An overview of the modification of g-C3N4 with high carbon containing materials for photocatalytic applications
Sulagna
Patnaik
,
Satyabadi
Martha
,
Saumyaprava
Acharya
and
K. M.
Parida
*
Centre for Nano Science and Nano Technology, Institute of Technical Education and Research, Siksha ‘O’ Anusandhan University, Bhubaneswar 751030, India. E-mail: kulamaniparida@soauniversity.ac.in; paridakulamani@yahoo.com; satyabadimartha@soauniversity.ac.in; Fax: +91-674-2350642; Tel: +91-674-2351777
First published on 28th December 2015
Abstract
In recent years, graphitic carbon nitride has become one of the very exciting sustainable materials, due to its unusual properties and promising applications as a heterogeneous catalyst in water splitting and organic contaminant degradation. A variety of modifications have been reported for this nanostructured material with the use of carbonaceous materials to enhance its potential applications. This review summarizes the ongoing developments towards the use of carbonaceous materials like activated carbon (AC), ordered mesoporous carbon (OMC), carbon nanotubes (CNTs), fullerene (C60) and graphene (GE) for the enhancement of the photocatalytic performance of metal free semiconductor photocatalysts because of their special structures and unique electronic properties. Also this review highlights the recent strategies aiming to promote the activity by coupling with polymers (having higher carbon content). Our study reveals that in addition to the charge transfer effects, morphological changes in g-C3N4 are also introduced by combination of g-C3N4 with carbonaceous materials to tailor its pristine properties and to extend its applications.
1. Introduction
Nowadays, the worldwide attention is focused on growing energy demands and increasing emission of greenhouse gases. To satisfy this rising energy demand with a minimum environmental impact, it is highly essential for chemists to develop a cost-effective sustainable method for utilizing solar energy for the betterment of energy and the environment. Thus the development of a suitable cost effective process in this regard is a great challenge. One of the most promising approaches is photocatalytic reactions assisted by a suitable semiconductor having a narrow band gap, which can absorb the visible light in the region (400 nm < λ < 800 nm). In the past few decades various homogeneous and heterogeneous photocatalytic systems such as metal oxides, metal oxynitrides, metal sulphides, metal oxysulphides and non-metal doped, transitional metal oxide modified or plasma based photocatalysts have been explored to harness solar energy.1–3 Although a good number of semiconductor photocatalysts are developed in this field, their photocatalytic activity under visible-light illumination is still limited as they are not suitable to utilize the whole range of visible light of solar spectra, as some of them have conduction bands lower than the reduction potential of water and are not photostable. To overcome this, very recently nanostructured polymeric g-C3N4 was developed which is a novel metal free visible light induced organic semiconductor photocatalyst with a suitable band gap energy of 2.7 eV. It is a promising high performance material due to its hardness, lightweight, non-toxicity, abundance, preparation from easily available starting materials and excellent stability under ambient conditions.4,5 This novel nanostructured material is found to have many potential applications such as energy conversion, purification of contaminated water, good performance in the photo-oxidation of dyes, as a base metal free catalyst for NO decomposition, as a reference material in differentiating oxygen activation sites for oxidation reactions, as a functional material to synthesise nanosized metal particles and has also achieved functionality as a stable photocatalyst for H2 evolution by splitting water under visible light irradiation and also in fuel cells.6–11 Although g-C3N4 has been explored as a material with promising potential in the photocatalysis field, the photocatalytic efficiency of neat g-C3N4 is still low, due to the high electron–hole recombination rate, the absorbance of only blue light of the solar spectrum (450 nm) which restricts the utilization of the broad spectrum of solar light, during synthesis the high degree of condensation of the monomers renders the material with a low surface area of g-C3N4 (∼10 m2 g−1) without forming textured pores and grain boundary effects which disrupt the delocalization of electrons. To improve the quantum efficiency and to promote the photocatalytic activity, strategies such as non-metal doping, transitional metal incorporation, conjugated polymer modification, coupling with other semiconductors, introduction of carbon-dopants and anchoring carbonaceousmaterials12–19 have been developed. The fusion of two π-conjugated systems not only stabilizes the hybrid but also enhances the utilization of solar spectra by extending the optical absorption to a longer wavelength region. Coupling with various carbon rich materials not only compensates for the disadvantages of individual semiconductor materials but also induces synergetic effects like visible light harnessing abilities, enhanced electron hole separation ability and improved photo stability.Many reviews20–23 and books have been published on photocatalytic application of semiconductors to solve the energy and environmental issues. In this review, we have highlighted a short overview of the recent significant progress on designing efficient carbonaceous materials and polymer (having higher carbon content) based g-C3N4 nanocomposites for hydrogen generation and pollutant degradation under visible-light irradiation. After introducing the synthesis of g-C3N4 based composites with carbonaceous materials, particular attention has been focused on physiochemical characterization and photocatalytic applications.
