Employing carboxylic acids in aryne multicomponent coupling triggered by aziridines/azetidines†
Tony
Roy
a,
Sachin Suresh
Bhojgude
a,
Trinadh
Kaicharla
a,
Manikandan
Thangaraj
a,
Bikash
Garai
b and
Akkattu T.
Biju
*a
aOrganic Chemistry Division, CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune-411008, India. E-mail: at.biju@ncl.res.in; Fax: +91-20-25902629; Tel: +91-20-25902441
bPhysical and Materials Chemistry Division, CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune-411008, India
First published on 16th November 2015
Abstract
The transition-metal-free aryne multicomponent coupling (MCC) involving carboxylic acids initiated by aziridines/azetidines has been reported. The use of aziridines as nucleophiles afforded N-aryl β-amino alcohol derivatives and the application of azetidines as nucleophilic triggers furnished N-aryl γ-amino alcohol derivatives in moderate to good yields. These reactions proceed under mild conditions and result in the formation of a new carbon–nitrogen bond and a new carbon–oxygen bond. The utility of carboxylic acids in aryne MCCs has been demonstrated, and the synthetic potential of phenols as acid surrogates in the present aryne MCCs has been realized.
Introduction
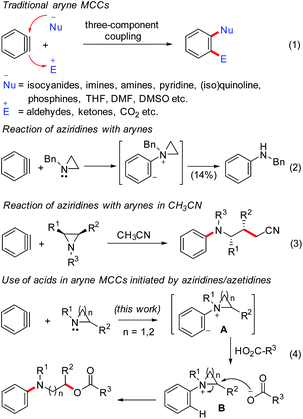
Among the various methods to build molecular complexity, the multicomponent couplings (MCCs) offer high levels of brevity and diversity, and are routinely used in medicinal and combinatorial chemistry.1,2 Engaging arynes in MCCs is one of the transition-metal-free protocols for the rapid access to complex 1,2-disubstituted benzene derivatives.3,4 Usually in aryne MCCs, a nucleophile (having no acidic protons) adds to aryne generating the aryl anion intermediate, which is trapped by a suitable electrophile (Scheme 1, eqn (1)).5 The commonly used nucleophiles are isocyanides,6 imines,7 amines,8 N-heterocycles9 such as pyridine, (iso)quinoline, phosphines,10 and solvents such as THF,6d DMF11 and DMSO.12 The electrophilic coupling partners usually used are aldehydes and ketones (including CO2),6a,13 activated imines and electron-deficient alkynes. Intriguingly, the utility of carboxylic acids as a third-component in aryne MCCs, to the best of our knowledge, is rare.14 Moreover, despite the utility of N-heterocycles in aryne MCCs, the use of aziridines as the nucleophilic trigger is limited.The generation of a zwitterion from aryne and N-benzyl aziridine and its subsequent rearrangement to N-benzyl aniline in 14% yield was known as early as 1972 (eqn (2)).15a Moreover, Cu-catalyzed aryne MCCs involving alkenyl aziridines and alkynes have been reported by Pineschi and co-workers.16 Recently, Larionov and co-workers disclosed the aryne MCCs initiated by aziridines, where the solvent CH3CN has been incorporated as the third-component resulting in the synthesis of N-aryl γ-aminobutyronitriles (eqn (3)).17a Very recently, we have developed the trifluoroacetic acid promoted aryne MCC involving arynes, aziridines and water for the synthesis of N-aryl β-amino alcohol derivatives.17b Herein, we report the aryne MCCs initiated by aziridines/azetidines, using carboxylic acids as the third-component.18 The reaction furnished either N-aryl β-amino alcohol derivatives or N-aryl γ-amino alcohol derivatives in moderate to good yields under mild conditions.
Mechanistically, the reaction of aziridines with arynes generates the zwitterionic intermediate A,15a which is sufficiently basic to deprotonate the carboxylic acid to form the quaternary ammonium salt B and the carboxylate anion. Nucleophilic attack of the carboxylate anion on salt B affords the N-aryl β-amino alcohol derivatives (eqn (4)). A new carbon–nitrogen bond as well as a new carbon–oxygen bond is formed in this reaction. In addition to the use of carboxylic acids as the third-component, the utility of phenols as the third-component in this aryne MCC has been demonstrated.
