Challenges and recent advances in MOF–polymer composite membranes for gas separation
Yuanyuan
Zhang
,
Xiao
Feng
,
Shuai
Yuan
,
Junwen
Zhou
and
Bo
Wang
*
Key Laboratory of Cluster Science, Ministry of Education of China, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, School of Chemistry, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: bowang@bit.edu.cn
First published on 24th March 2016
Abstract
Membrane technology has attracted tremendous attention in the field of gas separation due to its low cost and energy consumption. Polymer membranes are used in some industrial-scale gas separation processes, however, they often suffer a trade-off between permeability and selectivity. To overcome this limitation, porous materials with molecular sieve properties have been combined with polymers to give membranes with enhanced gas separation performance. Metal–organic frameworks (MOFs) are nanoporous materials possessing ultrahigh porosity, large surface area, structural diversity and rich functionalities, which make them promising candidates for gas separation. This review primarily focuses on the fabrication methods of MOF–polymer composite membranes including MOF-based mixed-matrix membranes (MMMs) and polymer supported MOF membranes. Recent progress in MOF membrane fabrication, incorporating the challenges and difficulties faced, are presented. Furthermore, corresponding solutions and strategies are given in detail to offer instructions to fabricate membranes with ideal morphology and performance.
1. Introduction
During the last two decades, membrane technology has played an increasingly crucial role in gas separation. Advantages including low cost, small footprint, and high efficiency energy as well as ease of processing and operation make it a competitive separation technology.1–3 So far, membrane-based gas separations have been applied in various industrial processes including H2/CO, H2/N2, and H2/CH4 separations, air separation, and natural gas sweetening.4 The processing and operation technology of polymer membranes is fairly mature in industry due to the advantages of high processability, economic feasibility, and low energy consumption.5 However, polymer membranes usually encounter a trade-off between permeability and selectivity (known as Robeson's upper bound), that is the improvement of permeability is often at the cost of selectivity.6,7 To overcome this obstacle, inorganic molecular sieve materials (i.e., zeolites, alumina, and ceramics) that can separate gas molecules via size exclusion mechanisms have been explored for their incorporation in polymeric materials. Among such porous inorganic materials, metal–organic frameworks (MOFs) have been evaluated as ideal candidates for membrane separation applications due to their well-defined and tunable pore size and pore environment.MOFs are nanoporous materials assembled using metal ions (or clusters) with organic linkers, combining the properties of both organic and inorganic components.8–11 Owing to their ultrahigh porosity, large surface area, well-defined open channels, structural diversity and rich functionalities, MOFs have developed applications in gas storage and separation,4,12–23 catalysis,24–26 sensing27–29 and many other fields. Compared with other inorganic fillers, MOFs are of interest for gas separation owing to the following characteristics: (1) a high degree of design: tremendous choice of linkers and metal ions can be employed to fabricate MOFs with diverse topologies, and the obtained MOFs can be further chemically modified through a post-synthetic modification (PSM) approach to tailor the pore sizes and inner surface properties; (2) enormous pores: MOFs possess a higher pore volume and lower density than conventional fillers, and thus they can have a larger impact on the hybrid membrane with the same mass loading.
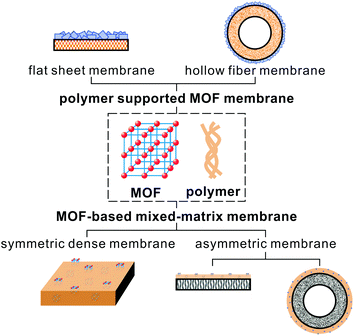
An increasing number of MOF-based membranes have been fabricated for gas separation, which can be divided into two types: continuous crystalline MOF thin films17,18,21–23,30–35 and mixed-matrix membranes (MMMs) with a MOF as filler.15,17–20,22 MOF thin films usually involve the deposition/growth of MOF crystals on a porous substrate. MOF-based mixed-matrix membranes consist of micro- or nano-particles of MOFs incorporated into a polymeric matrix. The merit of this strategy is the combination of processability of the polymers with the excellent gas separation capability of MOFs. Several excellent reviews have summarized this field, including both MOF thin films and MOF-based MMMs for gas separation.18,19,21,23,32 They place emphasis on the fabrication methods and the gas separation performance of the membranes. This review primarily focuses on the fabrication of MOF–polymer composite membranes, (1) MOF-based mixed-matrix membranes (MMMs): MOF particles incorporated into a polymeric matrix; (2) polymer supported MOF membranes: MOFs grown on a pre-formed polymer surface. Scheme 1 shows the different types of MOF–polymer composite membranes. Here, we present a summary of the major challenges and difficulties met during membrane fabrication and sum up the possible solutions and strategies based on reported studies (Fig. 1). We hope this review could offer meaningful and effective instructions to fabricate membranes with ideal morphology and performance, and inspire researchers with more strategies to tackle these challenges.
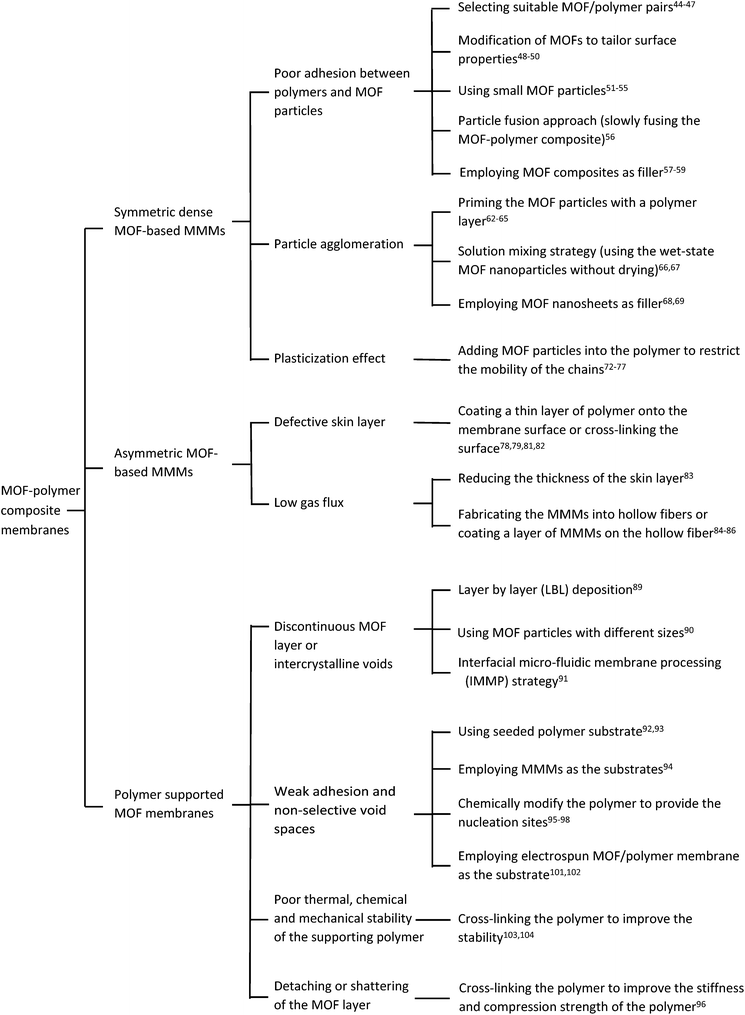
2. MOF-based mixed-matrix membranes
The incorporation of MOF particles into polymers can be seen as a promising way to surpass the trade-off between permeability and selectivity of polymer membranes,6,7 as well as overcoming the brittleness of inorganic films.36–38 The first report on the fabrication of MOF MMMs was in 2004.39 Yehia and coworkers incorporated a copper(II) biphenyl dicarboxylate–triethylenediamine MOF into a poly(3-acetoxyethylthiophene) (PEAT) matrix for gas separation, and the membrane exhibited improved CH4 selectivity compared to the pure polymer membrane. Since then, many MOF MMMs with attractive performances have been reported.15–20,22MOF MMMs can be classified into symmetric dense membranes and asymmetric membranes according to their morphology. Symmetric dense MOF-based MMMs are polymer membranes with dispersed MOF particles. Asymmetric membranes consist of a thin layer of polymer with MOF fillers supported on a porous support. In the following sections, we will discuss the challenges and corresponding solutions to improve the gas separation performance in these two types of MOF MMMs.
