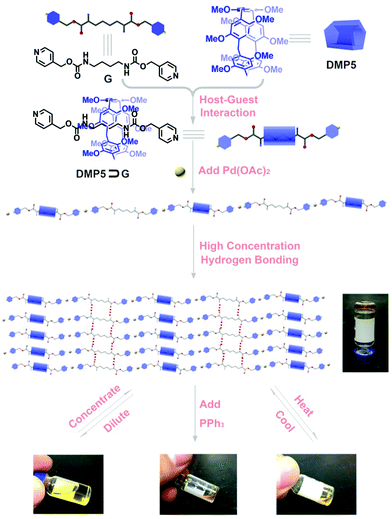
Stimuli-responsive supramolecular gel constructed by pillar[5]arene-based pseudo[2]rotaxanes via orthogonal metal–ligand coordination and hydrogen bonding interaction†
Xuan
Wu
,
Ying
Yu
,
Lei
Gao
,
Xiao-Yu
Hu
* and
Leyong
Wang
*
Key Laboratory of Mesoscopic Chemistry of MOE and Collaborative Innovation Center of Chemistry for Life Sciences, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023, China. E-mail: huxy@nju.edu.cn; lywang@nju.edu.cn; Fax: +86-(0)25-89687090; Tel: +86-(0)25-89682529
First published on 1st June 2016
Abstract
A pseudo[2]rotaxane formed from a dimethoxypillar[5]arene host and an amide-functionalized pyridine derivative guest was constructed in CHCl3, which could further assemble into a linear supramolecular polyrotaxane upon adding Pd(OAc)2via the incorporation of metal–ligand coordination. Furthermore, the obtained polyrotaxane could self-assemble to form a supramolecular gel at high concentration, which showed external stimuli-responsiveness.
Polyrotaxane1 constructed simply by incorporating rotaxane moieties into supramolecular polymers have attracted tremendous attention in the past two decades due to their unique structural features and wide potential applications in materials science, nanotechnology, and biological science.2 In order to efficiently construct various polyrotaxanes, scientists have developed many synthetic strategies, among which the most often used is the “threading-followed-by-stoppering” strategy that was first developed by Harada and coworkers by using a covalent polymer as an axle, which was threaded by cyclodextrin.2d However, the threading efficiency of macrocycles to the covalent polymers was relatively very low due to the chain length, concentration, and temperature,3 which greatly affected the physical and chemical properties of the obtained polyrotaxanes. Recently, with the rapid development of research in constructing various mechanically interlocked molecules (MIMs),4 a novel method for the fabrication of polyrotaxanes has been developed by using the strategy of “threading-followed-by-connecting”, by which well-defined, homogeneous, and dynamic supramolecular polyrotaxanes assisted by noncovalent interactions as the polymeric backbones could be achieved efficiently.5,6 Therefore, this strategy provides a more convenient way to construct smart supramolecular polymers due to the intrinsic reversibility of noncovalent interactions.
So far, although various supramolecular polyrotaxanes have been successfully constructed based on well-known macromolecules, such as crown ethers or cucurbituril (CB), by the incorporation of noncovalent metal–ligand coordinations,6–8 introducing new macromolecule moieties and novel noncovalent interactions into the realm of polyrotaxanes will no doubt expand the applications of polyrotaxanes and allow them to exhibit some unique stimuli-responsiveness. Pillar[n]arenes,9 as a new type of macrocyclic hosts, have a unique pillar architecture, easy functionalization, and outstanding properties in host–guest chemistry, which make them promising candidates for constructing various functional supramolecular materials,10 such as molecular machines,11 molecular recognition,12 ion transport,13 nanomaterials,14 and supramolecular polymers.15 Encouraged by the above exciting discovery, research on pillararene-based polyrotaxanes constructed by connecting rotaxanes with hydrogen bonding or metal–ligand coordination has been undertaken based on the “threading-followed-by-connecting” strategy; however, the yields of the obtained rotaxanes are usually very low, which has greatly restricted their potential applications.16 Therefore, it is essential to seek another efficient way to construct dynamic supramolecular polyrotaxanes. In our previous work on the construction of dynamic polyrotaxanes interlocked by orthogonal quadruple hydrogen bonding and pillararene-based host–guest interactions,16a we found that the hydrogen bonding between the ureidopyrimidinone motif and the oxygen atoms on pillararene could stabilize and thus facilitate the formation of pseudorotaxanes in high yields. Considering the hydrogen bond forming ability of the amide group, we wonder whether it is feasible to utilize an amide group-functionalized guest to enhance its bonding with a pillararene host, thus leading to the formation of stimuli-responsive supramolecular polyrotaxanes with high efficiency. Herein, we designed and synthesized a novel supramolecular polyrotaxane, which was constructed by directly connecting the obtained pseudo[2]rotaxanes formed from an amide group-functionalized pyridine derivative and dimethoxypillar[5]arene (DMP5)9a with a metal ion as linkage. More importantly, the obtained polyrotaxanes could further self-assemble to form a dynamic supramolecular gel at high concentration, which showed stimuli-responsiveness to external stimuli.