Results and discussion
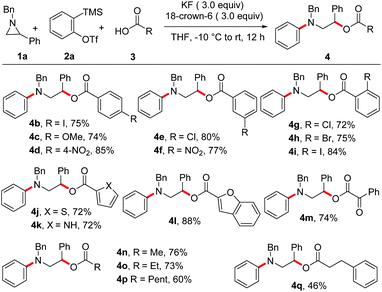
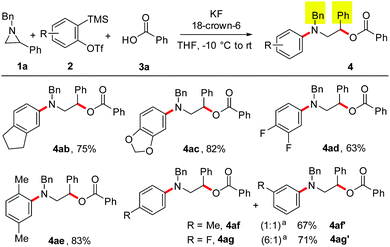
The present study was commenced by treating N-benzyl aziridine 1a and benzoic acid 3a with the aryne generated in situ from 2-(trimethylsilyl)aryl triflate 2a19 using KF in the presence of 18-crown-6. Under these conditions, a smooth reaction occurred leading to the formation of 2-(benzyl(phenyl)amino)-1-phenylethyl benzoate 4a in 83% yield (Scheme 2).20 Two-fold excess of KF and 18-crown-6 (relative to 2a) was needed for good conversion. Notably, the product derived from the rearrangement of the initially formed zwitterion (A) from 1a and aryne was not observed.15a Moreover, the fluorine incorporated product in the reaction of arynes with aziridines recently reported by Wu and Sha was not observed under the optimized conditions.21 As the benzoic acid and 2a were used in slight excess, the insertion product (phenyl benzoate) was observed in ∼10% yield under the present conditions.14,22After establishing the protocol for incorporating carboxylic acids in aryne MCCs, we then evaluated the scope of this reaction. First, the effect of various carboxylic acids was examined (Scheme 3). Gratifyingly, benzoic acids having electronically different substituents at the para-position of the ring were well-tolerated leading to the synthesis of the corresponding N-aryl β-amino alcohol derivatives in good yields irrespective of the substrate electronics (4b–4d). Moreover, benzoic acids with substituents at the meta- and ortho-positions of the ring underwent smooth MCCs affording the desired product in high yields (4e–4i). Additionally, heteroaromatic carboxylic acids and an α-keto acid could be employed as the third-component furnishing the expected MCC product in good yields (4j–4m). Furthermore, MCCs using a series of aliphatic acids resulted in the formation of the target products (4n–4q) in moderate to good yields demonstrating the versatility of the present reaction. Notably, stronger acids such as trifluoroacetic acid and trifluoromethanesulfonic acid did not afford the MCC product under the optimized conditions.17b
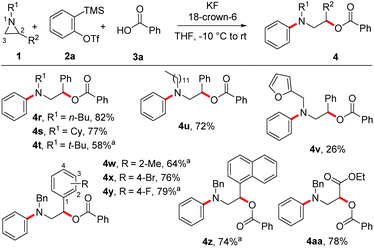
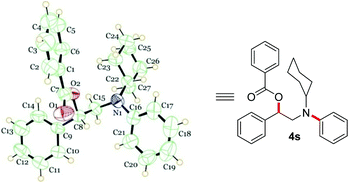
Next, the scope of this MCC with substituted aziridines was examined (Scheme 4). Interestingly, linear, branched and even sterically demanding substituents on aziridine nitrogen were tolerated (4r–4v). The corresponding products were isolated in moderate to good yields. In the case of 4s, the structure was confirmed by single-crystal X-ray analysis (Fig. 1).23 Moreover, aziridines with substitution at the 2-aryl ring underwent efficient MCC under the optimized conditions to afford N-aryl β-aryl functionalized β-amino alcohol derivatives 4w–4z in 64–79% yield. In addition, electron deficient ethyl 1-benzylaziridine-2-carboxylate afforded the desired product 4aa in 78% yield, thus expanding the scope of this MCC.