2.1. Symmetric dense MOF-based MMMs
Various polymers and MOFs have been employed to fabricate symmetric dense MOF MMMs, and the general approach for fabrication is a film-casting method (solvent-casting method). In a typical procedure, MOF particles are first dispersed in a solvent by stirring and/or sonication and then the polymer powders are added to form a homogeneously dispersed MOF/polymer solution. The polymer-MOF solution is casted on a flat surface usually with the help of a doctor blade system and the solvent is left to evaporate, followed by heating under vacuum to fully remove the solvent. Spin coating has also been applied as an optional way of casting to produce homogeneous MMMs.40Some reviews have described the non-ideal morphology of MMMs and their cause and the influence on the gas separation properties.41–43 Of these, the two main problems involved in fabricating MOF-based MMMs are: (1) the poor interaction between the MOF particles and polymer matrix resulting in non-selective defects; and (2) the agglomeration of the particles within the polymer that decreases the selectivity properties of the membranes. Another issue to be solved is the plasticization effect of the polymer under high pressure. Based on the above-mentioned challenges, researchers have proposed many strategies to modify the fabrication approach so as to address these issues and enhance the gas separation performance.
Selecting a suitable MOF/polymer pairing can overcome the poor compatibility between the filler and polymer to some extent. For example, non-covalent interactions, such as hydrogen bonding between the pendent groups anchored to the MOF and the side groups/chains on the polymer backbone, have been utilized to enhance the interface compatibility. Zornoza et al. reported a polysulfone (PSF) membrane containing flexible NH2-MIL-53(Al), and the hydrogen bonding between them allowed a high MOF loading of 25%.44 Cao and coworkers fabricated a compact MMM comprising an NH2-functionalized MOF and poly(methyl methacrylate) (PMMA). The obtained membrane exhibits a much higher permeability of H2 than the pure PMMA membrane.45 Tien-Binh and colleagues chose a polymer grafted with hydroxyl groups as the matrix with amine-functionalized MOFs as fillers. The hydrogen bonding between the amine groups and hydroxyl groups enhanced the interface interaction as expected.46
To further enhance the adhesion, Lin and colleagues chose the [Cd2L(H2O)]2·5H2O (Cd-6F) MOF with 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA) as the linker, which is also the moiety of the polymer matrix 4,4′-(hexafluoroisopropylidene)diphthalic anhydride-4,4′-oxydianiline (6FDA-ODA polyimide).47 Through in situ polymerization, Cd-6F could react with ODA from the polyimide (PI) chains forming –NH–CO– groups, thus achieving covalent bonds and eliminating interfacial voids (Fig. 2). Both the gas permeability and CO2 selectivity of the resulting membrane are much higher than those without interfacial interactions.
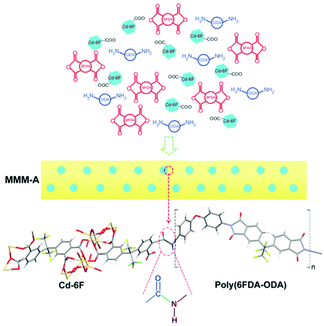 | ||
| Fig. 2 Diagram of the designed interaction between Cd-6F and 6FDA-ODA in MMM-A. Reprinted with permission.47 Copyright 2014 American Chemical Society. | ||
Utilization of a modification strategy to tailor the surface properties of MOFs is a more generally applicable way to enhance the adhesion between MOF particles and polymers.48,49 Venna et al. modified and optimized the surfaces of UiO-66-NH2 with different functional groups, such as phenyl acetyl groups, decanoyl acetyl groups, and succinic acid groups, to control the interaction with the Matrimid® polymer.49 The membrane containing MOFs modified with phenyl acetyl groups exhibited an increase of ∼200% in the permeability of CO2 and ∼25% in the ideal selectivity of CO2/N2 compared to the neat polymer membrane. This can be explained by the enhanced interactions and defect-free interface between the modified MOF and polymer, namely, the π–π stacking of the aromatic groups from the particles and the polymer chains and the hydrogen bonding between the imide and amide groups.
As demonstrated by Xin and coworkers, MOFs can also be decorated with polymers to enhance the interfacial adhesion.50 MIL-101 (Cr) was decorated with polyethylenimine (PEI) using a vacuum assisted method that facilitates the penetration of polymer chains into the cavity. The PEI-decorated MOF was incorporated into sulfonated poly(ether ether ketone) (SPEEK). Considering the reversible reaction between CO2 and amine groups, the amine groups in PEI are expected to help the transport of CO2. Due to the enhanced interfacial interaction as well as the assistance of the amine groups, membranes with PEI@MIL-101 (Cr) as filler showed an improvement of 128% and 102% in ideal selectivity for CO2/CH4 and CO2/N2, respectively, compared with the pure polymer membrane.
Another method attempted to achieve a defect-free interface is by employing smaller MOF particles.51–55 Smaller particles could offer a larger polymer/filler interfacial area and help fabricate thinner membranes. Reducing the particle size can be achieved either using a modified synthetic method or through post-treatment of the particles. Bae et al. diminished the size of ZIF-90 using a nonsolvent-induced crystallization strategy, and the nano-sized ZIF-90 particles were used to fabricate membranes with three kinds of PI (Ultem, Matrimid®, and (2,2-bis(3,4-carboxyphenyl) hexafluoropropane dianhydride–diaminomesitylene) (6FDA-DAM)).51 The ZIF-90/6FDA-DAM membrane showed a CO2 permeability reaching 720 Barrer that is 84% higher than the 6FDA-DAM membrane, and had a good CO2/CH4 mixed-gas selectivity of 37 at 25 °C, which transcends the upper bound for the polymer performance demonstrated in 1991. Ge et al. adopted a sonication treatment to reduce the size of Cu-BTC MOF and successfully fabricated Cu-BTC/PPO (poly(2,6-dimethyl-1,4-phenylene oxide)) composite membranes without noticeable interfacial defects. Compared with the membrane containing pristine Cu-BTC, increased selectivities of CO2 and H2 over N2 and CH4 were shown with size-reduced particles.52
A novel fusion method was developed by Shahid et al. to promote the interaction between the polymer matrix and MOF filler as well as to solve the distribution problem of the particles.56 Matrimid®5218 solution was first injected into water and formed spheres through solvent exchange and the obtained polymer particles were functionalized with imidazole groups and used for MMM fabrication. Then the ZIF-8 precursor solution was added to the polymer particle suspension leading to growth of the ZIF-8 in this system. Finally, the mixture was cast onto the substrate and subsequently left in a DMF vapour environment to slowly fuse the ZIF-8-surrounded polymer to form a defect-free MMM. The SEM image of the obtained membrane shows a good interfacial contact between the polymers and ZIF-8 nanocrystals; on the contrary, the membrane prepared using a conventional solution casting method presents a compatibility problem (Fig. 3). The membrane prepared with a fusion method shows a 100% improvement in CO2 permeability and a 65% increase in selectivity for CO2/CH4 compared to the Matrimid membrane. The in situ growth of MOF crystals in the polymer matrix and the slow fusion process of the polymer ensure a good contact between the filler and polymer, which reduces the possibility of gas molecules penetrating through defects and improves the selectivity.
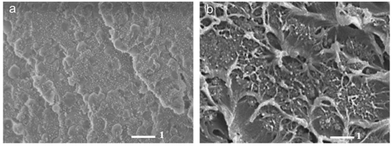 | ||
Fig. 3 MMM containing 30 wt% ZIF-8 prepared using (a) particle fusion and (b) conventional solution casting. (Magnification: (a): 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and (b): 20 000 and (b): 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Reprinted with permission.56 Copyright 2015 Elsevier B.V. 000). Reprinted with permission.56 Copyright 2015 Elsevier B.V. | ||
The approach to employ MOF composites or combine MOFs with other porous fillers to prepare MMMs also helps to achieve a better filler/polymer compatibility. Zhu and coworkers incorporated a novel carbon nanotube (CNT)/NH2-MIL-101(Al) composite into 6FDA-durene polyimide which showed good CO2 separation.57 The CNTs were first modified with carboxylic groups to provide nucleation sites, and then the in situ growth of NH2-MIL-101(Al) on the CNT-COOH was conducted using a solvothermal process. The prepared membrane shows a high permeability for CO2 and a good CO2/CH4 selectivity owing to the good adhesion and the functional groups brought by the filler, and is close to the Robeson upper bound (2008). Zornoza et al. used two kinds of filler (i.e. MOFs and zeolites) in the PSF taking advantage of the synergic effects of fillers with different properties, which may facilitate the dispersion and enhance gas separation performance.58 Later, the same group employed silica-(ZIF-8) core–shell spheres as the filler for PSF MMMs.59 First, mesoporous silica spheres (MSSs) were prepared and then the in situ growth of ZIF-8 was carried out on the MSSs to form a shell. The spherical composite MSS-Z8 was well dispersed in the polymer matrix with a good interaction at the interface. Due to the mesopores of the silica core and the micropores of the ZIF-8 shell, the MSS-Z8 membrane with 32 wt% loading exhibited an improvement of 300% in permeability for CO2 and a slight increase in selectivity for CO2/CH4 compared with pure PSF. Besides improving the interfacial interaction, preparation of novel MOF composites also offers a good way of tailoring the properties of fillers, i.e. introduction of functional groups and hierarchical pores, which favours the enhancement in selectivity.
