. Malcolmson, R. R. Schrock and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 943–953 CrossRef CAS PubMed; (c) S. J. Malcolmson, S. J. Meek, E. S. Sattely, R. R. Schrock and A. H. Hoveyda, Nature, 2008, 456, 933–937 CrossRef CAS PubMed; (d) R. R. Schrock and A. H. Hoveyda, Angew. Chem., Int. Ed., 2003, 42, 4592–4633 CrossRef CAS PubMed; (e) R. R. Schrock, Acc. Chem. Res., 1979, 12, 98–104 CrossRef CAS.
- (a) C. D. Montgomery, J. Chem. Educ., 2015, 92, 1653–1660 CrossRef CAS; (b) G. Frenking, M. Solá and S. F. Vyboishchikov, J. Organomet. Chem., 2005, 690, 6178–6204 CrossRef CAS; (c) S. F. Vyboishchikov and G. Frenking, Chem. – Eur. J., 1998, 4, 1439–1448 CrossRef CAS; (d) S. F. Vyboishchikov and G. Frenking, Chem. – Eur. J., 1998, 4, 1428–1438 CrossRef CAS; (e) G. Frenking and U. Pidun, J. Chem. Soc., Dalton Trans., 1997, 1653–1662 RSC.
- (a) M. A. Sierra, I. Fernández and F. P. Cossío, Chem. Commun., 2008, 4671–4682 RSC; (b) I. Fernández, M. A. Sierra, M. Gómez-Gallego, M. J. Mancheño and F. P. Cossío, Chem. – Eur. J., 2005, 11, 5988–5996 CrossRef PubMed.
- (a) Y.-T. Wu and A. de Meijere, Top. Organomet. Chem., 2004, 13, 21–57 CrossRef CAS; (b) A. de Meijere, H. Schirmer and M. Duetsch, Angew. Chem., Int. Ed., 2000, 39, 3964–4002 CrossRef CAS; (c) A. de Meijere, Pure Appl. Chem., 1996, 68, 61–72 CrossRef CAS.
- For selected reviews regarding the chemistry of Fischer carbene complexes: (a) E. Aguilar and L. A. López, Science of Synthesis, Knowledge Updates, 2014, vol. 2, pp. 1–137 Search PubMed; (b) M. A. Fernández-Rodríguez, P. García-García and E. Aguilar, Chem. Commun., 2010, 46, 7670–7687 RSC; (c) K. H. Dötz and J. Stendel, Chem. Rev., 2009, 109, 3227–3274 CrossRef PubMed; (d) J. Santamaría, Curr. Org. Chem., 2009, 13, 31–46 CrossRef; (e) J. Barluenga, M. A. Fernández-Rodríguez and E. Aguilar, J. Organomet. Chem., 2005, 690, 539–587 CrossRef CAS; (f) J. Barluenga, J. Santamaría and M. Tomás, Chem. Rev., 2004, 104, 2259–2284 CrossRef CAS PubMed.
- J. Barluenga, A. Diéguez, F. Rodríguez and F. J. Fañanás, Angew. Chem., Int. Ed., 2005, 44, 126–128 CrossRef CAS PubMed.
- N. Iwasawa, in Metal Vinylidenes and Allenylidenes for Catalysis, ed. C. Bruneau and P. Dixneuf, Wiley-WCH Verlag GmbH & Co. KGaA, Weinheim, 2008, pp. 159–191 Search PubMed.
- For selected examples, see: (a) K. Sangu, K. Fuchibe and T. Akiyama, Org. Lett., 2004, 6, 353–355 CrossRef CAS PubMed; (b) H. Fischer, H.-P. Volkland, A. Früh and R. Stumpf, J. Organomet. Chem., 1995, 491, 267–273 CrossRef CAS; (c) K. H. Dötz, M. Popall, G. Müller and K. Ackermann, J. Organomet. Chem., 1990, 383, 93–111 CrossRef; (d) S. J. Landon, P. M. Shulman and G. L. Geoffroy, J. Am. Chem. Soc., 1985, 107, 6739–6740 CrossRef CAS.
- For isolated examples of these compounds, see: (a) C. E. Strasser, E. Stander-Grobler, O. Schuster, S. Cronje and H. G. Raubenheimer, Eur. J. Inorg. Chem., 2009, 1905–1912 CrossRef CAS; (b) R. Aumann and P. Hinterding, Chem. Ber., 1992, 125, 2765–2772 CrossRef CAS.
