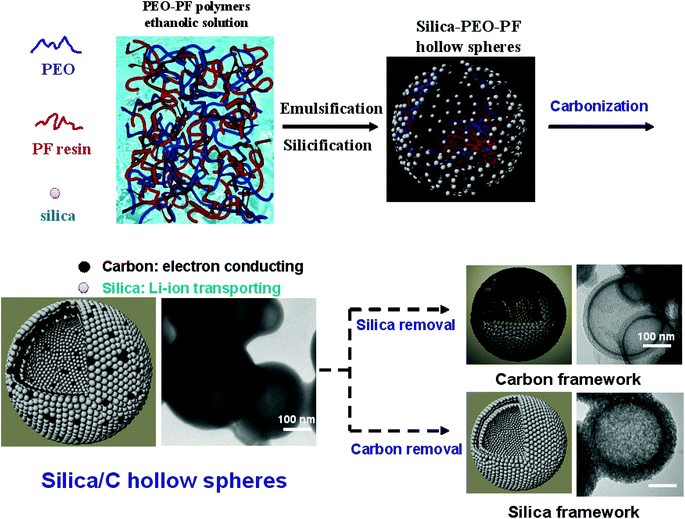
Mesoporous SiO2/carbon hollow spheres applied towards a high rate-performance Li-battery anode†
Chien-Wen
Wang
a,
Kung-Wen
Liu
b,
Wei-Fu
Chen
 c,
Jing-De
Zhou
a,
Hong-Ping
Lin
b,
Chun-Han
Hsu
c,
Jing-De
Zhou
a,
Hong-Ping
Lin
b,
Chun-Han
Hsu
 *ab and
Ping-Lin
Kuo
*a
*ab and
Ping-Lin
Kuo
*a
aDepartment of Chemical Engineering, National Cheng Kung University, Tainan, Taiwan, ROC. E-mail: chunhanhsu@gmail.com; Tel: +886-6-2757575 ext 65342
bDepartment of Chemistry, National Cheng Kung University, Tainan, Taiwan, ROC
cCenter for Condensed Matter Sciences, National Taiwan University, Taipei, Taiwan, ROC
First published on 21st September 2016
Abstract
Mesoporous SiO2/C hollow spheres have been successfully synthesized via a one-step template process and carbonization of a mesoporous SiO2/poly(ethylene oxide)/phenolic formaldehyde resin hollow nanocomposite, and then evaluated as anode materials for lithium-ion batteries. The continuous carbon framework significantly led the SiO2/C hollow spheres to reach a high conductivity (3.9 × 10−4 S cm−1) compared with the SiO2 hollow spheres (<10−9 S cm−1), furthermore, the unique hollow nanostructure with a large volume interior and numerous mesopores plugged with carbon in the silica shell, could accommodate the volume variation and improve the structural strain for Li ion conduction, as well as allow rapid access of Li ions during charge–discharge cycling. For battery applications, at 100 mA g−1 charge/discharge rates, the reversible capacity of this mesoporous SiO2/C anode (624 mA h g−1) is over ten times higher than that of the SiO2 anode (61 mA h g−1). More specifically, even under the high discharge rate of 3000 mA g−1, this SiO2/C hollow nanostructure exhibits a specific capacity of 582 mA h g−1, featuring a high retention of more than 90% of its low discharge rate of 100 mA g−1. This demonstrates that the effective conduction of electrons through the continuous carbon network and the fast transport of Li ions through the nanoscale SiO2 shell significantly contribute to the high-rate performance.