The most common and useful way to prevent aggregation is a “priming” technique.60,61 This method involves adding a small portion of polymer into the MOF particle dispersion at first, which could form a thin layer of polymer on the external surface of the particles so as to avoid agglomeration. Then the residual polymer powder is added and dissolved to prepare casting solutions. This strategy has been applied to many MOF MMM fabrication procedures.62–65 Ordoñez et al. prepared a series of ZIF-8/Matrimid® membranes with different MOF loadings (up to 80%) using the priming technique for gas separation.62 The permeability of all gases tested increased with filler loading. However, at high loadings of 50% and 60%, the permeability decreased and the selectivity increased, which can be explained by the molecular sieving role of the MOF particles. The results suggested that a higher loading of MOF particles is a prerequisite to achieve a molecular sieving effect.
A solution mixing strategy was reported by Song et al. to control the aggregation of particles.66 ZIF-8 nanocrystals were synthesized and the wet-state nanoparticles, without drying, were then directly incorporated into Matrimid®5218 solution to fabricate composite membranes. On evaporation, the polymer solution was tuned to a suitable concentration to minimize aggregation and sedimentation of the ZIF-8 particles. The ZIF-8 was well dispersed within the polymer matrix with fine adhesion at high loadings. A similar method has also been used to prepare ZIF-90-PBI (polybenzimidazole) composite membranes.67
Stand-alone MOF nanosheets can also be embedded into a polymer matrix with uniform occupation as demonstrated by Rodenas et al.68 As shown in Fig. 4, analysis of the FIB–SEM (tomographic focused ion beam scanning electron microscopy) tomograms indicates that the CuBDC nanosheets evenly occupy the cross-section of the PI membranes. Therefore, they expose a much larger surface area than bulk-type crystals, which enhances the molecule sieving efficiency, and contributes to the outstanding CO2/CH4 separation properties. At different transmembrane pressures, the selectivity for CO2/CH4 of the nanosheet CuBDC incorporated PI membrane is 20–80% higher than the native polymer membrane. Kang and coworkers also studied the influence of the filler structure on the gas separation performance.69 By employing the bulk crystal (BC), nanocubes (NC), and nanosheets (NS) of the MOF [Cu2(ndc)2(dabco)]n as fillers, the best performance is shown in the membranes with the nanosheets. The partially stacked state of the lamellar NS MOF fillers can enhance the sieving efficiency and reduce the nonselective gas permeating pathways, which proved to be beneficial to improve the selectivity.
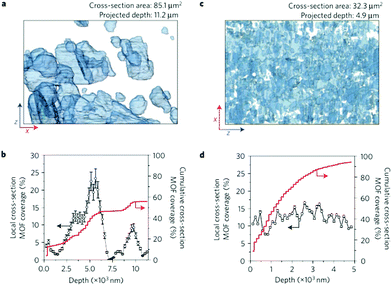 | ||
| Fig. 4 Image analysis of FIB–SEM tomograms for MOF–polymer composite membranes. (a, c) Full projections along the y direction of the reconstructed volumes for composite membranes containing bulk crystals (a) and nanosheets (c) of CuBDC MOF embedded in polyimide. The MOF particles are depicted as partially transparent to better perceive overlaps in the direction of the projection. (b, d) Evolution of the coverage of the membrane xz cross-section by the MOF particles for composite membranes containing bulk (b) and nanosheets (d) of CuBDC MOF embedded in polyimide. Error bars in b,d correspond to the standard deviation (%). Adapted and reprinted with permission.68 Copyright 2014 Nature Publishing Group. | ||
Poor interfacial compatibility usually leads to the formation of voids between the MOF particles and polymer, whereas particle agglomeration always results in defects between the fillers. However, in many cases, these two issues are closely related. Improving the adhesion between the MOF particles and polymer can also facilitate the dispersion to some extent, and vice versa. For example, the most commonly employed technique, “priming”, can control the dispersion of the particles by first coating a thin layer of polymer around the particles, which is beneficial for increasing the interaction between them.
Shahid and coworkers observed that upon the incorporation of MOF particles in the polymer membrane, the selectivity of the resulting MMM remained relatively high under high pressure.72 They deduced that the addition of MOF particles into the polymer restricts the mobility of the chains thus weakening the plasticization effect. Others also observed a similar phenomenon.73–77
2.2. Asymmetric MOF-based MMMs
Another type of MMM is an asymmetric membrane comprising a thin layer of polymer with MOF filler and a porous support. In most cases, the skin layer acts as a selective function, while the substrate plays the role of mechanical support. Compared with symmetric membranes, asymmetric membranes offer a lower gas resistance and higher particles loadings in the top layer. According to the geometry of the substrates, asymmetric MMMs can be sorted into flat sheet membranes and hollow fiber membranes (HFM).Basu et al. prepared asymmetric Matrimid®/Cu(BTC)3 and Matrimid®/PSF/Cu(BTC)3 MMMs supported on non-woven substrates via phase inversion.78 To further seal the surface defects, a thin layer of polydimethylsiloxane (PDMS) was deposited using spinning coating. The synthesized membrane shows enhanced CO2/CH4 and CO2/N2 selectivity and permeance with increasing filler loading. Later, this group incorporated three different MOFs into Matrimid® to fabricate both dense and asymmetric membranes with an improved thermal and mechanical performance.79 The resulting asymmetric membranes exhibited higher permeance than the dense membrane while maintaining the selectivity.
In order to improve the selectivity, cross-linked MMMs have been explored for gas separation. Shao and colleagues observed a high H2/CO2 selectivity of 102 in 6FDA-durene membranes, whose surface was cross-linked with ethylenediamine (EDA) vapor.80 To further make up for the loss in permeability, Wijenayake et al. embedded ZIF-8 into the matrix followed by surface cross-linking resulting in a 10-fold increase in the selectivities of H2/CO2, H2/N2, and H2/CH4 with a 45% reduction in H2 permeability compared to 6FDA-durene, which is close to the 2008 Robeson upper bound.81 The same group modified their method by spin coating a selective and cross-linkable polymer layer above the ZIF-8/6FDA-durene MMMs, followed by EDA cross-linking.82 The selective cross-linked layer contributes to the improved selectivity of the membrane for gas separation; however, this reduces the permeability of the MMMs at the same time. This cross-linking method is also an effective way to improve the mechanical, thermal and chemical stability of the membrane as we discuss in the sections 3.3 and 3.4.
A special morphology of asymmetric membranes is hollow fibers, which are a promising material for industrial gas separation with a large area per volume, good performance and low cost. In addition, hollow fiber membranes (HFM) can be easily assembled into a membrane module with high packing density for high production.
Hu et al. fabricated, for the first time, MOF–polymer MMM hollow fibers using a dry/wet-spinning method.84 Cu3(BTC)2/poly(amic acid) solution with a bore fluid solution (water) was extruded from the spinneret into a water quenching bath to form the fiber structure. The fiber was then heated to finish the imidation process. As can be seen in the SEM images (Fig. 5), a dense layer was produced at the outer and inner surfaces, which contributes to the high selectivity. The composite PI/Cu3(BTC)2 hollow fibers had a H2 permeance of 1266 GPU (1.5 times that of the pure PI) and selectivities for H2/CH4, H2/N2, H2/CO2, and H2/O2 of 240, 163, 28, and 42 (twice of the pure polymer), respectively. Dai and coworkers also embedded ZIF-8 nanoparticles into Ultem®1000 polymer and the obtained dual-layer fibers were post-treated with PDMS to seal the defects in the skin layer.85 Improvements in the permeance of CO2 and selectivity for CO2/N2 were observed in the hybrid membrane, being 85% and 20% higher than the pristine polymer membrane, respectively.