- K. Öfele, Angew. Chem., Int. Ed., 1968, 7, 950 CrossRef.
- (a) C. P. Casey and T. J. Burkhardt, J. Am. Chem. Soc., 1973, 95, 5833–5834 CrossRef CAS; (b) C. P. Casey, T. J. Burkhardt, C. A. Bunnell and J. C. Calabrese, J. Am. Chem. Soc., 1977, 99, 2127–2134 CrossRef CAS; (c) C. P. Casey, T. J. Burkhardt, S. M. Neumann, D. M. Scheck and H. E. Tuinstra, Inorg. Synth., 1979, 19, 180–183 CrossRef CAS.
- At that time, Fischer had reported the formation of 1,2-dimethoxy-1,1,2,2-tetraphenylethane, in addition to other products, by reaction of pentacarbonyl (1-methoxybenzylidene)chromium(0) with phenyllithium in ether at 0 °C; see: E. O. Fischer and S. Riedmuller, unpublished results referred to by E. O. Fischer, Pure Appl. Chem., 1972, 30, 353–372 CrossRef CAS.
- C. P. Casey, H. E. Tuinstra and M. C. Saeman, J. Am. Chem. Soc., 1976, 98, 608–609 CrossRef CAS.
- (a) E. O. Fischer, W. Held, S. Riedmuller and F. Kohler CrossRef, unpublished results referred to by E. O. Fisher, Angew. Chem., 1974, 86, 651–682 CrossRef; (b) E. O. Fischer, W. Held, F. R. Kreissl, A. Frank and G. Huttner, Chem. Ber., 1977, 110, 656–666 CrossRef CAS.
- F. R. Kreissl and W. Held, J. Organomet. Chem., 1975, 86, C10–C12 CrossRef CAS.
- E. O. Fischer and W. Held, J. Organomet. Chem., 1976, 112, C59–C62 CrossRef CAS.
- C. P. Casey, L. D. Albin and T. J. Burkhardt, J. Am. Chem. Soc., 1977, 99, 2533–2539 CrossRef CAS.
- J. Barluenga, A. Ballesteros, R. Bernardo de la Rúa, J. Santamaría, E. Rubio and M. Tomás, J. Am. Chem. Soc., 2003, 125, 1834–1842 CrossRef CAS PubMed.
- (a) N. Iwasawa, T. Ochiai and K. Maeyama, Organometallics, 1997, 16, 5137–5139 CrossRef CAS; (b) K. Fuchibe and N. Iwasawa, Tetrahedron, 2000, 56, 4907–4915 CrossRef CAS.
- N. Iwasawa, K. Maeyama and M. Saitou, J. Am. Chem. Soc., 1997, 119, 1486–1487 CrossRef CAS.
- J. Barluenga, R. Bernardo de la Rúa, D. de Sáa, A. Ballesteros and M. Tomás, Angew. Chem., Int. Ed., 2005, 44, 4981–4983 CrossRef CAS PubMed.
- J. Barluenga, D. de Sáa, A. Gómez, A. Ballesteros, J. Santamaría, A. de Prado, M. Tomás and A. L. Suárez-Sobrino, Angew. Chem., Int. Ed., 2008, 47, 6225–6228 CrossRef CAS PubMed.
- J. Levisalles, H. Rudler, Y. Jeannin and F. Daran, J. Organomet. Chem., 1979, 178, C8–C12 CrossRef CAS.
- (a) C. P. Casey and S. W. Polichnowski, J. Am. Chem. Soc., 1977, 99, 6097–6099 CrossRef CAS; (b) C. P. Casey, S. W. Polichnowski, A. Shusterman and C. R. Jones, J. Am. Chem. Soc., 1979, 101, 7282–7292 CrossRef CAS.
- H. Fischer, S. Zeuner and K. Ackermann, J. Chem. Soc., Chem. Commun., 1984, 684–686 RSC.
- H. Fischer and D. Reindl, J. Organomet. Chem., 1990, 385, 351–361 CrossRef CAS.
- K. H. Dötz and J. Pfeiffer, Chem. Commun., 1996, 895–896 RSC.
- J. Pfeiffer and K. H. Dötz, Organometallics, 1998, 17, 4353–4361 CrossRef CAS.