2. Brief sketch of the properties of carbonaceous materials
Carbon nanostructured materials are very important in many research fields due to their unique properties like chemical stability, good conductivity and high surface area. It has been found that different modified carbonaceous semiconductors are found to accelerate transfer of electrons from the surface of the photocatalyst to the liquid–solid interface due to the electron transportability of carbon. For which graphitic carbon nitride can be coupled with various carbonaceous materials having higher carbon content, like ordered mesoporous carbon,24–29 multiwalled carbon nanotubes,30–37 fullerene38,39 and graphene40–55 and polymers56 like C-PDA,57 PANI,58 P3HT,59 7,7,8,8-tetracyanoquinodimethane (TCNQ)60 to enhance its photocatalytic activity.These are particularly important carbon nanostructures which possess a number of active adsorption sites for the reactants, good electron hole separation and enhanced visible-light absorption by sensitization. When coupled with g-C3N4, the photocatalytic activity of the composites is effectively improved. The electronic integration of the graphitic carbon nitride with different carbon species having unpaired electrons in the lattice significantly enhances the delocalisation and hence the potential applications. An optimum amount of dopants favours efficient electron hole separation and shifts the optical absorption to a lower energy region with the increasing carbon content. This may be due to the extension of the pi-conjugated structure towards the carbon dopants.
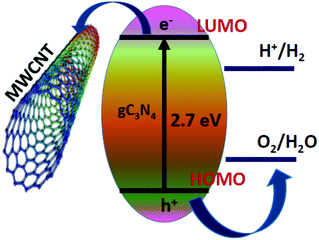
Ordered mesoporous carbon has a tubular structure with a large pore size, increased surface area and systematically arranged mono-disperse mesopores which provide a good opportunity for preparing nanocomposites with different semiconductor photocatalysts. The composite of ordered mesoporous carbon (OMC) with g-C3N4 acts as an efficient photocatalyst for contaminant degradation. Another carbon species multi-walled carbon nanotubes (MWCNTs)30–37 are also good candidates for the improvement of photocatalysis. Usually, multi-walled carbon nanotubes are used as electron sinks to improve the photogenerated charge carrier separation and delay their recombination (Fig. 1).
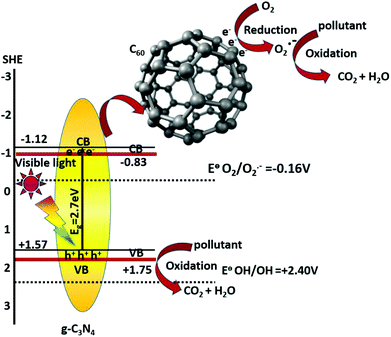
Among the use of different types of carbonaceous materials such as graphene (GE), fullerene (C60), carbon nanotubes (CNTs) and activated carbon (AC), fullerene has attracted considerable attention for the enhancement of photocatalytic performances of semiconductors because of its unique electronic properties and special structures.38 Fullerenes (C60) with unique electronic properties represent another allotrope of carbon. C60 with 60 π-electrons consists of 30 bonding molecular orbitals, and helps in electron transfer reduction.39 The structure of C60 behaves as an excellent electron acceptor, which induces a rapid photoinduced charge separation and retards the charge recombination. When coupled with fullerene, the C60/g-C3N4 composite behaves as a highly efficient photocatalyst for the degradation of organic pollutants. Another important 2D carbon nanostructured network of graphene possesses a hexagonal structure containing sp2-hybridized carbon atoms. Graphene also exhibits unique properties, such as an exceptional conductivity (106 S cm−1), a large specific surface area (2630 m2 g−1), a fast mobility of charge carriers at room temperature (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1) and a good optical transmittance (97.7%) compared with other carbon nanostructures. Based on several theoretical and experimental research studies it has been reported that graphene helps in the sensitization of several semiconducting materials.40–55
000 cm2 V−1 s−1) and a good optical transmittance (97.7%) compared with other carbon nanostructures. Based on several theoretical and experimental research studies it has been reported that graphene helps in the sensitization of several semiconducting materials.40–55