The scope of this reaction with differently substituted arynes was also studied (Scheme 5). Electronically different symmetrical 4,5-disubstituted arynes generated from their precursors underwent efficient MCCs with aziridine 1a and benzoic acid to afford the functionalized aromatic amines in good yields (4ab–4ad). In addition, the 3,6-dimethyl aryne generated from its precursor afforded the desired product 4ae in 83% yield. As expected, the 4-methyl benzyne furnished an inseparable mixture of regioisomers 4af and 4af′ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and in 67% yield. Furthermore, an inseparable mixture of regioisomers 4ag and 4ag′ in a 6
1 ratio and in 67% yield. Furthermore, an inseparable mixture of regioisomers 4ag and 4ag′ in a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and in 71% yield was obtained by using 4-fluorobenzyne as the aryne component in this reaction.
1 ratio and in 71% yield was obtained by using 4-fluorobenzyne as the aryne component in this reaction.
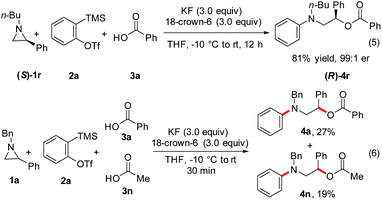
Further experiments have shed light on the mechanism of this transformation. The reaction of enantiomerically pure aziridine (S)-1r with 2a and 3a under the optimized conditions afforded the chiral amino alcohol derivative (R)-4r in 81% yield and 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er (Scheme 6, eqn (5)). The formation of (R)-4r in high er rules out the possibility of SN1 opening of intermediate B (eqn (4)). The enantiomerically pure (S)-1r was prepared by the reaction of commercially available (R)-phenyloxirane with n-butylamine followed by the cyclization of the intermediate β-amino alcohol derivative.24 Moreover, a competition experiment carried out using 1a and aryne generated from 2a with aromatic acid 3a and aliphatic acid 3n revealed that 3a reacted ∼1.5 times faster than 3n when the reaction was quenched after 30 minutes under the optimized conditions (eqn (6)). A similar result was obtained when the reaction was quenched after 60 minutes.20 These results tend to indicate that the aromatic acid being more strong can protonate the aryl anion intermediate A (eqn (4)) faster than the aliphatic acid.
1 er (Scheme 6, eqn (5)). The formation of (R)-4r in high er rules out the possibility of SN1 opening of intermediate B (eqn (4)). The enantiomerically pure (S)-1r was prepared by the reaction of commercially available (R)-phenyloxirane with n-butylamine followed by the cyclization of the intermediate β-amino alcohol derivative.24 Moreover, a competition experiment carried out using 1a and aryne generated from 2a with aromatic acid 3a and aliphatic acid 3n revealed that 3a reacted ∼1.5 times faster than 3n when the reaction was quenched after 30 minutes under the optimized conditions (eqn (6)). A similar result was obtained when the reaction was quenched after 60 minutes.20 These results tend to indicate that the aromatic acid being more strong can protonate the aryl anion intermediate A (eqn (4)) faster than the aliphatic acid.
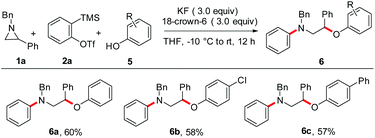
Encouraged by the success of the efficient aryne MCCs using carboxylic acids as the third-component, we continued our studies using phenols as acid surrogates in the present MCC. In a pilot experiment, treatment of 1a with aryne generated from 2a and phenol 5a under optimized reaction conditions resulted in the synthesis of N-benzyl-N-(2-phenoxy-2-phenylethyl)aniline 6a in 60% yield (Scheme 7). In this case, the aziridine–aryne adduct A (eqn (3)) was protonated by phenol followed by the nucleophilic attack by the phenoxide ion resulting in the formation of 6.25 As in the case of reaction with acids, the O-arylated phenol was also observed in ∼15% yield.14 The reaction worked reasonably well with 4-Cl and 4-Ph substituted phenols, and the desired products were isolated in moderate yields.