As shown in Scheme 1, a well-designed guest molecule, G, bearing a pyridine motif at both ends was successfully synthesized (Scheme S1, ESI†). Initially, the host–guest interaction between DMP5 and G was studied by 1H NMR experiments. The 1H NMR spectra of G in the absence and presence of the DMP5 host were recorded, as shown in Fig. 1, from which remarkable upfield chemical shifts and a broadening effect of the methylene protons (Hd, He, and Hf) on G could be observed in the presence of DMP5 due to the inclusion-induced shielding effects; whereas, no obvious change was observed for the protons Ha, Hb, and Hc on G. The above results indicated that the pillar[5]arene host DMP5 (the ‘wheel’) was fully threaded by guest G (the ‘axle’) with the alkyl chain in the cavity of DMP5 and the pyridine motif out of its cavity, generating a supramolecular pseudorotaxane DMP5⊃G, as shown in Scheme 1. From the 2D NOESY spectrum (Fig. S5, ESI†), obvious NOE correlation signals could be observed between the protons He–f on G and H1 on DMP5, which also confirmed the formation of the DMP5⊃G pseudorotaxane.
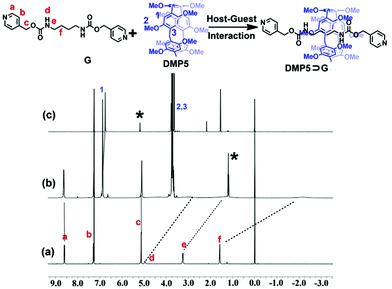 | ||
| Fig. 1 1H NMR spectra (300 MHz, CDCl3, 298 K) of (a) G (10 mM), (b) G (10 mM) + DMP5 (8 mM), (c) DMP5 (8 mM). (“*” indicates the solvent residue). | ||
The stoichiometry of complexation between DMP5 and G was further investigated by Job's plot method based on 1H NMR experiments (Fig. S6 and S7, ESI†), which indicated the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between DMP5 and G. Moreover, the ESI-MS spectrum of an equimolar mixture of DMP5 and G showed one intense peak at m/z 1109.40 for the 1
1 binding stoichiometry between DMP5 and G. Moreover, the ESI-MS spectrum of an equimolar mixture of DMP5 and G showed one intense peak at m/z 1109.40 for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex ([DMP5 + G + H]+), which also indicated the formation of 1
1 host–guest complex ([DMP5 + G + H]+), which also indicated the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex between DMP5 and G (Fig. S8, ESI†). To determine the association constant for DMP5⊃G in CHCl3, 1H NMR titration experiments with a constant concentration of DMP5 and varying concentrations of G were carried out in CDCl3 (Fig. S9, ESI†). By a non-linear curve-fitting method, the association constant (Ka) of DMP5⊃G was determined to be (3.62 ± 0.04) × 102 M−1 (Fig. S10, ESI†).
1 host–guest complex between DMP5 and G (Fig. S8, ESI†). To determine the association constant for DMP5⊃G in CHCl3, 1H NMR titration experiments with a constant concentration of DMP5 and varying concentrations of G were carried out in CDCl3 (Fig. S9, ESI†). By a non-linear curve-fitting method, the association constant (Ka) of DMP5⊃G was determined to be (3.62 ± 0.04) × 102 M−1 (Fig. S10, ESI†).