- H. Berke, P. Härter, G. Huttner and L. Zsolnai, Z. Naturforsch., 1981, 36b, 929–937 CAS.
- A mechanism analogous to the one proposed for the synthesis of other cyclobutenylidene complexes may be operating; see ref. 8b.
- K. Miki, T. Yokoi, F. Nishino, K. Ohe and S. Uemura, J. Organomet. Chem., 2002, 645, 228–234 CrossRef CAS.
- J. Takaya, H. Kusama and N. Iwasawa, Chem. Lett., 2004, 33, 16–17 CrossRef CAS.
- C. P. Casey and T. J. Burkhardt, J. Am. Chem. Soc., 1974, 96, 7808–7809 CrossRef CAS.
- J. L. Herrison and Y. Chauvin, Macromol. Chem. Phys., 1970, 141, 161–176 CrossRef.
- J. McGinnis, T. J. Katz and S. Hurwitz, J. Am. Chem. Soc., 1976, 98, 605–1486 CrossRef CAS.
- T. J. Katz, J. McGinnis and C. Altus, J. Am. Chem. Soc., 1976, 98, 606–608 CrossRef CAS.
- M. P. Doyle, J. H. Griffin, V. Bagheri and R. L. Dorow, Ofrganometallics, 1984, 33, 53–61 CrossRef.
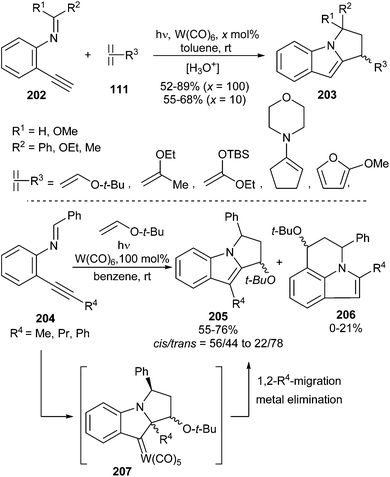
- J. Barluenga, E. Tudela, R. Vicente, A. Ballesteros and M. Tomás, Chem. – Eur. J., 2011, 17, 2349–2352 CrossRef CAS PubMed.
- H. Fischer and J. Hofmann, Chem. Ber., 1991, 124, 981–988 CrossRef CAS.
- (a) J. Barluenga, E. Tudela, R. Vicente, A. Ballesteros and M. Tomás, Angew. Chem., Int. Ed., 2011, 50, 2107–2110 CrossRef CAS PubMed; (b) E. Tudela, J. González, R. Vicente, J. Santamaría, M. A. Rodríguez and A. Ballesteros, Angew. Chem., Int. Ed., 2014, 53, 12097–12100 CrossRef CAS PubMed.
- H. Fischer, W. Bidell and J. Hofmann, J. Chem. Soc., Chem. Commun., 1990, 858–859 RSC.
- K. Miki, F. Nishino, K. Ohe and S. Uemura, J. Am. Chem. Soc., 2002, 124, 5260–5261 CrossRef CAS PubMed.
- For selected reviews on enyne metathesis see: (a) I. Dragutan, V. Dragutan, A. Demonceau and L. Delaude, Curr. Org. Chem., 2013, 17, 2678–2720 CrossRef CAS; (b) C. Fischmeister and C. Bruneau, Beilstein J. Org. Chem., 2011, 7, 156–166 CrossRef CAS PubMed.
- T. J. Katz and T. M. Sivavec, J. Am. Chem. Soc., 1985, 107, 737–738 CrossRef CAS.
- A. Pérez, J. L. Serrano, T. Sierra, A. Ballesteros, D. de Sáa and J. Barluenga, J. Am. Chem. Soc., 2011, 133, 8110–8113 CrossRef PubMed.
- H. Fischer, J. Schmid and R. Märkl, J. Chem. Soc., Chem. Commun., 1985, 572–573 RSC.
- H. Fischer, S. Zeuner and J. Riede, Angew. Chem., Int. Ed., 1984, 23, 726–727 CrossRef.
- H. Fischer and S. Zeuner, Z. Naturforsch., B: Anorg. Chem. Org. Chem., 1985, 40, 954–960 Search PubMed.