1. Introduction
For lithium-ion batteries (LIBs), high safety, high capacity and high rate performance have been considered as essential requirements for the application to hybrid-electric vehicles and electric vehicles.1–3 Silicon-based materials have been extensively studied for use as anodes in LIBs due to their high theoretical capacity (4200 mA h g−1), high safety and low-cost.4–6 However, the drastic volume variation during operation leads to their remarkable capacity fading.Among the silicon-based materials, SiO2 is cost-effective, environmentally-friendly and one of the most abundant materials on Earth. However, of the SiO2 anode-material characteristics responsible for the rate capability of batteries, the low electrical conductivity (10−10–10−15 S cm−1) and lithium ion diffusion coefficient are two of the most important issues.7–10 The hollowing of electrode materials remarkably increased the rate-performance,11–16 and even achieved a capacity of 119 mA h g−1 at a current rate of 10 C even after 1200 cycles for a hollow TiO2 anode. Furthermore, it has been reported that SiO2 exhibits Li reactivity in the amorphous state such as a hollow structure and a thin film, but the processes required complex procedures employing high cost precursors.17–19
We herein report a simple, cost-effective and inexpensive material to prepare mesoporous SiO2/C hollow spheres as the anode material for LIBs, for which, sodium silicate and phenolic formaldehyde (PF) resin were used as a silica and carbon precursor, respectively. These mesoporous SiO2/C hollow spheres not only offer enhanced electronic conductivity through the continuous carbon network, but also improve Li ion diffusion via the nanoscale SiO2/C shell.
2. Experimental
2.1 Preparation of mesoporous SiO2/C hollow nanocomposites
According to Lin,20–24 the typical synthesis of SiO2/C composites is as follows: poly(ethylene oxide) (PEO, Acrös, MW 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) (1.0 g) and PF (1.0 g; PF2180, phenol/aldehyde, 0.8–0.9; MW ca. 96
000) (1.0 g) and PF (1.0 g; PF2180, phenol/aldehyde, 0.8–0.9; MW ca. 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; Chung-Chun Plastic, Taiwan) polymers were dissolved in a mixture of ethanol (20.0 g) and water (10.0 g) to form a clear solution. Then, the PF–PEO blended solution was quickly poured into an acidified sodium silicate aqueous solution (pH = 2.0) at 40 °C, which was prepared by adjusting the pH value of a mixture of sodium silicate (27 wt% SiO2, 4.0 g, Sigma-Aldrich) and water (150.0 g) to 6.0 and aging for 3 min. A light-yellow precipitate formed within a few seconds. Filtration, washing, and drying at 100 °C yield the PEO–PF/silica hollow spheres. The SiO2/C hollow spheres were formed after pyrolysis at 800 °C under a N2 atmosphere for 2 h; in contrast, the SiO2 hollow spheres are produced after calcination at 600 °C under an O2 atmosphere for 12 h. For comparison, the carbon hollow spheres were prepared by removing the SiO2 from SiO2/C hollow spheres under 5.0 M NaOH treatments.
000; Chung-Chun Plastic, Taiwan) polymers were dissolved in a mixture of ethanol (20.0 g) and water (10.0 g) to form a clear solution. Then, the PF–PEO blended solution was quickly poured into an acidified sodium silicate aqueous solution (pH = 2.0) at 40 °C, which was prepared by adjusting the pH value of a mixture of sodium silicate (27 wt% SiO2, 4.0 g, Sigma-Aldrich) and water (150.0 g) to 6.0 and aging for 3 min. A light-yellow precipitate formed within a few seconds. Filtration, washing, and drying at 100 °C yield the PEO–PF/silica hollow spheres. The SiO2/C hollow spheres were formed after pyrolysis at 800 °C under a N2 atmosphere for 2 h; in contrast, the SiO2 hollow spheres are produced after calcination at 600 °C under an O2 atmosphere for 12 h. For comparison, the carbon hollow spheres were prepared by removing the SiO2 from SiO2/C hollow spheres under 5.0 M NaOH treatments.