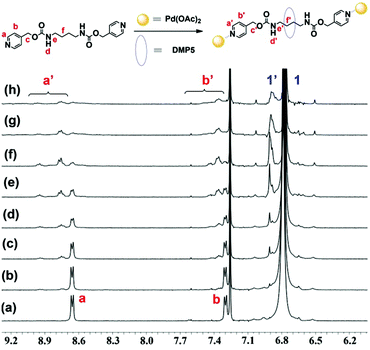
After constructing the pseudo[2]rotaxane formed between DMP5 and G, we wondered whether we could fabricate supramolecular polyrotaxanes by connecting the obtained pseudo[2]rotaxanes with metal ions, such as Pd(OAc)2. So in the following work, we first investigated the binding ratio between Pd(OAc)2 and G. To a mixed solution of G and DMP5 ([G]/[DMP5] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) in CDCl3, different amounts of Pd(OAc)2 were added, and the 1H NMR spectra were recorded. As shown in Fig. 2, upon gradually increasing the amount of Pd(OAc)2 added, both the protons Ha and Hb on the pyridine motif of G showed remarkable downfield shifts, and exhibited a slow exchange process within NMR timescales. When the molar ratio of [Pd(OAc)2]/[G] was increased to 1
5) in CDCl3, different amounts of Pd(OAc)2 were added, and the 1H NMR spectra were recorded. As shown in Fig. 2, upon gradually increasing the amount of Pd(OAc)2 added, both the protons Ha and Hb on the pyridine motif of G showed remarkable downfield shifts, and exhibited a slow exchange process within NMR timescales. When the molar ratio of [Pd(OAc)2]/[G] was increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the chemical shifts of Ha and Hb did not change any more, indicating that the binding ratio between Pd(OAc)2 and G was 1
1, the chemical shifts of Ha and Hb did not change any more, indicating that the binding ratio between Pd(OAc)2 and G was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Furthermore, the protons H1 on DMP5 were also split into two sets of peaks, one for the free DMP5 and the other for the complexed DMP5 host in the polyrotaxane backbone, which indicated that supramolecular polyrotaxane with metal ion (Pd(OAc)2) as the linkage was formed. Based on the integration ratio (4
1. Furthermore, the protons H1 on DMP5 were also split into two sets of peaks, one for the free DMP5 and the other for the complexed DMP5 host in the polyrotaxane backbone, which indicated that supramolecular polyrotaxane with metal ion (Pd(OAc)2) as the linkage was formed. Based on the integration ratio (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4) between Hf′ and Ha′ (or Hb′) (Fig. S12, ESI†), it could be further concluded that about 60% of G was threaded into the cavity of DMP5, which might be the reason why the protons Ha and Hb on the pyridine motif became broadened and showed multi-peaks. The above results indicated that Pd(OAc)2 could serve as the linkage group to efficiently connect the obtained DMP5⊃G pseudo[2]rotaxanes, leading to the formation of supramolecular polyrotaxanes.
2.4) between Hf′ and Ha′ (or Hb′) (Fig. S12, ESI†), it could be further concluded that about 60% of G was threaded into the cavity of DMP5, which might be the reason why the protons Ha and Hb on the pyridine motif became broadened and showed multi-peaks. The above results indicated that Pd(OAc)2 could serve as the linkage group to efficiently connect the obtained DMP5⊃G pseudo[2]rotaxanes, leading to the formation of supramolecular polyrotaxanes.
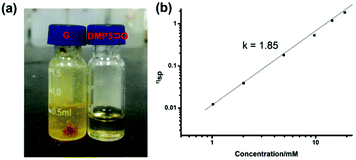
It was found that when Pd(OAc)2 was mixed with G in CHCl3, a yellow precipitate was formed immediately. However, if Pd(OAc)2 was added to the CHCl3 solution containing G and DMP5 ([G]/[DMP5] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), no precipitation could be observed, and the solution became very viscous (Fig. 3a), which was visual evidence confirming the formation of pillar[5]arene-contained supramolecular polyrotaxanes in solution. To further investigate the polymeric properties of the obtained supramolecular polyrotaxanes, viscosity measurements were performed in CHCl3 by using a micro-Ubbelohde viscometer. A double-logarithmic plot of specific viscosity versusG concentration was obtained, as shown in Fig. 3b. It was found that the curve slope from dilute concentration to 20 mM was determined to be 1.85. Upon continual increasing the concentration, the solution became too viscous to be measured. As a control, the CHCl3 solution containing both G and DMP5 did not become viscous, even at a very high concentration (30 mM). Therefore, it could be concluded that supramolecular polyrotaxanes could be formed only when assisted by metal–ligand coordination.