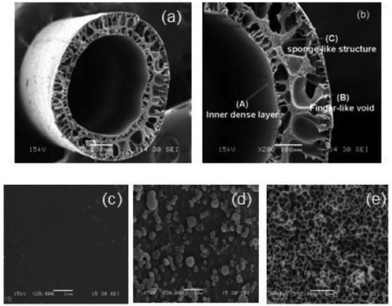 | ||
| Fig. 5 SEM images of hollow-fiber PI–Cu3(BTC)2 MMMs: (a, b) cross section, (c) outer surface, (d) surface of finger-like void, and (e) sponge-like structure. Reprinted with permission.84 Copyright 2010 American Chemical Society. | ||
MMMs can also be used as a layer coated on hollow fiber membranes to heighten the gas separation performance. Zulhairun and coworkers coated PSF asymmetric hollow fiber membranes with a PDMS/Cu3(BTC)2 layer via dip-coating.86 On the one hand, the coating can seal the imperfections on the surface and improve the selectivity. On the other hand, it also provides protection to the polymer membranes during testing.
To sum up, as micro- or meso-porous materials with open channels and narrow pore size distributions, MOFs can indeed increase the selectivity and permeability of polymer membranes through the synthesis of MMMs. To further improve the gas separation performance of MMMs, enhancing the MOF loading amount is highly desirable. However, it will inevitably lead to the loss of its mechanical property to form a stand-alone membrane. Therefore, the amount of MOF and polymer in the MMM should be balanced, and problems that are related to the voids between the particles or at the interfaces need to be considered. Although the above-mentioned methods can overcome partial obstacles, time-consuming processes, large cost and unsatisfactory performance still impede the development of MOF-based MMMs for real applications. Furthermore, polymer chains may clog the pores of MOFs so that the molecular sieving function of the MOFs cannot be fully utilized in these MMMs. Utilizing macroporous substrates as supports and MOF membranes as sieving layers will offer an alternative methodology.
3. Polymer supported MOF membranes
Various substrates have been used to fabricate supported MOF membranes. Inorganic supports like alumina, silica, zeolites, and titania have been widely explored for MOF growth; however, the brittleness and high cost of these materials hinder their further application. Given the scalability, low cost and well-known processing technique of polymers, it is desirable to fabricate MOF membranes supported on polymeric materials. Furthermore, MOF particles are expected to interact more easily with polymer substrates due to their hybrid nature. Based on the structure of the polymer employed, these membranes are generally divided into flat sheet membranes and hollow fiber membranes (HFM). A general method for the preparation of polymer supported MOF membranes is to first anchor the MOF seeds onto the polymer substrate, followed by growing MOF particles to form thin films. Other methods have been also investigated and will be discussed below.Yao and coworkers prepared a MOF film supported on a flexible polymer substrate through a contra-diffusion method for the first time.87 A porous nylon membrane was used as the substrate and placed in the middle of a homemade diffusion cell to separate the metal and ligand solutions (Fig. 6). In this process, Zn2+ and Hmim (2-methylimidazolate) diffuse through the pores of the support and crystallize on both sides, thus forming layers of ZIF-8. By changing the concentrations of the metal and ligand solutions and the reaction time, different sizes of crystal and thickness of the MOF layer can be obtained. The membranes were tested in gas permeation experiments and exhibited high H2 permeance with modest H2/N2 ideal selectivities that were slightly higher than the Knudsen diffusion selectivity.
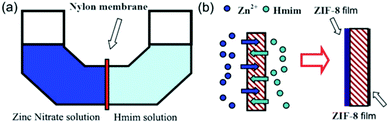 | ||
| Fig. 6 (a) Diffusion cell for ZIF-8 film preparation and (b) the schematic formation of ZIF-8 films on both sides of the nylon support via contra-diffusion of Zn2+ and Hmim through the pores of the nylon support. Reprinted with permission.87 Copyright 2011 Royal Society of Chemistry. | ||
Mao and colleagues reported a pressure-assisted method possessing the potential for the scale-up fabrication of MOF/polymer HFMs88 (Fig. 7). Copper hydroxide nanostrands (CHNs) were filtered on PVDF (polyvinylidene fluoride) hollow fibers under pressure and acted as a copper source for HKUST-1 synthesis. Then the filtered PVDF hollow fiber with the CHN layer was placed in a H3BTC solution for reaction under room temperature with the pump on. A continuous HKUST-1 layer was grown and firmly adhered on the PVDF surface, and the obtained membranes were evaluated for gas separation tests with both inside-out and outside-in modes. The pressure-assisted method proved to be a promising way to achieve the scale-up preparation of MOF/polymer HFMs.
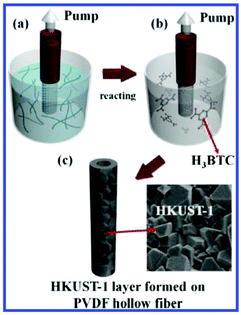 | ||
Fig. 7 Preparation process of HKUST-1 membrane on the surface of PVDF hollow fibers. (a) Filtering CHNs on PVDF hollow fibers. (b) After filtering for 30 min, reaction with 20 mL of a 20 mM H3BTC ethanol/water solution with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at room temperature, and (c) after a certain time, HKUST-1 layers were formed on the PVDF hollow fiber support. A vacuum pressure of 90 kPa was maintained during the whole process. Reprinted with permission.88 Copyright 2014 American Chemical Society. 1 at room temperature, and (c) after a certain time, HKUST-1 layers were formed on the PVDF hollow fiber support. A vacuum pressure of 90 kPa was maintained during the whole process. Reprinted with permission.88 Copyright 2014 American Chemical Society. | ||
To fabricate supported membranes with ideal morphology and excellent gas separation capability, there are still many difficulties ahead, including (1) imperfect coverage of MOF particles on the substrate or a discontinuous MOF layer that will weaken the selectivity; (2) weak adhesion between the polymer substrate and MOF film that will lead to the formation of nonselective voids; (3) detaching or shattering of the MOF layer from the support because of the difference in mechanical properties; and (4) poor thermal, chemical and mechanical stability of the polymer must be enhanced to give a good, stable performance.
3.1. Discontinuous MOF layer or intercrystalline voids
In any case, an inter-grown and continuous selective layer without cracks is a precondition for outstanding and stable gas separation. To achieve this aim, researchers are trying to fabricate membranes with high MOF loadings and complete coverage of the MOF layer.Nagaraju et al. synthesized ZIF-8 and CuBTC on asymmetric PSF ultrafiltration membranes through in situ crystallization followed by layer by layer (LBL) deposition.89 The substrate was first dipped in a BTC solution and a Cu(NO3)2 solution separately for 12 h, and then washed with water and dried. This procedure was repeated for 3 cycles for the in situ growth of MOF seeds. Then, the LBL deposition was carried out by immersing the membrane in BTC solution, followed by suspension in a solution containing both BTC and Cu(NO3)2. To increase the crystal coverage on the support and fabricate a dense MOF layer, the LBL process was repeated 3 times. Composite membranes with different MOFs showed decreased permeance and better selectivity for H2/CO2 and H2/C3H6 than the PSF membrane due to the selective function of the MOF layer. For example, the selectivity of CuBTC@PSF for H2/CO2 and H2/C3H6 increased by 132% and 140%, respectively, compared with the pristine PSF membrane.
The strategy of using MOF particles with different sizes through in situ crystallization to eliminate defects and grow a compact layer of MOF was demonstrated by Cacho-Bailo and cowokers.90 By varying the molar ration of the metal ion and ligand in the solution, a continuous and thick ZIF-8 layer with both nano- and micro-sized particles was grown on the porous PSF substrate. The high selectivities for H2/CH4 (10.5 ± 0.6) and H2/N2 (12.4 ± 0.9) reveal the molecular sieving effect is achieved by the ZIF-8 layers.