2.2 Methods of characterization
Morphological characterization was performed by scanning electron microscopy (SEM) using a JEOL JEM6700 FE-SEM operating at 10 kV. Size and structure characterization were performed by transmission electron microscopy (TEM) using a Hitachi H-7500 microscope operating at 80 kV. Specific surface areas of the prepared carbon spheres were determined using the Brunauer–Emmett–Teller (BET) method by using a Micromeritics ASAP 2020 instrument. X-ray powder diffraction (XRD) was performed by using a Rigaku RINT2100 X-ray diffractometer with Cu-Kα radiation operated at 30 kV and 30 mA while thermogravimetric analysis was conducted by using a thermogravimetric analyzer (TGA Q-500) over a temperature range of 50–800 °C at a heating rate of 20 °C min−1. A micro Raman spectrometer from Renishaw with a He–Ne laser source with a wavelength of 633 nm was used to determine the structure of the SiO2/carbon hollow spheres. The electronic conductivity of the samples was measured with a four-point probe (Elchema CM-308, USA). Measurement specimens were made by casting samples on a glass substrate with a 5 wt% polytetrafluoroethylene binder to form 1.2 mm carbon films.2.3 Electrochemical measurements
Electrodes and coin cells (CR2032) were prepared as follows. To construct the tested anode, active material (70 wt%), super P (15 wt%, a conductive carbon additive) and polyvinylidene fluoride (PVDF) (15 wt%) were thoroughly mixed. Then, N-methyl-pyrrolidone (NMP) was added to the above mixture as a binder solvent. After magnetic stirring for 10 h, the resulting slurry was coated onto a copper current collector and dried under vacuum at 120 °C for 12 h. Finally the coin cells were assembled in a dry and inert glove box with the prepared anode with a capacity density of ca. 2 mA h cm−2 for SiO2/C active materials, lithium metal, and Celgard 2400 polypropylene separator. The assembled cells were cycled at different charge–discharge rates in a potential range of 0.05–3.0 V on a BAT-750B cell test instrument (AcuTech Systems Co. Ltd). Specific capacity was calculated based on the mass of active materials with the carbon included. 1.0 M LiPF6 EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) was used as the electrolyte.
1 by volume) was used as the electrolyte.
3. Results and discussion
The one-step template synthesis approach of the mesoporous SiO2/C hollow spheres is schematically illustrated in Fig. 1. The procedure starts with the structure-directing polymer, PEO, blended with a carbon precursor, namely PF resin. Subsequently, the blended solution was mixed with a sodium silicate solution to form SiO2/PEO/PF hollow spheres, which then underwent carbonization to form the mesoporous SiO2/C hollow-sphere composite. The detailed synthetic process and chemical compositions are described elsewhere.21 A plausible mechanism was proposed addressing the formation of the hollow spheres. When the PEO–PF solution was added into a highly diluted sodium silicate aqueous solution, the hydrophobic PF polymers induced the formation of PF–PEO emulsions. At the same time, the silica species, which assemble with the PEO polymers, attach onto the outer surface of the PEO–PF emulsions. After fast further silica condensation, the PEO–PF emulsions were solidified prior to the PF phase-separation, and then the SiO2/PEO/PF hollow spheres were generated.Due to the SiO2/C nanoscale-shell dimensions, Li ions can readily transport into and out of the shell; furthermore, electrons can be effectively conducted into the insulating silica shell through the carbon network during operation. Via these two characteristics, it is expected that the improved Li ion transport and electron conduction will intensify battery performance, particularly at high charge–discharge rates. For comparison, the SiO2 hollow spheres were prepared from the SiO2/PEO/PF composite by further calcinations to remove the organic template, and the carbon hollow spheres were prepared by silica etching from this SiO2/C hollow composite.