5), no precipitation could be observed, and the solution became very viscous (Fig. 3a), which was visual evidence confirming the formation of pillar[5]arene-contained supramolecular polyrotaxanes in solution. To further investigate the polymeric properties of the obtained supramolecular polyrotaxanes, viscosity measurements were performed in CHCl3 by using a micro-Ubbelohde viscometer. A double-logarithmic plot of specific viscosity versusG concentration was obtained, as shown in Fig. 3b. It was found that the curve slope from dilute concentration to 20 mM was determined to be 1.85. Upon continual increasing the concentration, the solution became too viscous to be measured. As a control, the CHCl3 solution containing both G and DMP5 did not become viscous, even at a very high concentration (30 mM). Therefore, it could be concluded that supramolecular polyrotaxanes could be formed only when assisted by metal–ligand coordination.
Meanwhile, a dynamic light scattering (DLS) experiment was also conducted to investigate the size of the formed supramolecular aggregates (Fig. 4b). The DLS result showed that the average diameter of the obtained polyrotaxanes was 2074 nm ([G] = 15 mM, [G]/[DMP5]/[Pd(OAc)2] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), further indicating the formation of large aggregates in the above solution. Moreover, the scanning electron microscope (SEM) image of a rod-like fiber drawn from a very concentrated solution containing G, DMP5, and Pd(OAc)2 in chloroform further provided direct physical evidence for the formation of such supramolecular polymers, which generally result from the entanglement of linear or cross-linked macro-sized aggregates.
1), further indicating the formation of large aggregates in the above solution. Moreover, the scanning electron microscope (SEM) image of a rod-like fiber drawn from a very concentrated solution containing G, DMP5, and Pd(OAc)2 in chloroform further provided direct physical evidence for the formation of such supramolecular polymers, which generally result from the entanglement of linear or cross-linked macro-sized aggregates.
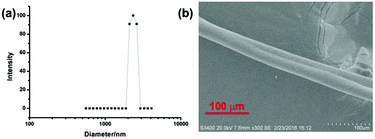 | ||
| Fig. 4 (a) DLS result of supramolecular polyrotaxanes in CHCl3, (b) SEM image of the supramolecular polyrotaxanes drawn from the concentrated solution in CHCl3. | ||
The macroscopic properties of the obtained supramolecular polymers at high concentration were further investigated. Supramolecular polymers were prepared by dissolving the monomers in CHCl3 at 45.0 °C ([G] = 45 mM, [G]/[DMP5]/[Pd(OAc)2] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), so that a very viscous polymeric solution could be obtained. Upon gradually cooling to room temperature, such a viscous solution transforms into a transparent gel, as shown in Scheme 1. The formation of a supramolecular gel might be caused by further self-assembly between the amide groups on G through hydrogen bonding interactions, which was certified by varying concentration 1H NMR experiments (Fig. S13, ESI†). From the 1H NMR spectra, it was found that the chemical shift of proton Hd assigned to the amide group on G exhibited concentration-dependent properties, which provided solid evidence for the formation of hydrogen bonding interactions between G molecules.17 Based on the integration of the protons in Fig. S12 (ESI†), we could further conclude that about 40% of G was not encapsulated into the cavity of DMP5, thus intermolecular hydrogen bonding interactions might exist between the formed polyrotaxane backbones, leading to the formation of the supramolecular gel. Moreover, the rheology results further gave detailed information on the formation of the polyrotaxane-based supramolecular gel. As shown in Fig. 5, the oscillatory experiment suggested that the obtained gel exhibited a network behavior with the storage modulus G′ larger than the loss modulus G′′ at high oscillatory frequency (from 0.6 to 100 rad s−1), but lower than the loss modulus G′′ at low frequency (less than 0.6 rad s−1) at 25 °C (with a crossover point at G′ = G′′ = 4887 Pa). Moreover, the storage modulus G′ reached to a plateau value at about 104 Pa (termed as the plateau modulus G0) at high frequency, indicating that the sample exhibited moderate mechanical properties. The above observations further confirmed the formation of a supramolecular polymeric network gel with good mechanical properties.18