Brown and coworkers developed an interfacial micro-fluidic membrane processing (IMMP) strategy to grow continuous ZIF-8 membranes on the inner surfaces of poly(amide–imide) (Torlon) hollow fibers.91 As shown in Fig. 8, zinc nitrate was dissolved in a mixed organic solvent with low concentration, and the solution could be flowed through the bore of the fiber at different rates. Hmim was dissolved in water at a high concentration, and the solution was placed in the chamber around the fiber shell. By controlling the flow rate of the bore solution, ZIF-8 membranes with different structures could be obtained. A combination of the flowing and static states of the bore solution proved to be the best conditions to give an inter-grown and continuous ZIF-8 membrane. The key points of the methods include the in situ growth of ZIF-8, the solvent interface and controlled microfluidic conditions. Then a lumen capping step was applied by infiltrating PDMS into the fiber, and high separation factors for H2/C3H8 and C3H6/C3H8 of 370 at 120 °C and 12 at 25 °C, respectively, were achieved by the PDMS-sealed fiber.
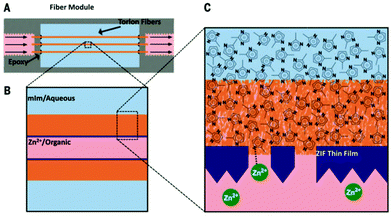 | ||
| Fig. 8 Scheme depicting the interfacial microfluidic membrane processing (IMMP) approach for MOF membranes in hollow fibers. (A) Side view of a series of fibers mounted in the IMMP reactor. (B) The Zn2+ ions are supplied in a 1-octanol solution (light red) flowing through the bore of the fiber, whereas the methylimidazole linkers are supplied on the outer (shell) side of the fiber in an aqueous solution (light blue). (C) Magnified view of the fiber support during synthesis. In this example, the membrane forms on the inner surface of the fiber by reaction of the two precursors to form a polycrystalline ZIF-8 layer (dark blue). Reprinted with permission.91 Copyright 2014 The American Association for the Advancement of Science. | ||
From a macroscopic perspective, the above-mentioned strategies can prevent the formation of a discontinuous MOF layer, however, invisible intercrystalline defects are hard to avoid. One of the merits of polymer supported MOF films is the higher flux compared with MOF MMMs, but intercrystalline nonselective voids inevitably decrease the selectivity for gas separation. One possible solution is to utilize MOF sheets of nanometer thickness to prepare ultrathin molecular sieve membranes without ordered lamellar stacking of the nanosheets, which may suppress the formation of intercrystalline voids and increase both the permeability and selectivity.
3.2. Weak adhesion and non-selective void spaces
The adhesion between the top layer and support determines the integrity of the whole membrane and the separation selectivity. Weak adhesion could cause the formation of a non-selective transport path for gas molecules, resulting in degradation of the separation ability. To strengthen the adhesion between the selective top layer and the porous substrate, Ge et al. fabricated a ZIF-8 film on a seeded polyethersulfone (PES) substrate.92 The porous PES with a side pore size similar to ZIF-8 nanocrystals was first rubbed with dry ZIF-8 seeds, and then immersed in a precursor solution to grow ZIF-8 layers through a hydrothermal method. The PES-supported ZIF-8 membranes were tested for gas separation and showed a H2 permeance of 4 × 10−7 mol m−2 s−1 Pa−1 at 333 K. Compared to the membrane prepared from seedless growth, the seeded membrane had a higher selectivity for H2/N2 due to the elimination of the cracks between the MOF layer and substrate.Brown et al. also synthesized dense ZIF membranes on polymeric hollow fibers by employing a dip-coating technique.93 Nano-sized ZIF-90 particles were first synthesized, and Torlon fibers were seeded by dipping into a suspension of ZIF-90. The second growth of the ZIF-90 nanocrystals on the fiber was conducted in a test tube at low temperature and the whole process is technologically scalable. The gas permeation test proceeded at 35 and 70 °C, and the membrane presented comparable results due to the complete coverage of the polycrystalline ZIF-90 without prominent defects.
Li et al. employed MMMs as seeded substrates to fabricate a trinity of MOF membranes.94 A PSF hollow fiber substrate was first coated with a PDMS/Cu3(BTC)2 layer to increase the adhesion of the MOF layer, and subsequently immersed in a Cu3(BTC)2 mother solution to form a continuous MOF layer. The integrity of the trinity membranes proved to have excellent gas separation capabilities for H2/N2 and N2/CO2. The MMM substrates offer nucleation sites for the growth of MOF nanocrystals and a selective layer that makes up for the loss in selectivity caused by the intercrystalline voids in the MOF layer. The results suggest that the combination of MMMs and a dense MOF layer contributes a lot to the excellent selectivity.
Another way to improve the integrity of the entire membrane and avoid cracks is to chemically modify the polymer, and the modification method includes a solution phase and a vapor phase modification. Centrone et al. successfully grew MIL-47 on polyacrylonitrile (PAN) substrates using microwave irradiation.95 The nitrile group of PAN is converted to carboxylic acid during the process to provide nucleation sites and proved to be a prerequisite for growth. A similar strategy has also been used to fabricate ZIF-8-PAN hollow fiber membranes.96 The polymer can also be coated on various substrates and then modified for the growth of MOF crystals. Ben and colleagues spin coated poly(methyl methacrylate) (PMMA) on substrates such as Si wafers, centrifuge tubes, toys and stainless steel nets, and then converted the PMMA into poly(methacrylic acid) (PMAA) using concentrated sulfuric acid.97 The carboxylic groups on the PMAA layer act as heterogeneous nucleation sites for the formation of the HKUST-1 layer. This method proved to be a general and convenient way to grow MOF membranes on many other materials. The sample with the stainless steel net as the substrate was tested for gas separation and showed high stability.
Considering that modification in the solution phase may cause swelling of the polymer and need further processing to remove the chemical modifier, Shamsaei et al. proposed using EDA vapour to modify the polymer membrane of bromomethylated poly(2,6-dimethyl-1,4-phenylene oxide) (BPPO), promoting the formation and adhesion of ZIF-8.98 This vapor modification method is surface-limiting and proceeds under mild conditions, and does not cause swelling or affect the structure of the polymer. The modified substrate was then immersed in a ZIF-8 precursor solution under room temperature. A continuous and thin layer of ZIF-8 without any visible defects can be observed on top of the support, which contributes to the high permeance with high selectivity for H2 of the composite membrane.
Fibrous membranes are considered as promising materials for gas separation due to the high surface to volume ratio and porosity. Electrospinning is a simple and widely employed method to fabricate fibers with various compositions and tunable morphologies. Ostermann and Rose first reported the preparation of MOF–polymer composite fibers using an electrospinning technique.99,100 Wu et al. employed MOF/polymer electrospun fiber mats as skeletons for the further growth of MOF crystals to fabricate free-standing membranes.101 Specifically, a HKUST-1/PS solution was first electrospun into nanofibers and collected to form a mat of certain thickness, and the HKUST-1 precursor sol was dripped slowly onto the mats. Then the membrane with precursor sol was heated to help the growth of the MOF crystals. A ZIF-8 membrane fabricated in the same way was used for the separation of N2/CO2, and the results proved that the membrane is highly permeant due to the voids between the disordered fibers. Similarly, Fan and colleagues produced supported MOF membranes by depositing electrospun fibers on macroporous SiO2 wafers and porous Al2O3 tubes, followed by secondary growth of the crystals.102 As indicated by the gas separation tests, the synthesized ZIF-8 membranes could act as molecular sieves.