Fig. 2a–c display the SEM images of the SiO2/C hollow spheres, SiO2 hollow spheres and carbon hollow spheres, respectively. All the three types of samples show a similar sphere morphology with an average diameter of 0.5–1.0 μm. In addition, Fig. 2d displays the SEM images corresponding to C, O, and Si EDX elemental mappings of the SiO2/C hollow spheres, further demonstrating the homogeneous distribution of C and SiO2 in the nanocomposite. The hollow structure was proved by the TEM images, as shown in Fig. 3a–c, in which the shell diameters are all less than 50 nm. Both the silica framework and carbon framework show a hollow sphere structure, which provides evidence of the continuous structure of silica and carbon in the SiO2/C composite. After removing the organic species or silica from the SiO2/C hollow spheres (Fig. 3d), both SiO2 hollow spheres (Fig. 3e) and carbon hollow spheres (Fig. 3f) were found to have a porous structure. Furthermore, the TEM images corresponding to C, O, and Si EDX elemental mappings of the single SiO2/C hollow sphere confirm the homogeneous distribution of C and SiO2 in the nanocomposite as shown in Fig. S1.†
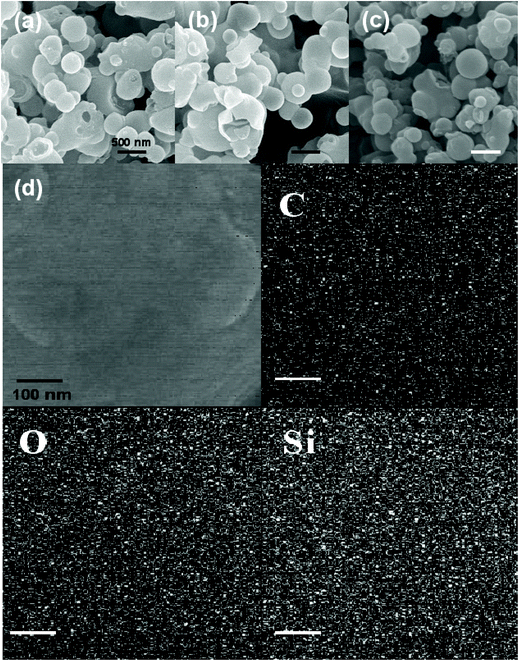 | ||
| Fig. 2 SEM images of (a) SiO2/C hollow spheres, (b) SiO2 hollow spheres, (c) carbon hollow spheres and (d) corresponding C, O, and Si EDX elemental mappings of SiO2/C hollow spheres. | ||
 | ||
| Fig. 3 TEM images of (a, d) SiO2/C hollow spheres, (b, e) SiO2 hollow spheres and (c, f) carbon hollow spheres. | ||
The porous properties of the SiO2/C hollow spheres and SiO2 hollow spheres were further characterized by measuring the N2 adsorption–desorption isotherms, as shown in Fig. 4a, which revealed the characteristics of a type IV curve, confirming the mesoporous structure of the SiO2 shell. The surface areas, calculated with the BET model, are 8.7 and 288.6 m2 g−1 for of SiO2/C and SiO2 hollow spheres, respectively. The low surface area of SiO2/C hollow spheres may be due to the carbon which effectively plugs the pores on the surface of the SiO2 hollow spheres and therefore the void volume inside is not accessible to the N2. Furthermore, a pore-size range from 1.5–7 nm for the hollow SiO2 spheres can be clearly distinguished from the pore-size distribution curve, as shown in Fig. 4b. The remarkably large surface area and porous structure of the SiO2 hollow spheres are derived by removal of the continuous carbon network of hollow SiO2/C spheres from the mesoporous shells. This hollow nanostructure has a large interior volume and a nanoscale shell, which efficiently transports electrons and Li ions inside the SiO2/C composite.
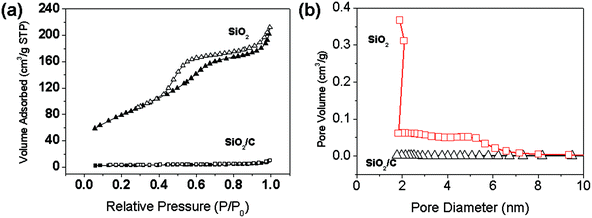 | ||
| Fig. 4 (a) N2 adsorption and desorption isotherms and (b) BJH pore size distribution curves of SiO2/C hollow spheres, and SiO2 hollow spheres. | ||
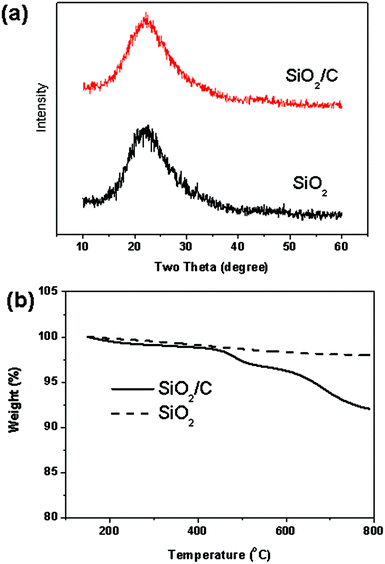