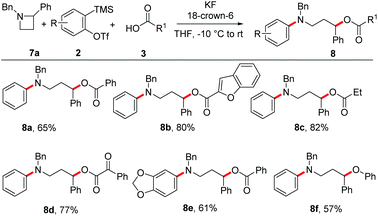
It was found that the present aryne MCCs are not limited to N-substituted aziridines as nucleophilic triggers, but instead N-substituted azetidines afforded the corresponding N-aryl γ-amino alcohol derivatives in good yields (Scheme 8). The reaction worked well with aromatic, heteroaromatic and aliphatic carboxylic acids and in all cases, the desired product was formed in good yields (8a–8c). In addition, α-ketoacid and 4,5-disubstituted symmetrical aryne generated from the precursor also underwent smooth aryne MCCs under the present conditions using azetidine as the nucleophilic trigger (8d–8e). In addition, phenol was also used as the acid surrogate in this reaction leading to the formation of the phenoxy derivative 8f in 57% yield.
Conclusions
In conclusion, we have developed a practical and efficient MCC involving arynes, aziridines/azetidines and carboxylic acids. The reaction resulted in a transition-metal-free route to N-aryl β-amino alcohol derivatives and N-aryl γ-amino alcohol derivatives in good yields. The utility of carboxylic acids in aryne MCCs has been demonstrated and phenols can also be used as acid surrogates in this reaction. Given the importance of amino alcohol derivatives in biological systems, the MCC approach presented herein is likely a method to access these compounds under mild conditions.Experimental section
General procedure for the MCC involving aziridine, aryne and carboxylic acids
To a flame-dried screw-capped test tube equipped with a magnetic stir bar was added KF (0.087 g, 1.5 mmol) and 18-crown-6 (0.396 g, 1.5 mmol) inside a glove box. Carboxylic acid 3 (0.75 mmol) was added outside the glove box under an argon atmosphere. The mixture was dissolved in THF (2.0 mL) under an argon atmosphere and continued stirring for five minutes at 30 °C. After five minutes of stirring, aziridine 1 (0.5 mmol) was added. Then the reaction mixture was cooled to −10 °C and kept stirring for five minutes. To the stirring solution, aryne precursor 2 (0.75 mmol) was added. Then the reaction mixture was slowly warmed to rt and kept stirring for 12 h. After 12 h the reaction was stopped, the solvent was evaporated and the crude residue was pre-adsorbed on silica gel and purified by flash column chromatography (pet. ether/EtOAc = 98/2) on silica gel to afford the corresponding N-aryl β-amino alcohol derivatives 4 in moderate to good yields.General procedure for the MCC involving aziridine, aryne and phenols
To a flame-dried screw-capped test tube equipped with a magnetic stir bar was added KF (0.087 g, 1.5 mmol) and 18-crown-6 (0.396 g, 1.5 mmol) inside a glove box. Phenol 5 (0.75 mmol) was added outside the glove box under an argon atmosphere. The mixture was dissolved in THF (2.0 mL) under an argon atmosphere and continued stirring for five minutes at 30 °C. After five minutes of stirring, aziridine 1 (0.5 mmol) was added. Then the reaction mixture was cooled to −10 °C and kept stirring for five minutes. To the stirring solution, aryne precursor 2 (0.75 mmol) was added. Then the reaction mixture was slowly warmed to rt and kept stirring for 12 h. After 12 h the reaction was stopped, the solvent was evaporated and the crude residue was pre-adsorbed on silica gel and purified by flash column chromatography (pet. ether/EtOAc = 98/2) on silica gel to afford the corresponding N-aryl β-amino alcohol derivatives 6 in moderate to good yields.General procedure for the MCC involving azetidine, aryne and carboxylic acids/phenol
To a flame-dried screw-capped test tube equipped with a magnetic stir bar was added KF (87 mg, 1.5 mmol) and 18-crown-6 (396 mg, 1.5 mmol) inside a glove box. Carboxylic acid/phenol 3/5 (0.75 mmol) was added outside the glove box under an argon atmosphere. The mixture was dissolved in THF (2.0 mL) under an argon atmosphere and continued stirring for five minutes at 30 °C. After five minutes of stirring, azetidine 7 (0.5 mmol) was added. Then the reaction mixture was cooled to −10 °C and kept stirring for five minutes. To the stirring solution, aryne precursor 2 (0.75 mmol) was added. Then the reaction mixture was slowly warmed to rt and kept stirring for 12 h. After 12 h the reaction was stopped, the solvent was evaporated and the crude residue was pre-adsorbed on silica gel and purified by flash column chromatography (pet. ether/EtOAc = 98/2) on silica gel to afford the corresponding N-aryl γ-amino alcohol derivatives 8 in moderate to good yields.Acknowledgements
Financial support from the SERB-DST, Government of India (Grant No. SR/S1/OC/12/2012) is kindly acknowledged. T. R. thanks CSIR-OSDD for the Tata-CSIR-OSDD fellowship (tcof-6) and DST for the Inspire fellowship. M. T., T. K., and S. S. B. thank CSIR for the research fellowships. We thank Dr Rahul Banerjee for the support with X-ray analysis, Dr P. R. Rajamohanan and Mr Sanoop B. Nair for the excellent NMR support, Mrs B. Santhakumari for the HRMS data, and Mr Anup Bhunia and Mr Santhivardhana Reddy Yetra for the useful discussion.Notes and references
- For reviews, see: (a) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef PubMed; (b) C. de. Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969 RSC; (c) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef PubMed; (d) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS PubMed; (e) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (f) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef.