3.3. Poor thermal, chemical and mechanical stability of the supporting polymer
Problems including poor thermal stability and chemical resistance of the polymers remain to be addressed for their further application. On the one hand, MOFs synthesized under harsh conditions such as high temperatures and using organic solvents are usually difficult to grow on polymer surfaces. On the other hand, the obtained composite membranes may not show stable performance due to the instability of the polymer substrates especially under harsh test conditions. Besides the careful selection of the polymer, cross-linking has emerged as an effective way to enhance the thermal and chemical stability of polymers.103,104 Li et al. cross-linked PVDF hollow fibers using amines that also provided nucleation sites at the same time.103 The modified PVDF is highly stable even in DMF under high temperature, and was successfully used as a substrate to grow ZIF-7 layers. Since ZIF-7 possesses a smaller aperture (0.3 nm), a high permeance of H2 (13.29 × 10−7 mol s−1 m−2 Pa−1) is achieved by the ZIF-7/PVDF composite membrane with a superior ideal selectivity for H2/N2 (18.30) and H2/CO2 (16.32).3.4. Detaching or shattering of the MOF layer
Polymer substrates tend to be much more flexible than the MOF layers; therefore, there is a possibility that the stiff crystalline MOF layer may be shattered or detached from the substrate. Furthermore, polymers may swell or shrink under some conditions, which will also lead to the same problem. As reported by Li and coworkers, PAN was heated to facilitate crosslinking between the nitrile groups, thus enhancing the stiffness and compression strength as well as the chemical and mechanical stability of the polymer.96 A stiff PAN hollow fiber membrane with heterogeneous nucleation sites was used for the growth of the ZIF-8 layer and the obtained membrane achieved a high H2 permeance with a good separation capability for N2/H2.In summary, compared with MMMs, polymer supported MOF membranes usually possess a higher permeability for gas molecules by taking full advantage of the open channels of MOFs. There are still some problems hindering the advance of this field, for example, (1) defects or intercrystalline voids in the MOF layer decrease the selectivity; (2) voids between the MOF layer and polymer support layer resulting from low adhesion also lead to a low separation performance; and (3) poor mechanical properties and thermo- or chemo-stabilities of the polymer layer bring limitations for the choice of MOF. Many efforts have been made to address these problems and offer promising hope for the future.
4. Conclusion and outlook
Membrane-based gas separation stands out from other separation techniques with its low cost of operating and processing, ease of operation and energy saving with small footprint. MOFs possess a wide of range of pore sizes and shapes, surface properties and high degree of control over pore functionality at the molecular level, thus presenting a new class of highly attractive membrane material. The combination of MOFs and flexible polymers to fabricate composite membranes is a promising strategy to break the trade-off between permeability and selectivity and realize industrial application.There are many issues to be addressed to fabricate desirable MOF–polymer composite membranes. For instance, MOF-based MMMs face challenges in preparing membranes with well-dispersed particles, perfect interfacial adhesion between the polymer and MOF, and defect-free membrane surface as well as a high flux. As we have mentioned above, a lot of strategies have been proposed to tackle these obstacles. An alternative way is co-polymerizing MOFs and conventional polymers/monomers together to yield an approximately homogenous phase, and the covalent bonds between the MOF fillers and polymer matrix can greatly improve the interfacial interactions. We have developed a post-synthetic polymerization (PSP) strategy to covalently link MOF particles with polymer chains by direct copolymerization of the MOF and butyl methacrylate (BMA) monomer under UV light, thus enhancing the interaction between the MOF and polymer.105 The results demonstrated that the MOFs were uniformly dispersed within the matrix without obvious aggregation. It is worth noting that the photo-polymerization is a fast and facile method to prepare a flexible and stand-alone membrane without the need for further purification and re-shaping procedures. Although the membrane configuration requires further optimization for application in gas separation, the PSP method exhibits the potential to achieve defect-free structures with a facile preparation technique and may shed light on the development of new MOF membranes.
As for polymer supported MOF membranes, the key is to enhance the adhesion between the substrate and the MOF layer, and create nucleation sites for MOF growth. Though researchers have tried to grow continuous MOF layers without defects on the polymer support, intercrystalline voids are hard to avoid and thus influence the selectivity of the membranes. Besides employing disordered MOF nanosheets to form ultrathin membranes with high permeability and selectivity as we have mentioned before, another possible choice is to modify the surface of MOF nanoparticles with long alkyl chains. These long chains may reduce the chance of gas molecules passing through the intercrystalline defects.
The addition of MOF fillers is expected to offer excellent selectivity properties stemming from their narrow pore size distribution and shape discrimination ability. Basic requirements for MOFs to be incorporated in polymer membranes include thermal and mechanical stability, ease of synthesis and resistance to humidity. Furthermore, MOFs with suitable pore size and functional groups with regard to targeted gas pair are preferred. MOFs with a pore size matching the kinetic diameters of the gas pair tend to achieve molecular sieving when processed into membranes. By choosing MOFs with desirable functionalities or introducing certain groups by post-synthetic modification, the affinity between the gases and MOFs can be easily controlled. Characteristics like open metal sites and flexibility in MOFs are also crucial for gas separation.
The large-scale production of MOFs with outstanding gas separation performance still remains a challenge. The raw materials, especially the organic ligands, are expensive and the synthetic methods are in most cases complicated and tedious, which makes the realization of MOF production for industrial processes even harder. Although methods such as electrolysis and twin screw extrusion can achieve the mass preparation of some particular MOFs, the types of MOF that can be synthesized using these processes are rather limited. Currently most MOFs with excellent sieving properties cannot be produced on a large scale, and so more versatile methods need to be developed for industrial purposes.
On the other hand, it is also challenging to achieve a continuous production of MOF membranes in an industrial process. Recently, our group reported a scalable and facile hot-pressing (HoP) method to make MOF coatings.106 A high temperature and pressure are applied at the same time to promote the rapid growth of MOFs on various substrates. This novel and facile method can be used for the continuous production of MOF membranes with a roll-to-roll machine, which sheds light on the development of the fabrication technique of MOF membranes.
Molecular sieve nanosheets (MSNs) are a promising field in membrane gas separation owing to their ultrahigh permeability. Exfoliated zeolite nanosheets,107 graphene oxide (GO) nanosheets,108 and MOF nanosheets109 have been tested for gas separation and show remarkable performance. These nanosheets can also be combined with polymer substrates to give flexible membranes, however, there are few chemically and dynamically stable two-dimensional MOFs available for exfoliation. Therefore, the design and synthesis of stable and exfoliatable 2D MOFs are highly desired.
In summary, we have reviewed the recent progress in the fabrication of MOF–polymer composite membranes for gas separation. Researchers have come up with many ideas and strategies to improve processing techniques and eliminate defects, and prove that MOF-composite membranes are indeed promising for real-world applications. Despite the many problems still to be solved to achieve industrialization and commercial applications, we remain optimistic that the continued investigation of this promising field will realize their practical application in the future.
List of abbreviations
| PAET | Poly(3-acetoxyethylthiophene) |
| PSF | Polysulfone |
| 6FDA-ODA | 4,4′-(Pexafluoroisopropylidene)diphthalic anhydride-4,4′-oxydianiline |
| PI | Polyimide |
| PEI | Polyethylenimine |
| SPEEK | Sulfonated poly(ether ether ketone) |
| 6FDA-DAM | (2,2-Bis(3,4-carboxyphenyl) hexafluoropropane dianhydride–diaminomesitylene) |
| PPO | (Poly(2,6-dimethyl-1,4-phenylene oxide)) |
| PBI | Polybenzimidazole |
| PDMS | Polydimethylsiloxane |
| PVDF | Polyvinylidene fluoride |
| PES | Polyethersulfone |
| PAN | Polyacrylonitrile |
| EDA | Ethylenediamine |
| PMMA | Poly(methyl methacrylate) |
| PMAA | Poly(methacrylic acid) |
| BMA | Butyl methacrylate |
| BPPO | Bromo-methylated poly(2,6-dimethyl-1,4-phenylene oxide) |
| BDC | 1,4-Benzenedicarboxylate |
| BTC | 1,3,5-Benzentricarboxylic acid |
| Hmim | 2-Methylimidazolate |
| NMP | 1-Methyl-2-pyrrolidone |
Acknowledgements
This work was financially supported by the 973 Program 2013CB834704, Provincial Key Project of China (grant no. 7131253), the National Natural Science Foundation of China (grant no. 21471018, 21404010, 21201018, 21490570) and 1000 Plan (Youth).References
- R. W. Baker, Ind. Eng. Chem. Res., 2002, 41, 1393 CrossRef CAS.
- P. Bernardo, E. Drioli and G. Golemme, Ind. Eng. Chem. Res., 2009, 48, 4638 CrossRef CAS.
- W. J. Koros and G. K. Fleming, J. Membr. Sci., 1993, 83, 1 CrossRef CAS.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed.
- M. Ulbricht, Polymer, 2006, 47, 2217 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 1991, 62, 165 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed.
- G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230 CrossRef PubMed.
- H. C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415 RSC.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- L. J. Murray, M. Dincă and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC.