SiO2 has been reported to exhibit Li reactivity in the amorphous states, such as a hollow structure and a thin film. Herein, the crystallographic structure of the material was analyzed by XRD patterns, as shown in Fig. 5a. As can be seen, there is a weak broadening band between 20° and 25°, which means that both SiO2/C hollow spheres and SiO2 hollow spheres possess the amorphous structure. For carbon content measurements, the TGA curves of the SiO2/C and SiO2 composites are shown in Fig. 5b. In order to completely remove the carbon component, the SiO2/C and SiO2 composites were sintered under flowing oxygen within a temperature range of 50–800 °C. The weight changes in the SiO2/C and SiO2 composites were 8 and 2 wt%, respectively. Combining the weight loss from the decomposition of the amorphous silica upon heating in air, the weight loss suggests a carbon content of about 6 wt% for the SiO2/carbon sample. Furthermore, the peaks at 103.2 eV for Si 2p and 532.45 eV for O 1s in XPS spectra (Fig. S2†) mean that SiOx/C hollow spheres possess the SiO2 state. The above result corresponded with the Si and O molar ratio from EDX analysis in Fig. S1.†
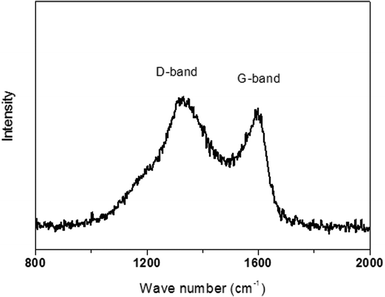
Electrode conductivity is a very important factor for electron transport. In order to analyze the structure of the resultant nano-composite, the SiO2/C hollow spheres were further investigated using Raman spectroscopy (Fig. 6) and conductivity measurements. The vibration band around 1580 cm−1 (G-band), ascribed to the interplane sp2 C–C stretching, is the characteristic feature of graphite carbon. Another vibration band around 1340 cm−1 (D-band) is due to the defects within the carbon. Thus, the presence of a strong G-band reflects a high graphitized degree in the hollow SiO2/C spheres. From the conductivity measurements, the significantly better conductivity of the SiO2/C composite (3.9 × 10−4 S cm−1) compared with the silica hollow spheres (<10−9 S cm−1) is derived from the continuous carbon framework.
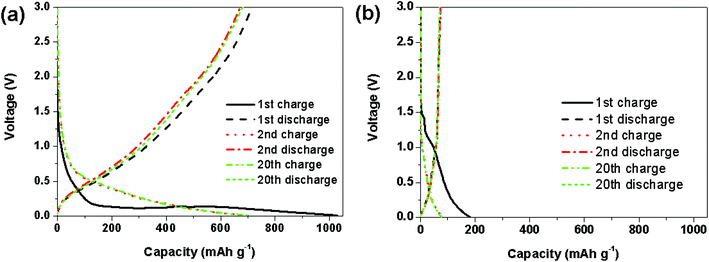
In the present study, we investigated, for the first time, the charge/discharge performance of the mesoporous SiO2/C hollow spheres as the anode material. Fig. 7a and b offer the charge/discharge voltage profiles of different cycles using the mesoporous SiO2/C hollow spheres and mesoporous SiO2 hollow spheres, respectively. For the SiO2/C hollow spheres, a plateau at 0.5 V can be observed only in the first charge voltage profile. The charge and discharge capacities of the 1st cycle are 1050 and 690 mA h g−1, respectively, with a low initial Coulombic efficiency of 66%; the charge capacity of the 2nd cycle is 700 mA h g−1. This irreversible capacity can be attributed to the formation of the solid-electrolyte-interface (SEI) layer and the irreversible electrochemical reactions between lithium ions and SiO2.19 In the following discharge/charge process, the voltage profiles show a similar shape and the electrochemical performance of the SiO2/C hollow spheres becomes stable. For comparison, the reversible capacities of the hollow SiO2 spheres are 61 mA h g−1, as shown in Fig. 7b. The reversible capacity of the SiO2/C spheres (624 mA h g−1) is over ten times higher than that of the SiO2 hollow spheres. Furthermore, the irreversible capacity of about 30% is smaller than the other silica material in the literature. This demonstrates the efficient electron transport capability into the SiO2/C composite through the carbon network, which is essential for improvement of the electrochemical performance.