- (a) R. S. Menon and V. Nair, Modifications of the Ugi reaction, in Science of Synthesis, Multicomponent Reactions I, ed. T. J. J. Muller, Thieme Chemistry, 2014, p. 503 Search PubMed; (b) Multicomponent Reactions, ed. J. Zhu and H. Bienaymé, Wiley-VCH, Weinheim, 2005 Search PubMed.
- For recent reviews on aryne chemistry, see: (a) A. V. Dubrovskiy, N. A. Markina and R. C. Larock, Org. Biomol. Chem., 2013, 11, 191 RSC; (b) C. Wu and F. Shi, Asian J. Org. Chem., 2013, 2, 116 CrossRef CAS; (c) D. Pérez, D. Peña and E. Guitián, Eur. J. Org. Chem., 2013, 5981 CrossRef; (d) P. M. Tadross and B. M. Stoltz, Chem. Rev., 2012, 112, 3550 CrossRef CAS PubMed; (e) C. M. Gampe and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 3766 CrossRef CAS PubMed; (f) A. Bhunia, S. R. Yetra and A. T. Biju, Chem. Soc. Rev., 2012, 41, 3140 RSC; (g) K. Okuma, Heterocycles, 2012, 85, 515 CrossRef CAS; (h) H. Yoshida, J. Ohshita and A. Kunai, Bull. Chem. Soc. Jpn., 2010, 83, 199 CrossRef CAS. For a review on hetarynes, see: (i) A. E. Goetz, T. K. Shah and N. K. Garg, Chem. Commun., 2015, 51, 34 RSC.
- (a) H. Yoshida, Aryne-based Multicomponent Reactions, in Multicomponent Reactions in Organic Synthesis, ed. J. Zhu, Q. Wang and M.-X. Wang, Wiley-VCH, Weinheim, 2015, p. 39 Search PubMed; (b) T. Kaicharla and A. T. Biju, Transition-Metal-Free Synthesis of Benzo-Fused Five and Six-Membered Heterocycles Employing Arynes, in Green Synthetic Approaches for Biologically Relevant Heterocycles, ed. G. Brahmachari, Elsevier, 2014, p. 45 Search PubMed; (c) Y. Chen and R. C. Larock, Arylation Reactions Involving the Formation of Arynes, in Modern Arylation Methods, ed. L.Ackermann, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2009, p. 401 Search PubMed.
- For a highlight on aryne three-component coupling, see: (a) S. S. Bhojgude and A. T. Biju, Angew. Chem., Int. Ed., 2012, 51, 1520 CrossRef CAS PubMed. See also: (b) A. Bhunia and A. T. Biju, Synlett, 2014, 608 CAS.