- J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed.
- I. Erucar, G. Yilmaz and S. Keskin, Chem. – Asian J., 2013, 8, 1692 CrossRef CAS PubMed.
- B. R. Pimentel, A. Parulkar, E. K. Zhou, N. A. Brunelli and R. P. Lively, ChemSusChem, 2014, 7, 3202 CrossRef CAS PubMed.
- D. Bradshaw, A. Garai and J. Huo, Chem. Soc. Rev., 2012, 41, 2344 RSC.
- W. Li, Y. Zhang, Q. Li and G. Zhang, Chem. Eng. Sci., 2015, 135, 232 CrossRef CAS.
- H. B. Tanh Jeazet, C. Staudt and C. Janiak, Dalton Trans., 2012, 41, 14003 RSC.
- B. Zornoza, C. Tellez, J. Coronas, J. Gascon and F. Kapteijn, Microporous Mesoporous Mater., 2013, 166, 67 CrossRef CAS.
- E. Adatoz, A. K. Avci and S. Keskin, Sep. Purif. Technol., 2015, 152, 207 CrossRef CAS.
- J. Yao and H. Wang, Chem. Soc. Rev., 2014, 43, 4470 RSC.
- M. Shah, M. C. McCarthy, S. Sachdeva, A. K. Lee and H.-K. Jeong, Ind. Eng. Chem. Res., 2012, 51, 2179 CrossRef CAS.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC.
- A. Aijaz, A. Karkamkar, Y. J. Choi, N. Tsumori, E. Ronnebro, T. Autrey, H. Shioyama and Q. Xu, J. Am. Chem. Soc., 2012, 134, 13926 CrossRef CAS PubMed.
- H. Q. Xu, J. Hu, D. Wang, Z. Li, Q. Zhang, Y. Luo, S. H. Yu and H. L. Jiang, J. Am. Chem. Soc., 2015, 137, 13440 CrossRef CAS PubMed.
- B. Chen, L. Wang, Y. Xiao, F. R. Fronczek, M. Xue, Y. Cui and G. Qian, Angew. Chem., Int. Ed., 2009, 48, 500 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed.
- Y. Guo, X. Feng, T. Han, S. Wang, Z. Lin, Y. Dong and B. Wang, J. Am. Chem. Soc., 2014, 136, 15485 CrossRef CAS PubMed.
- M. Tu, S. Wannapaiboon and R. A. Fischer, Inorg. Chem. Front., 2014, 1, 442 RSC.
- A. Betard and R. A. Fischer, Chem. Rev., 2012, 112, 1055 CrossRef CAS PubMed.
- S. Qiu, M. Xue and G. Zhu, Chem. Soc. Rev., 2014, 43, 6116 RSC.
- O. Shekhah, J. Liu, R. A. Fischer and C. Wöll, Chem. Soc. Rev., 2011, 40, 1081 RSC.
- D. Zacher, O. Shekhah, C. Wöll and R. A. Fischer, Chem. Soc. Rev., 2009, 38, 1418 RSC.
- J.-L. Zhuang, A. Terfort and C. Wöll, Coord. Chem. Rev., 2016, 307, 391 CrossRef CAS.
- P. S. Goh, A. F. Ismail, S. M. Sanip, B. C. Ng and M. Aziz, Sep. Purif. Technol., 2011, 81, 243 CrossRef CAS.
- T.-S. Chung, L. Y. Jiang, Y. Li and S. Kulprathipanja, Prog. Polym. Sci., 2007, 32, 483 CrossRef CAS.
- K. Hunger, N. Schmeling, H. B. Jeazet, C. Janiak, C. Staudt and K. Kleinermanns, Membranes, 2012, 2, 727 CrossRef CAS PubMed.
- T. J. P. H. Yehia, J. P. Ferraris, K. J. Balkus and I. H. Musselman, Polym. Prepr., 2004, 45, 35 Search PubMed.
- P. Burmann, B. Zornoza, C. Téllez and J. Coronas, Chem. Eng. Sci., 2014, 107, 66 CrossRef CAS.
- T. T. Moore and W. J. Koros, J. Mol. Struct., 2005, 739, 87 CrossRef CAS.
- R. Nasir, H. Mukhtar, Z. Man and D. F. Mohshim, Chem. Eng. Technol., 2013, 36, 717 CrossRef CAS.
- G. Dong, H. Li and V. Chen, J. Mater. Chem. A, 2013, 1, 4610 CAS.
- B. Zornoza, A. Martinez-Joaristi, P. Serra-Crespo, C. Tellez, J. Coronas, J. Gascon and F. Kapteijn, Chem. Commun., 2011, 47, 9522 RSC.
- L. Cao, K. Tao, A. Huang, C. Kong and L. Chen, Chem. Commun., 2013, 49, 8513 RSC.
- N. Tien-Binh, H. Vinh-Thang, X. Y. Chen, D. Rodrigue and S. Kaliaguine, J. Mater. Chem. A, 2015, 3, 15202 CAS.
- R. Lin, L. Ge, L. Hou, E. Strounina, V. Rudolph and Z. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 5609 CAS.
- M. W. Anjum, F. Vermoortele, A. L. Khan, B. Bueken, D. E. De Vos and I. F. Vankelecom, ACS Appl. Mater. Interfaces, 2015, 7, 25193 CAS.
- S. R. Venna, M. Lartey, T. Li, A. Spore, S. Kumar, H. B. Nulwala, D. R. Luebke, N. L. Rosi and E. Albenze, J. Mater. Chem. A, 2015, 3, 5014 CAS.
- Q. Xin, J. Ouyang, T. Liu, Z. Li, Z. Li, Y. Liu, S. Wang, H. Wu, Z. Jiang and X. Cao, ACS Appl. Mater. Interfaces, 2015, 7, 1065 CAS.
- T. H. Bae, J. S. Lee, W. Qiu, W. J. Koros, C. W. Jones and S. Nair, Angew. Chem., Int. Ed., 2010, 49, 9863 CrossRef CAS PubMed.
- L. Ge, W. Zhou, V. Rudolph and Z. Zhu, J. Mater. Chem. A, 2013, 1, 6350 CAS.
- J. Sánchez-Laínez, B. Zornoza, Á. Mayoral, Á. Berenguer-Murcia, D. Cazorla-Amorós, C. Téllez and J. Coronas, J. Mater. Chem. A, 2015, 3, 6549 Search PubMed.
- W. S. Chi, S. Hwang, S.-J. Lee, S. Park, Y.-S. Bae, D. Y. Ryu, J. H. Kim and J. Kim, J. Membr. Sci., 2015, 495, 479 CrossRef CAS.
- B. Seoane, J. M. Zamaro, C. Téllez and J. Coronas, RSC Adv., 2011, 1, 917 RSC.
- S. Shahid, K. Nijmeijer, S. Nehache, I. Vankelecom, A. Deratani and D. Quemener, J. Membr. Sci., 2015, 492, 21 CrossRef CAS.
- R. Lin, L. Ge, S. Liu, V. Rudolph and Z. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 14750 CAS.
- B. Zornoza, B. Seoane, J. M. Zamaro, C. Tellez and J. Coronas, ChemPhysChem, 2011, 12, 2781 CrossRef CAS PubMed.
- S. Sorribas, B. Zornoza, C. Téllez and J. Coronas, J. Membr. Sci., 2014, 452, 184 CrossRef CAS.
- R. Mahajan and W. J. Koros, Ind. Eng. Chem. Res., 2000, 39, 2692 CrossRef CAS.
- R. Mahajan and W. J. Koros, Polym. Eng. Sci., 2002, 42, 1420 CAS.
- M. J. C. Ordoñez, K. J. Balkus, J. P. Ferraris and I. H. Musselman, J. Membr. Sci., 2010, 361, 28 CrossRef.
- R. Adams, C. Carson, J. Ward, R. Tannenbaum and W. Koros, Microporous Mesoporous Mater., 2010, 131, 13 CrossRef CAS.
- J. O. Hsieh, K. J. Balkus, J. P. Ferraris and I. H. Musselman, Microporous Mesoporous Mater., 2014, 196, 165 CrossRef CAS.
- F. Dorosti, M. Omidkhah and R. Abedini, Chem. Eng. Res. Des., 2014, 92, 2439 CrossRef CAS.