Furthermore, the rate characteristics of the SiO2/C and SiO2 anodes are shown in Fig. 8a. Under low charge/discharge rate of 100 mA g−1, the specific capacity of the SiO2/carbon hollow spheres is 624 mA h g−1. More specifically, at the high discharge rate of 3000 mA g−1, the SiO2/C nanostructure exhibits a high retention of more than 90%, with a specific capacity of 582 mA h g−1. To our knowledge, this remarkable capacity retention is larger than the previous reports of SiO2-based anode material, such as nano-SiO2 in hard carbon, SiO2 film, carbon-coated SiO2 nanoparticles and milled quartz.7–10,25 Considering the SiO2 intrinsic properties of poor electron and lithium ion conduction, the significantly enhanced performance of the hollow SiO2/C composites at high charge/discharge currents is promising. The most notable feature of this SiO2/C nanocomposite is the high retention ability, even at 3000 mA g−1. For comparison, the physical properties and rate performance test of the carbon hollow sphere (Fig. S3†), after removal of SiO2, is added in the ESI.† Comparing with the SiO2/C anode, the carbon hollow sphere anode shows low rate performance with the 3 A g−1/0.1 A g−1 capacity retention of only 50%. Furthermore, the cyclic test of the SiO2/C hollow spheres and SiO2 hollow spheres at the discharge rate of 100 mA g−1 shown in Fig. 8b indicates that SiO2/C hollow spheres exhibited a very high and stable discharge capacity with almost no discernible capacity decay.
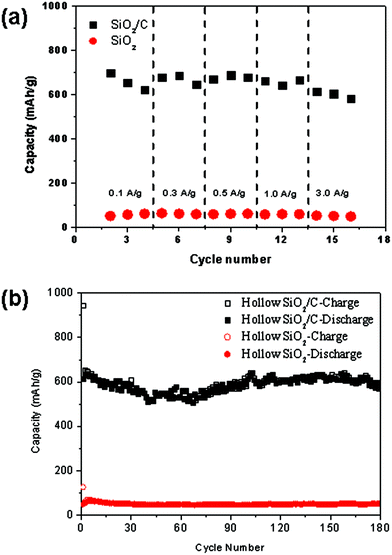 | ||
| Fig. 8 (a) Rate performance and (b) cycle life of SiO2/C hollow nanocomposites, and SiO2 hollow spheres. | ||
The interfacial stability between the electrodes and the electrolyte is an important factor of battery performance and impacts the cycling stability of the cells. The impedances of the SiO2/C and SiO2 hollow sphere cell before and after the cycling were measured by the electrochemical impedance spectrum method in the open circuit potential. All samples show typical EIS plots and can be fitted by the equivalent circuit, as shown in Fig. 9 and summarized in Table S1.† The intercept of the spectra with the real axis reflects the bulk resistance (Rb), and the difference of Rb after charge–discharge cycles for the SiO2/C and SiO2 hollow sphere cells is 0.9 and 1.8 Ω, respectively. The semicircle of the Nyquist plot at the high and middle-frequency regions is due to the charge transfer resistance between the electrolyte and active materials (Rct). Obviously, the SiO2/C cell contributed to a low charge transfer resistance of 54.9 Ω after cycling. In contrast, the SiO2 cell showed significant increase in interfacial resistance of 102.9 Ω after cyclic tests. The results demonstrate that our fabricated SiO2/C hollow structure exhibits high interfacial stability, which is a highly desirable property for electrode materials in LIBs.