1), so that a very viscous polymeric solution could be obtained. Upon gradually cooling to room temperature, such a viscous solution transforms into a transparent gel, as shown in Scheme 1. The formation of a supramolecular gel might be caused by further self-assembly between the amide groups on G through hydrogen bonding interactions, which was certified by varying concentration 1H NMR experiments (Fig. S13, ESI†). From the 1H NMR spectra, it was found that the chemical shift of proton Hd assigned to the amide group on G exhibited concentration-dependent properties, which provided solid evidence for the formation of hydrogen bonding interactions between G molecules.17 Based on the integration of the protons in Fig. S12 (ESI†), we could further conclude that about 40% of G was not encapsulated into the cavity of DMP5, thus intermolecular hydrogen bonding interactions might exist between the formed polyrotaxane backbones, leading to the formation of the supramolecular gel. Moreover, the rheology results further gave detailed information on the formation of the polyrotaxane-based supramolecular gel. As shown in Fig. 5, the oscillatory experiment suggested that the obtained gel exhibited a network behavior with the storage modulus G′ larger than the loss modulus G′′ at high oscillatory frequency (from 0.6 to 100 rad s−1), but lower than the loss modulus G′′ at low frequency (less than 0.6 rad s−1) at 25 °C (with a crossover point at G′ = G′′ = 4887 Pa). Moreover, the storage modulus G′ reached to a plateau value at about 104 Pa (termed as the plateau modulus G0) at high frequency, indicating that the sample exhibited moderate mechanical properties. The above observations further confirmed the formation of a supramolecular polymeric network gel with good mechanical properties.18
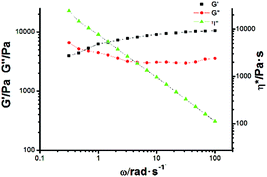 | ||
Fig. 5 Oscillation frequency sweep of polyrotaxane-based supramolecular gel at 23 °C (storage modulus G′ (black, ■), loss modulus G′′ (red,  ), complex viscosity (green, ), complex viscosity (green,  )). )). | ||
Furthermore, the obtained supramolecular gel exhibited reversible gel–sol phase transitions upon heating and cooling, which could be reversibly achieved many times (Fig. 6b). Also, reversible gel–sol phase transitions could be achieved by diluting and concentrating. Upon the addition of CHCl3, the obtained gel turns into solution after intensively shaking, and a supramolecular gel would be formed again by gradually evaporating the added CHCl3, and such a reversible cycle could be repeated many times (Fig. 6a). The above reversible transformations might result from the dynamic nature of the noncovalent hydrogen bonding interactions among the linear polymeric chains, since the strength might be weakened by heating or diluting, resulting in the transformation from a gel to sol. Whereas, upon cooling or concentrating, reversible recovery of the intermolecular hydrogen bonding interactions would lead to the reformation of a supramolecular gel. However, if the competitive ligand triphenylphosphine (PPh3) was added to the diluted solution or to the viscous solution at high temperature, the obtained solution could not form a supramolecular gel any more upon concentrating or cooling due to the competitive binding of PPh3 with Pd(OAc)2, resulting in destroying the polyrotaxanes.
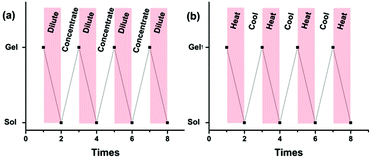 | ||
| Fig. 6 Reversible stimuli-responsive gel–sol transformation: (a) concentration-dependent transformation, (b) temperature-dependent transformation. | ||
Conclusions
In summary, a novel supramolecular polyrotaxane was successfully constructed by directly connecting the obtained pseudo[2]rotaxanes formed from an amide-functionalized pyridine derivative guest and a DMP5 host with a metal ion as linkage, which resulted in further gelation at high concentration assisted by the intermolecular hydrogen bonding interactions. Moreover, the obtained supramolecular polymeric network gel constructed by the orthogonal noncovalent interactions exhibited thermo- and diluting-induced reversible gel–sol transformation, whereas, adding the competitive ligand PPh3 resulted in the irreversible transformation. Further study on the macroscopic responsive behaviors of the supramolecular gel was conducted as well, which suggested its potential application as a smart material.Acknowledgements
We are grateful for the National Natural Science Foundation of China (no. 21572101, 21472089) and Jiangsu Provincial Natural Science Foundation of China (BK20140595).Notes and references
- (a) A. Harada, A. Hashidzume, H. Yamaguchi and Y. Takashima, Chem. Rev., 2009, 109, 5974 CrossRef CAS PubMed; (b) F. M. Raymo and J. F. Stoddart, Chem. Rev., 1999, 99, 1643 CrossRef CAS PubMed; (c) A. Harada, Y. Takashima and H. Yamaguchi, Chem. Soc. Rev., 2009, 38, 875 RSC; (d) H.-Y. Gong, B. M. Rambo, E. Karnas, V. M. Lynch and J. L. Sessler, Nat. Chem., 2010, 2, 406 CrossRef CAS PubMed.