- For selected reports, see: (a) T. Kaicharla, M. Thangaraj and A. T. Biju, Org. Lett., 2014, 16, 1728 CrossRef CAS PubMed; (b) J. Li, S. Noyori, K. Nakajima and Y. Nishihara, Organometallics, 2014, 33, 3500 CrossRef CAS; (c) F. Sha, L. Luling Wu and X. Huang, J. Org. Chem., 2012, 77, 3754 CrossRef CAS PubMed; (d) H. Yoshida, Y. Asatsu, Y. Mimura, Y. Ito, J. Ohshita and K. Takaki, Angew. Chem., Int. Ed., 2011, 50, 9676 CrossRef CAS PubMed; (e) K. M. Allan, C. D. Gilmore and B. M. Stoltz, Angew. Chem., Int. Ed., 2011, 50, 4488 CrossRef CAS PubMed; (f) H. Yoshida, Y. Ito and J. Ohshita, Chem. Commun., 2011, 47, 8512 RSC; (g) F. Sha and X. Huang, Angew. Chem., Int. Ed., 2009, 48, 3458 CrossRef CAS PubMed; (h) H. Yoshida, H. Fukushima, J. Ohshita and A. Kunai, Angew. Chem., Int. Ed., 2004, 43, 3935 CrossRef CAS PubMed.
- (a) Y. Zhou, Y. Chi, F. Zhao, W.-X. Zhang and Z. Xi, Chem. – Eur. J., 2014, 20, 2463 CrossRef CAS PubMed; (b) H. Yoshida, H. Fukushima, J. Ohshita and A. Kunai, J. Am. Chem. Soc., 2006, 128, 11040 CrossRef CAS PubMed.
- (a) C. E. Hendrick, S. L. McDonald and Q. Wang, Org. Lett., 2013, 15, 3444 CrossRef CAS PubMed; (b) H. Yoshida, T. Morishita and J. Ohshita, Org. Lett., 2008, 10, 3845 CrossRef CAS PubMed; (c) H. Yoshida, T. Morishita, H. Fukushima, J. Ohshita and A. Kunai, Org. Lett., 2007, 9, 3367 CrossRef CAS PubMed; (d) T. Morishita, H. Fukushima, H. Yoshida, J. Ohshita and A. Kunai, J. Org. Chem., 2008, 73, 5452 CrossRef CAS PubMed.
- (a) A. Bhunia, T. Roy, P. Pachfule, P. R. Rajamohanan and A. T. Biju, Angew. Chem., Int. Ed., 2013, 52, 10040 CrossRef CAS PubMed; (b) A. Bhunia, D. Porwal, R. G. Gonnade and A. T. Biju, Org. Lett., 2013, 15, 4620 CrossRef CAS PubMed. For related works, see: (c) F. Nawaz, K. Mohanan, L. Charles, M. Rajzmann, D. Bonne, O. Chuzel, J. Rodriguez and Y. Coquerel, Chem. – Eur. J., 2013, 19, 17578 CrossRef CAS PubMed; (d) P. Liu, M. Lei and L. Hu, Tetrahedron, 2013, 69, 10405 CrossRef CAS; (e) M. Jeganmohan, S. Bhuvaneswari and C.-H. Cheng, Chem. – Asian J., 2010, 5, 153 CrossRef CAS PubMed; (f) M. Jeganmohan and C.-H. Cheng, Chem. Commun., 2006, 2454 RSC.
- (a) A. Bhunia, T. Kaicharla, D. Porwal, R. G. Gonnade and A. T. Biju, Chem. Commun., 2014, 50, 11389 RSC; (b) A. Bhunia, T. Roy, R. G. Gonnade and A. T. Biju, Org. Lett., 2014, 16, 5132 CrossRef CAS PubMed.
- (a) E. Yoshioka, H. Tamenga and H. Miyabe, Tetrahedron Lett., 2014, 55, 1402 CrossRef CAS; (b) C. Zhou, J. Wang, J. Jin, P. Lu and Y. Wang, Eur. J. Org. Chem., 2014, 1832 CrossRef CAS; (c) E. Yoshioka, H. Tanaka, S. Kohtani and H. Miyabe, Org. Lett., 2013, 15, 3938 CrossRef CAS PubMed; (d) E. Yoshioka, S. Kohtani and H. Miyabe, Angew. Chem., Int. Ed., 2011, 50, 6638 CrossRef CAS PubMed; (e) H. Yoshida, Y. Ito and J. Ohshita, Chem. Commun., 2011, 47, 8512 RSC; (f) E. Yoshioka, S. Kohtani and H. Miyabe, Org. Lett., 2010, 12, 1956 CrossRef CAS PubMed.