- For selected reviews on the Dötz reaction, see: (a) L. Chupak, in Name Reactions for Carbocyclic Ring Formations, ed. J. J. Li, John Wiley & Sons, Inc, Hoboken, N. J., 2010, pp. 309–323 Search PubMed; (b) M. L. Waters and W. D. Wulff, Org. React., 2008, 70, 121–623 Search PubMed; (c) A. Minatti and K. H. Dötz, Top. Organomet. Chem., 2004, 13, 123–156 CrossRef CAS; (d) K. H. Dötz and P. Tomuschat, Chem. Soc. Rev., 1999, 28, 187–198 RSC.
- J. Barluenga, P. García-García, D. de Sáa, M. A. Fernández-Rodríguez, R. Bernardo de la Rúa, A. Ballesteros, E. Aguilar and M. Tomás, Angew. Chem., Int. Ed., 2007, 46, 2610–2612 CrossRef CAS PubMed.
- H. Fischer, A. Schlageter, W. Bidell and A. Früh, Organometallics, 1993, 10, 389–391 CrossRef.
- K. Weiss and P. Kindl, Angew. Chem., Int. Ed., 1984, 23, 629–630 CrossRef.
- K. Weiss and K. Hoffmann, in Advances in Metal Carbene Chemistry, ed. U. Shubert, Kluwer Academic Publishers, Dordrecht, The Netherlands, 1989, pp. 351–354 Search PubMed.
- J. Barluenga, A. Gómez, J. Santamaría and M. Tomás, J. Am. Chem. Soc., 2009, 131, 14628–14629 CrossRef CAS PubMed.
- I. Funes-Ardoiz, J. González, J. Santamaría and D. Sampedro, J. Org. Chem., 2016, 81, 1565–1570 CrossRef CAS PubMed.
- (a) J. Barluenga, A. Gómez, J. Santamaría and M. Tomás, Angew. Chem., Int. Ed., 2010, 49, 1306–1308 CrossRef CAS PubMed; (b) I. Funes-Ardoiz and D. Sampedro, J. Org. Chem., 2014, 79, 11824–11828 CrossRef CAS PubMed.
- J. González, A. Gómez, I. Funes-Ardoiz, J. Santamaría and D. Sampedro, Chem. – Eur. J., 2014, 20, 7061–7068 CrossRef PubMed.
- H. Kusama, J. Tayaka and N. Iwasawa, J. Am. Chem. Soc., 2002, 124, 11592–11593 CrossRef CAS PubMed.
- (a) H. Kusama, Y. Miyashita, J. Tayaka and N. Iwasawa, Org. Lett., 2006, 8, 289–292 CrossRef CAS PubMed; (b) T. Ohyama, M. Uchida, H. Kusama and N. Iwasawa, Chem. Lett., 2015, 44, 1850–1853 Search PubMed.
- H. Kusama, Y. Suzuki, J. Tayaka and N. Iwasawa, Org. Lett., 2006, 8, 895–898 CrossRef CAS PubMed.
- J. Takaya, Y. Miyashita, H. Kusama and N. Iwasawa, Tetrahedron, 2011, 67, 4455–4466 CrossRef CAS.
- (a) T. Miura, K. Kiyota, H. Kusama and N. Iwasawa, J. Organomet. Chem., 2007, 692, 562–568 CrossRef CAS; (b) T. Miura, K. Kiyota, H. Kusama and N. Iwasawa, Org. Lett., 2005, 7, 1445–1447 CrossRef CAS PubMed; (c) N. Iwasawa, T. Miura, K. Kiyota, H. Kusama, K. Lee and P. H. Lee, Org. Lett., 2002, 4, 4463–4466 CrossRef CAS PubMed; (d) H. Kusama, H. Yamabe and N. Iwasawa, Org. Lett., 2002, 4, 2569–2571 CrossRef CAS PubMed; (e) N. Iwasawa, K. Maeyama and H. Kusama, Org. Lett., 2001, 3, 3871–3873 CrossRef CAS PubMed; (f) K. Maeyama and N. Iwasawa, J. Am. Chem. Soc., 1998, 120, 1928–1929 CrossRef CAS.
- For related and selected references by other authors regarding tungsten carbonyl complexes catalysed cyclizations involving acetylenes and where non-stabilized carbene complexes have not been proposed as reaction intermediates, see: M. Gulías, J. R. Rodríguez, L. Castedo and J. L. Mascareñas, Org. Lett., 2003, 5, 1975–1977 CrossRef PubMed.