- (a) R. S. Forgan, J.-P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434 CrossRef CAS PubMed; (b) K. Kim, Chem. Soc. Rev., 2002, 31, 96 RSC; (c) V. N. Vukotic and S. J. Loeb, Chem. Soc. Rev., 2012, 41, 5896 RSC; (d) A. Harada, J. Li and M. Kamachi, Nature, 1992, 356, 325 CrossRef CAS.
- (a) A. Harada and M. Kamachi, Macromolecules, 1990, 23, 2821 CrossRef CAS; (b) S. Kamitori, O. Matsuzaka, S. Kondo, S. Muraoka, K. Okuyama, K. Noguchi, M. Okada and A. Harada, Macromolecules, 2000, 33, 1500 CrossRef CAS.
- J.-P. Collin, C. Dietrich-Buchecker, P. Gaviña, M. C. Jimenez-Molero and J.-P. Sauvage, Acc. Chem. Res., 2001, 34, 477 CrossRef CAS PubMed.
- (a) G. Du, E. Moulin, N. Jouault, E. Buhler and N. Giuseppone, Angew. Chem., Int. Ed., 2012, 51, 12504 CrossRef CAS PubMed; (b) P. Wei, X. Yan and F. Huang, Chem. Commun., 2014, 50, 14105 RSC.
- (a) G. J. E. Davidson and S. J. Loeb, Angew. Chem., Int. Ed., 2003, 42, 74 CrossRef CAS; (b) D. J. Hoffart and S. J. Loeb, Angew. Chem., Int. Ed., 2005, 44, 901 CrossRef CAS PubMed; (c) D. J. Hoffart, J. Tiburcio, A. de la Torre, L. K. Knight and S. J. Loeb, Angew. Chem., Int. Ed., 2008, 47, 97 CrossRef CAS PubMed; (d) Y. Liu, Y.-L. Zhao, H.-Y. Zhang and H.-B. Song, Angew. Chem., Int. Ed., 2003, 42, 3260 CrossRef CAS PubMed; (e) K.-M. Park, D. Whang, E. Lee, J. Heo and K. Kim, Chem. – Eur. J., 2002, 8, 498 CrossRef CAS PubMed; (f) V. N. Vukotic and S. J. Loeb, Chem. – Eur. J., 2010, 16, 13630 CrossRef CAS PubMed; (g) D. Whang, Y.-M. Jeon, J. Heo and K. Kim, J. Am. Chem. Soc., 1996, 118, 11333 CrossRef CAS.
- Y.-M. Jeon, D. Whang, J. Kim and K. Kim, Chem. Lett., 1996, 25, 503 CrossRef.
- (a) S. J. Loeb and J. A. Wisner, Angew. Chem., Int. Ed., 1998, 37, 2838 CrossRef CAS; (b) S. J. Loeb and J. A. Wisner, Chem. Commun., 1998, 34, 2757 RSC.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T.-A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 CrossRef CAS PubMed; (b) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721 CrossRef CAS PubMed.
- (a) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294 CrossRef CAS PubMed; (b) T. Ogoshi and T.-A. Yamagishi, Eur. J. Org. Chem., 2013, 2961 CrossRef CAS.
- (a) T. Ogoshi, T. Akutsu, D. Yamafuji, T. Aoki and T.-A. Yamagishi, Angew. Chem., Int. Ed., 2013, 52, 8111 CrossRef CAS PubMed; (b) M. Ni, X.-Y. Hu, J. Jiang and L. Wang, Chem. Commun., 2014, 50, 1317 RSC; (c) N. L. Strutt, R. S. Forgan, J. M. Spruell, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 5668 CrossRef CAS PubMed; (d) Y. Guan, P. Liu, C. Deng, M. Ni, S. Xiong, C. Lin, X.-Y. Hu, J. Ma and L. Wang, Org. Biomol. Chem., 2014, 12, 1079 RSC; (e) X. Wu, M. Ni, W. Xia, X.-Y. Hu and L. Wang, Org. Chem. Front., 2015, 2, 1013 RSC; (f) S. Dong, J. Yuan and F. Huang, Chem. Sci., 2014, 5, 247 RSC.