- (a) H.-Y. Li, L.-J. Xing, M.-M. Lou, H. Wang, R.-H. Liu and B. Wang, Org. Lett., 2015, 17, 1098 CrossRef CAS PubMed; (b) F.-L. Liu, J.-R. Chen, Y.-Q. Zou, Q. Wei and W.-J. Xiao, Org. Lett., 2014, 16, 3768 CrossRef CAS PubMed.
- (a) W.-J. Yoo, T. V. Q. Nguyen and S. Kobayashi, Angew. Chem., Int. Ed., 2014, 53, 10213 CrossRef CAS PubMed; (b) H. Yoshida, T. Morishita and J. Ohshita, Org. Lett., 2008, 10, 3845 CrossRef CAS PubMed; (c) H. Yoshida, H. Fukushima, J. Ohshita and A. Kunai, J. Am. Chem. Soc., 2006, 128, 11040 CrossRef CAS PubMed.
- For the insertion of arynes into the O–H bond of carboxylic acids, see: (a) Z. Liu and R. C. Larock, Org. Lett., 2004, 6, 99 CrossRef CAS PubMed; (b) Z. Liu and R. C. Larock, J. Org. Chem., 2006, 71, 3198 CrossRef CAS PubMed.
- (a) A. G. Giumanini, J. Org. Chem., 1972, 37, 513 CrossRef CAS. For a report on the reaction of arynes with 1-azirines, see: (b) V. Nair and K. H. Kim, J. Org. Chem., 1975, 40, 3784 CrossRef CAS.
- F. Berti, P. Crotti, G. Cassano and M. Pineschi, Synlett, 2012, 2463 CAS.
- (a) D. Stephens, Y. Zhang, M. Cormier, G. Chavez, H. Arman and O. V. Larionov, Chem. Commun., 2013, 49, 6558 RSC; (b) T. Roy, D. R. Baviskar and A. T. Biju, J. Org. Chem., 2015, 80, 11131 CrossRef CAS PubMed. For a review on ring opening of non-activated 2-substituted aziridines, see: (c) S. Stanković, M. D'hooghe, S. Catak, H. Eum, M. Waroquier, V. V. Speybroeck, N. De Kimpe and H.-J. Ha, Chem. Soc. Rev., 2012, 41, 643 RSC.
- For related aryne MCCs triggered by cyclic ethers, see: (a) K. Okuma, Y. Fukuzaki, A. Nojima, K. Shioji and Y. Yokomori, Tetrahedron Lett., 2008, 49, 3063 CrossRef CAS; (b) K. Okuma, H. Hino, A. Sou, N. Nagahara and K. Shioji, Chem. Lett., 2009, 38, 1030 CrossRef CAS.
- (a) Y. Himeshima, T. Sonoda and H. Kobayashi, Chem. Lett., 1983, 1211 CrossRef CAS. For a simplified procedure, see: (b) D. Peña, A. Cobas, D. Pérez and E. Guitián, Synthesis, 2002, 1454 Search PubMed.
- For details, see the ESI.†.
- (a) C.-Y. Tang, G. Wang, X.-Y. Yang, X.-Y. Wu and F. Sha, Tetrahedron Lett., 2014, 55, 6447 CrossRef CAS; (b) For the reaction of arynes with 1-azirines, see: V. Nair and K. H. Kim, J. Org. Chem., 1975, 40, 3784 CrossRef CAS.
- It may be mentioned that performing the reaction at 30 °C afforded 4a in 64% yield along with the O–H insertion product in 23% yield. Decreasing the reaction temperature improved the yield of 4a.
- CCDC 1050176 (4s).
- Y. Du, Y. Wu, A. H. Liu and L. N. He, J. Org. Chem., 2008, 73, 4709 CrossRef CAS PubMed.
- For an early report on the use of phenol in aryne MCCs, see: R. S. Pal and M. M. Bokadia, Polym. J. Chem., 1978, 52, 1473 CAS . See also: nucleophilic coupling with arynes. S. V. Kessar, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, England, 1991, vol. 4, p. 483 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details on experimental procedures, characterization data of all compounds, and single crystal X-ray data of compound 4s. CCDC 1050176. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00328h |
| This journal is © the Partner Organisations 2016 |