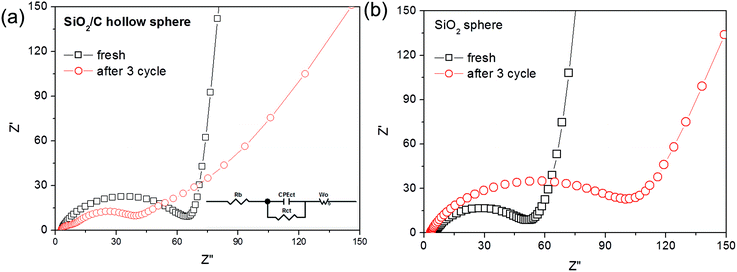 | ||
| Fig. 9 Nyquist plots of EIS spectra and the equivalent circuit of (a) SiO2/C and (b) SiO2 cells before cycling and at the 3rd cycle stage. | ||
4. Conclusions
In conclusion, a mesoporous SiO2/C hollow composite was synthesized and used as the anode material for LIBs. According to the TEM images, nitrogen adsorption/desorption isotherms, and silica removal results, the SiO2/C composites have a hollow morphology with a continuous silica and carbon framework. From the XRD results, all intense peaks were well indexed to the amorphous structure. From TGA analysis, the carbon content of the SiO2/C was about 6 wt%. Under all charge/discharge rates, the specific capacities of the SiO2/C core–shell composites are over ten times higher than that of the SiO2 material. More specifically, at the high discharge rate of 3000 mA g−1, the SiO2/C nanostructure exhibits a high retention of more than 90%, with a specific capacity of 582 mA h g−1. To our knowledge, this remarkable capacity retention is larger than the previous reports. This indicates that the efficient transport capability of Li ions into the SiO2/C shells of the hollow structure and the superior electronic conduction inside the continuous carbon network essentially intensify the electrochemical performance.Acknowledgements
The authors would like to thank the Ministry of Science and Technology, Taipei, R. O. C. for their generous financial support of this research.References
- D. Andre, S. J. Kim, P. Lamp, S. F. Lux, F. Maglia, O. Paschos and B. Stiaszny, Future Generations of Cathode Materials: An Automotive Industry Perspective, J. Mater. Chem. A, 2015, 3, 6709–6732 CAS.
- L. Croguennec and M. R. Palacin, Recent Achievements on Inorganic Electrode Materials for Lithium-Ion Batteries, J. Am. Chem. Soc., 2015, 137, 3140–3156 CrossRef CAS PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Nanomaterials for Rechargeable Lithium Batteries, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- M. Y. Ge, J. P. Rong, X. Fang and X. W. Zhang, Porous Doped Silicon Nanowires for Lithium Ion Battery Anode with Long Cycle Life, Nano Lett., 2012, 12, 2318–2323 CrossRef CAS PubMed.
- A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, High-Performance Lithium-Ion Anodes Using A Hierarchical Bottom-Up Approach, Nat. Mater., 2010, 9, 353–358 CrossRef CAS PubMed.
- N. Liu, H. Wu, M. T. McDowell, Y. Yao, C. Wang and Y. Cui, A Yolk-Shell Design for Stabilized and Scalable Li-Ion Battery Alloy Anodes, Nano Lett., 2012, 12, 3315–3321 CrossRef CAS PubMed.
- W. S. Chang, C. M. Park, J. H. Kim, Y. U. Kim, G. Jeong and H. J. Sohn, Quartz (SiO2): A New Energy Storage Anode Material for Li-Ion Batteries, Energy Environ. Sci., 2012, 5, 6895–6899 CAS.
- Q. Sun, B. Zhang and Z. W. Fu, Lithium Eectrochemistry of SiO2 Tin Film Electrode for Lithium-Ion Batteries, Appl. Surf. Sci., 2008, 254, 3774–3779 CrossRef CAS.
- N. Yan, F. Wang, H. Zhong, Y. Li, Y. Wang, L. Hu and Q. Chen, Hollow Porous SiO2 Nanocubes towards High-Performance Anodes for Lithium-Ion Batteries, Sci. Rep., 2013, 3, 1568 Search PubMed.
- Z. Favors, W. Wang, H. H. Bay, A. George and M. Ozken, Stable cycling of SiO2 Nanotubes as High-Performance Anodes for Lithium-ion Batteries, Sci. Rep., 2014, 4, 4605 Search PubMed.
- F. Wang, J. Wang, H. Ren, H. Tang, R. Yu and D. Wang, Multi-shelled LiMn2O4 hollow microspheres as superior cathode materials for lithium-ion batteries, Inorg. Chem. Front., 2016, 3, 365–369 RSC.
- H. Ren, J. Sun, R. Yu, M. Yang, L. Gu, P. Liu, H. Zhao, D. Kisailus and D. Wang, Controllable synthesis of mesostructures from TiO2 hollow to porous nanospheres with superior rate performance for lithium ion batteries, Chem. Sci., 2016, 7, 793–798 RSC.