- (a) P. Wang, Z. Li and X. Ji, Chem. Commun., 2014, 50, 13114 RSC; (b) W. Xia, X.-Y. Hu, Y. Chen, C. Lin and L. Wang, Chem. Commun., 2013, 49, 5085 RSC; (c) S. Sun, X.-Y. Hu, D. Chen, J. Shi, Y. Dong, C. Lin, Y. Pan and L. Wang, Polym. Chem., 2013, 4, 2224 RSC.
- (a) W. Si, L. Chen, X.-B. Hu, G. Tang, Z. Chen, J.-L. Hou and Z.-T. Li, Angew. Chem., Int. Ed., 2011, 50, 12564 CrossRef CAS PubMed; (b) W. Si, Z.-T. Li and J.-L. Hou, Angew. Chem., Int. Ed., 2014, 53, 4578 CrossRef CAS PubMed.
- (a) Y. Chang, K. Yang, P. Wei, S. Huang, Y. Pei, W. Zhao and Z. Pei, Angew. Chem., Int. Ed., 2014, 53, 13126 CrossRef CAS PubMed; (b) X.-Y. Hu, K. Jia, Y. Cao, Y. Li, S. Qin, F. Zhou, C. Lin, D. Zhang and L. Wang, Chem. – Eur. J., 2015, 21, 1208 CrossRef CAS PubMed; (c) Y. Cao, X.-Y. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y.-Z. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762 CrossRef CAS PubMed; (d) Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542 CrossRef CAS PubMed; (e) G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711 CrossRef CAS PubMed; (f) X. Wu, Y. Li, C. Lin, X.-Y. Hu and L. Wang, Chem. Commun., 2015, 51, 6832 RSC.
- (a) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397 CrossRef CAS PubMed; (b) T. Ogoshi, H. Kayama, D. Yamafuji, T. Aoki and T.-A. Yamagishi, Chem. Sci., 2012, 3, 3221 RSC; (c) X.-Y. Hu, X. Wu, S. Wang, D. Chen, W. Xia, C. Lin, Y. Pan and L. Wang, Polym. Chem., 2013, 4, 4292 RSC; (d) X.-Y. Hu, P. Zhang, X. Wu, W. Xia, T. Xiao, J. Jiang, C. Lin and L. Wang, Polym. Chem., 2012, 3, 3060 RSC; (e) N. L. Strutt, H. Zhang, M. A. Giesener, J. Leia and J. Fraser Stoddart, Chem. Commun., 2012, 48, 1647 RSC; (f) Z.-Y. Li, Y. Zhang, C.-W. Zhang, L.-J. Chen, C. Wang, H. Tan, Y. Yu, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 136, 8577 CrossRef CAS PubMed; (g) N. Song, D.-X. Chen, Y.-C. Qiu, X.-Y. Yang, B. Xu, W. Tian and Y.-W. Yang, Chem. Commun., 2014, 50, 8231 RSC.
- (a) X.-Y. Hu, X. Wu, Q. Duan, T. Xiao, C. Lin and L. Wang, Org. Lett., 2012, 14, 4826 CrossRef CAS PubMed; (b) L. Gao, Z. Zhang, B. Zheng and F. Huang, Polym. Chem., 2014, 5, 5734 RSC.
- (a) J. W. Steed, Chem. Soc. Rev., 2010, 39, 3686 RSC; (b) M. R. Panman, B. H. Bakker, D. den Uyl, E. R. Kay, D. A. Leigh, W. J. Buma, A. M. Brouwer, J. A. J. Geenevasen and S. Woutersen, Nat. Chem., 2013, 5, 929 CrossRef CAS PubMed.
- (a) X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS PubMed; (b) W. Qigang, J. L. Mynar, M. Yoshida, L. Eunji, L. Myongsoo, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterizations, NMR spectra, and the calculation of binding constant. See DOI: 10.1039/c6qo00197a |
| This journal is © the Partner Organisations 2016 |