- Q. Song, S. K. Nataraj, M. V. Roussenova, J. C. Tan, D. J. Hughes, W. Li, P. Bourgoin, M. A. Alam, A. K. Cheetham, S. A. Al-Muhtaseb and E. Sivaniah, Energy Environ. Sci., 2012, 5, 8359 CAS.
- T. Yang and T.-S. Chung, J. Mater. Chem. A, 2013, 1, 6081 CAS.
- T. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, F. X. Llabrés i Xamena and J. Gascon, Nat. Mater., 2015, 14, 48 CrossRef CAS PubMed.
- Z. Kang, Y. Peng, Z. Hu, Y. Qian, C. Chi, L. Y. Yeo, L. Tee and D. Zhao, J. Mater. Chem. A, 2015, 3, 20801 CAS.
- A. Bos, I. G. M. Pünt, M. Wessling and H. Strathmann, J. Membr. Sci., 1999, 155, 67 CrossRef CAS.
- A. F. Ismail and W. Lorna, Sep. Purif. Technol., 2002, 27, 173 CrossRef CAS.
- S. Shahid and K. Nijmeijer, J. Membr. Sci., 2014, 470, 166 CrossRef CAS.
- X. Guo, H. Huang, Y. Ban, Q. Yang, Y. Xiao, Y. Li, W. Yang and C. Zhong, J. Membr. Sci., 2015, 478, 130 CrossRef CAS.
- E. V. Perez, K. J. Balkus, J. P. Ferraris and I. H. Musselman, J. Membr. Sci., 2009, 328, 165 CrossRef CAS.
- S. Shahid and K. Nijmeijer, J. Membr. Sci., 2014, 459, 33 CrossRef CAS.
- T. Rodenas, M. van Dalen, P. Serra-Crespo, F. Kapteijn and J. Gascon, Microporous Mesoporous Mater., 2014, 192, 35 CrossRef CAS.
- J. A. Thompson, J. T. Vaughn, N. A. Brunelli, W. J. Koros, C. W. Jones and S. Nair, Microporous Mesoporous Mater., 2014, 192, 43 CrossRef CAS.
- S. Basu, A. Cano-Odena and I. F. J. Vankelecom, J. Membr. Sci., 2010, 362, 478 CrossRef CAS.
- S. Basu, A. Cano-Odena and I. F. J. Vankelecom, Sep. Purif. Technol., 2011, 81, 31 CrossRef CAS.
- L. Shao, C.-H. Lau and T.-S. Chung, Int. J. Hydrogen Energy, 2009, 34, 8716 CrossRef CAS.
- S. N. Wijenayake, N. P. Panapitiya, S. H. Versteeg, C. N. Nguyen, S. Goel, K. J. Balkus, I. H. Musselman and J. P. Ferraris, Ind. Eng. Chem. Res., 2013, 52, 6991 CrossRef CAS.
- S. N. Wijenayake, N. P. Panapitiya, C. N. Nguyen, Y. Huang, K. J. Balkus, I. H. Musselman and J. P. Ferraris, Sep. Purif. Technol., 2014, 135, 190 CrossRef CAS.
- J. Benito, M. Fenero, S. Sorribas, B. Zornoza, K. J. Msayib, N. B. McKeown, C. Téllez, J. Coronas and I. Gascón, Colloids Surf., A, 2015, 470, 161 CrossRef CAS.
- J. Hu, H. Cai, H. Ren, Y. Wei, Z. Xu, H. Liu and Y. Hu, Ind. Eng. Chem. Res., 2010, 12605 CrossRef CAS.
- Y. Dai, J. R. Johnson, O. Karvan, D. S. Sholl and W. J. Koros, J. Membr. Sci., 2012, 401–402, 76 CrossRef CAS.
- A. K. Zulhairun, Z. G. Fachrurrazi, M. Nur Izwanne and A. F. Ismail, Sep. Purif. Technol., 2015, 146, 85 CrossRef CAS.
- J. Yao, D. Dong, D. Li, L. He, G. Xu and H. Wang, Chem. Commun., 2011, 47, 2559 RSC.
- Y. Mao, J. Li, W. Cao, Y. Ying, L. Sun and X. Peng, ACS Appl. Mater. Interfaces, 2014, 6, 4473 CAS.
- D. Nagaraju, D. G. Bhagat, R. Banerjee and U. K. Kharul, J. Mater. Chem. A, 2013, 1, 8828 CAS.
- F. Cacho-Bailo, B. Seoane, C. Téllez and J. Coronas, J. Membr. Sci., 2014, 464, 119 CrossRef CAS.
- A. J. Brown, N. A. Brunelli, K. Eum, F. Rashidi, J. R. Johnson, W. J. Koros, C. W. Jones and S. Nair, Science, 2014, 345, 72 CrossRef CAS PubMed.
- L. Ge, W. Zhou, A. Du and Z. Zhu, J. Phys. Chem. C, 2012, 116, 13264 CAS.
- A. J. Brown, J. R. Johnson, M. E. Lydon, W. J. Koros, C. W. Jones and S. Nair, Angew. Chem., Int. Ed., 2012, 51, 10615 CrossRef CAS PubMed.
- W. Li, G. Zhang, C. Zhang, Q. Meng, Z. Fan and C. Gao, Chem. Commun., 2014, 50, 3214 RSC.
- A. Centrone, Y. Yang, S. Speakman, L. Bromberg, G. C. Rutledge and T. A. Hatton, J. Am. Chem. Soc., 2010, 132, 15687 CrossRef CAS PubMed.
- W. Li, Z. Yang, G. Zhang, Z. Fan, Q. Meng, C. Shen and C. Gao, J. Mater. Chem. A, 2014, 2, 2110 CAS.
- T. Ben, C. Lu, C. Pei, S. Xu and S. Qiu, Chem. – Eur. J., 2012, 18, 10250 CrossRef CAS PubMed.
- E. Shamsaei, Z. X. Low, X. Lin, A. Mayahi, H. Liu, X. Zhang, J. Zhe Liu and H. Wang, Chem. Commun., 2015, 51, 11474 RSC.
- R. Ostermann, J. Cravillon, C. Weidmann, M. Wiebcke and B. M. Smarsly, Chem. Commun., 2011, 47, 442 RSC.
- M. Rose, B. Böhringer, M. Jolly, R. Fischer and S. Kaskel, Adv. Eng. Mater., 2011, 13, 356 CrossRef CAS.
- Y.-n. Wu, F. Li, H. Liu, W. Zhu, M. Teng, Y. Jiang, W. Li, D. Xu, D. He, P. Hannam and G. Li, J. Mater. Chem., 2012, 22, 16971 RSC.
- L. Fan, M. Xue, Z. Kang, H. Li and S. Qiu, J. Mater. Chem., 2012, 22, 25272 RSC.
- W. Li, Q. Meng, C. Zhang and G. Zhang, Chem. – Eur. J., 2015, 21, 7224 CrossRef CAS PubMed.
- W. Li, P. Su, G. Zhang, C. Shen and Q. Meng, J. Membr. Sci., 2015, 495, 384 CrossRef CAS.
- Y. Zhang, X. Feng, H. Li, Y. Chen, J. Zhao, S. Wang, L. Wang and B. Wang, Angew. Chem., Int. Ed., 2015, 54, 4259 CrossRef CAS PubMed.
- Y. Chen, S. Li, X. Pei, J. Zhou, X. Feng, S. Zhang, Y. Cheng, H. Li, R. Han and B. Wang, Angew. Chem., Int. Ed., 2016, 55, 3419 CrossRef CAS PubMed.
- K. Varoon, X. Zhang, B. Elyassi, D. D. Brewer, M. Gettel, J. A. L. S. Kumar, S. Maheshwari, A. Mittal, C.-Y. Sung, M. Cococcioni, L. F. Francis, A. V. McCormick, K. A. Mkhoyan and M. Tsapatsis, Science, 2011, 334, 72 CrossRef CAS PubMed.
- H. Li, Z. Song, X. Zhang, Y. Huang, S. Li, Y. Mao, H. J. Ploehn, Y. Bao and M. Yu, Science, 2013, 342, 95 CrossRef CAS PubMed.
- Y. Peng, Y. Li, Y. Ban, H. Jin, W. Jiao, X. Liu and W. Yang, Science, 2014, 346, 1356 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2016 |