- H. Ren, R. Yu, J. Wang, Q. Jin, M. Yang, D. Mao, D. Kisailus, H. Zhao and D. Wang, Multishelled TiO2 hollow microshperes as anodes with superior reversible capacity for lithium ion batteries, Nano Lett., 2014, 14, 6679–6684 CrossRef CAS PubMed.
- S. Xu, C. M. Hessel, H. Ren, R. Yu, Q. Jin, M. Yang, H. Zhao and D. Wang, α-Fe2O3 multi-shelled hollow microspheres for lithium batteries anodes with superior capacity and charge retention, Energy Environ. Sci., 2014, 7, 632–637 CAS.
- J. Wang, N. Yang, H. Tang, Z. Dong, Q. Jin, M. Yang, D. Kisailus, H. Zhao, Z. Tang and D. Wang, Accurate Control of Multishelled Co3O4 Hollow Microspheres as High-Performance Anode Materials in Lithium-Ion Batteries, Angew. Chem., Int. Ed., 2013, 52, 6417–6420 CrossRef CAS PubMed.
- M. H. Lee, J. Y. Kim and H. K. Song, A hollow sphere secondary structure of LiFePO4 nanoparticles, Chem. Commun., 2010, 46, 6795–6797 RSC.
- M. Sasidharan, D. Liu, N. Gunawardhana, M. Yoshio and K. Nakashima, Synthesis, Characterization and Application for Lithium-Ion Rechargeable Batteries of Hollow Silica Nanospheres, J. Mater. Chem., 2011, 21, 13881–13888 RSC.
- B. K. Guo, J. Shu, Z. X. Wang, H. Yang, L. H. Shi, Y. N. Liu and L. Q. Chen, Electrochemical Reduction of Nano-SiO2 in Hard Carbon as Anode Material for Lithium Ion Batteries, Electrochem. Commun., 2008, 10, 1876–1878 CrossRef CAS.
- Q. Chen and K. Sieradzki, Spontaneous Evolution of Bicontinuous Nanostructures in Dealloyed Li-Based Systems, Nat. Mater., 2013, 12, 1102–1106 CrossRef CAS PubMed.
- S. H. Wu, C. Y. Mou and H. P. Lin, Synthesis of Mesoporous Silica Nanoparticles, Chem. Soc. Rev., 2013, 42, 3862–3875 RSC.
- C. Y. Chang-Chien, C. H. Hsu, T. Y. Lee, C. W. Liu, S. H. Wu, H. P. Lin, C. Y. Tang and C. Y. Lin, Synthesis of Carbon and Silica Hollow Spheres with Mesoporous Shells using Polyethylene Oxide/Phenol Formaldehyde Polymer Blend. Eur, J. Inorg. Chem., 2007, 24, 3798–3804 Search PubMed.
- C. H. Hsu, J. Y. Jan, H. P. Lin and P. L. Kuo, Nitrogen-Doped Mesoporous Carbon Hollow Spheres as A Novel Carbon Support for Oxygen Reduction Reaction, New J. Chem., 2014, 38, 5521–5526 RSC.
- C. H. Hsu, H. Y. Liao and P. L. Kuo, High-Speed Lithium-Ion Transfer inside Mesoporous Core–Shell LiFePO4/Carbon-Sphere Cathodes, Energy Technol., 2014, 2, 409–413 CrossRef CAS.
- Y. C. Chen, W. T. Chiu, J. C. Chen, C. S. Chang, L. H. C. Wang, H. P. Lin and H. C. Chang, The Photothermal Effect of Silica–Carbon Hollow Sphere–Concanavalin A on Liver Cancer Cells, J. Mater. Chem. B, 2015, 3, 2447–2454 RSC.
- Y. Yao, J. J. Zhang, L. G. Xue, T. Huang and A. S. Yu, Carbon-coated SiO2 nanoparticles as an anode material for lithium ion batteries, J. Power Sources, 2011, 196, 10240–10243 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qi00125d |
| This journal is © the Partner Organisations 2016 |