- T. Miura, H. Murata, K. Kiyota, H. Kusama and N. Iwasawa, J. Mol. Catal. A: Chem., 2004, 213, 59–71 CrossRef CAS.
- Unpublished result referred by H. Kusama, Y. Onizawa and N. Iwasawa in ref. 66.
- H. Kusama, Y. Onizawa and N. Iwasawa, J. Am. Chem. Soc., 2006, 128, 16500–16501 CrossRef CAS PubMed.
- Y. Onizawa, M. Hara, T. Hashimoto, H. Kusama and N. Iwasawa, Chem. – Eur. J., 2010, 16, 10785–10796 CrossRef CAS PubMed.
- Y. Karibe, H. Kusama and N. Iwasawa, Angew. Chem., Int. Ed., 2012, 51, 6214–6218 CrossRef CAS PubMed.
- Y. Onizawa, H. Kusama and N. Iwasawa, J. Am. Chem. Soc., 2008, 130, 802–803 CrossRef CAS PubMed.
- H. Kusama, Y. Karibe, R. Imai, Y. Onizawa, H. Yamabe and N. Iwasawa, Chem. – Eur. J., 2011, 17, 4839–4848 CrossRef CAS PubMed.
- (a) N. Iwasawa, M. Shido and H. Kusama, J. Am. Chem. Soc., 2001, 123, 5814–5815 CrossRef CAS PubMed; (b) H. Kusama, H. Funami, M. Shido, Y. Hara, J. Tayaka and N. Iwasawa, J. Am. Chem. Soc., 2005, 127, 2709–2716 CrossRef CAS PubMed.
- For the synthesis of pyranylidene complexes from group six metal carbonyl complexes and β-ethynyl α,β-unsaturated carbonyl compounds, see: K. Ohe, K. Miki, T. Yokoi, F. Nishino and S. Uemura, Organometallics, 2000, 19, 5525–5528 CrossRef CAS.
- K. Ito, Y. Hara, S. Mori, H. Kusama and N. Iwasawa, Chem. – Eur. J., 2009, 15, 12408–12416 CrossRef CAS PubMed.
- A. Grandmarre, H. Kusama and N. Iwasawa, Chem. Lett., 2007, 36, 66–67 CrossRef CAS.
- K. Ohe, T. Yokoi, K. Miki, F. Nishino and S. Uemura, J. Am. Chem. Soc., 2002, 124, 526–527 CrossRef CAS PubMed.
- K. Maeyama and N. Iwasawa, J. Org. Chem., 1999, 64, 1344–1346 CrossRef CAS.
- T. Miura and N. Iwasawa, J. Am. Chem. Soc., 2002, 124, 518–519 CrossRef CAS PubMed.
- For a revision on transmetallation from group VI carbene complexes, see: M. Gómez-Gallego, M. J. Mancheño and M. A. Sierra, Acc. Chem. Res., 2005, 38, 44–53 CrossRef PubMed.
- B. H. Edward and M. D. Rausch, J. Organomet. Chem., 1981, 210, 91–96 CrossRef.
- For recent reviews, see: (a) R. J. Harris and R. A. Widenhoefer, Chem. Soc. Rev., 2016, 45, 4533–4551 RSC; (b) Y. Wang, M. E. Muratore and A. M. Echavarren, Chem. – Eur. J., 2015, 21, 7332–7339 CrossRef CAS PubMed; (c) R. Dorel and A. M. Echavarren, J. Org. Chem., 2015, 80, 7321–7332 CrossRef CAS PubMed; (d) D. Qian and J. Zhang, Chem. Soc. Rev., 2015, 44, 677–698 RSC; (e) L. Zhang, Acc. Chem. Res., 2014, 47, 877–888 CrossRef CAS PubMed; (f) H.-S. Yeom and S. Shin, Acc. Chem. Res., 2014, 47, 966–977 CrossRef CAS PubMed.
- G. Seidel, B. Gabor, R. Goddard, B. Heggen, W. Thiel and A. Fürstner, Angew. Chem., Int. Ed., 2014, 53, 879–882 CrossRef CAS PubMed.
- G. Seidel and A. Fürstner, Angew. Chem., Int. Ed., 2014, 53, 4807–4811 CrossRef CAS PubMed.
Footnote |
| † Dedicated to Professor Dr Barry M. Trost on the occasion of his 75th birthday. |
| This journal is © the Partner Organisations 2016 |