To improve the efficiency of g-C3N4, it was also coupled with different types of polymers like C-PDA, PANI, P3HT, TCNQ etc.57 PDA (polydopamine) is utilized as a conductive carbon material to coat the surface of g-C3N4.58 Polyaniline (PANI) is one of the most important conducting polymers because of its unique structure, electron delocalization, chemical stability, easy preparation and simple doping chemistry. Furthermore, PANI due to its conjugated pi-electron system behaves as an electron donor upon light irradiation and is known as a good hole conductor. When it is coupled with g-C3N4 the synergistic effect between g-C3N4 and PANI is found to improve the photogenerated charge carrier separation. Most of the biopolymers readily extend hierarchical structures in the presence of water through the formation of coiled coils and double or triple helices, which exhibit different chemical functionalities by introducing carboxylate, hydroxyl groups and thus strongly bind to metal cations forming gels having high thermal stability. The biopolymer-activation of g-C3N4 simultaneously acts as a soft-template to form sponge-like structures and also incorporate active carbon-dopant sites. The resulting biopolymer-activated sponge-like g-C3N4 nanostructured material enhances the photocatalytic activity and finds its application as a photocathode material.59 P3HT is another π-conjugated polymeric organic semiconductor material and can become conductive when electrons are delocalised from the conjugated π-orbitals via doping or by coupling with other semiconductors, which finds applications as bioelectronics, biosensing, bioimaging, conductive polymers, donor materials etc. P3HT is a p-type semiconductor with higher hole carrier mobility with a suitable band gap of 1.9–2.1 eV. When p-type poly-3-hexylthiophene (P3HT) is coupled with n-type g-C3N4 nanoplates at the nanoscale, it results in effective phase separation at the interface. The ideal nanostructure heterojunctions favor charge transfer due to the extended conjugation in the structure of the donor molecules at the interface and result in effective photoinduced charge separation.60 7,7,8,8-Tetracyanoquinodimethane (TCNQ) also behaves as a powerful electron acceptor due to the presence of a highly conjugated π electron system. TCNQ and its anions have strong π–π stacking interactions with other conjugative π-structured materials to form charge-transfer (CT) complexes. The conjugated π electron system of TCNQ drastically changes its electrical, magnetic and electrochemical properties by forming charge-transfer (CT) complexes. Due to the enhanced electron channelization different conjugative π-structure materials form hybrid photocatalysts with TCNQ and exhibit superior photocatalytic activity due to the reduced photoinduced electron hole recombination rate. In addition to that, the band structure of these hybrid semiconductor composites is also adjusted to show remarkably enhanced photocatalytic performance. The strong π–π stacking interactions between g-C3N4 and TCNQ having conjugated structures result in an ideal combination to make a hybrid semiconductor photocatalyst.
3. Composites of g-C3N4-carbonaceous materials: an approach towards synthesis
By preparing the g-C3N4-based composite the photocatalytic activity of g-C3N4 was dramatically enhanced due to decrease in the band gap position of the composite, increase in electron channelization and hence charge carrier separation. Different groups of scientists reported the construction of g-C3N4-based composite photocatalysts with various carbonaceous materials and carbon rich polymers.Ordered mesoporous carbon (OMC)/g-C3N4 composites were synthesized by a simple low-temperature thermal condensation method. Shi et al.24 synthesised OMC by using SBA-15, sucrose and H2SO4 (98%) and then OMC/g-C3N4 composites were synthesized by mixing the specified amounts of C/SBA-15 with melamine and subjected to calcination. Suryawanshi et al.30 synthesized an organic metal-free nanocomposite of g-C3N4 with functionalized multiwalled carbon nanotubes (MWCNTs) by adding functionalized MWCNTs in situ to 1.5 M aqueous solution of cyanamide by the hydrothermal method, to maximise its photocatalytic H2 evolution under visible light. There is a 100% improvement of its activity for an optimum of 0.5% MWCNT/g-C3N4 nanocomposites. According to Chai et al.38 C60/g-C3N4 composites were synthesized by a simple adsorption method by using an appropriate amount of C-60 and 50 mL toluene which was sonicated for 1 h so that C60 gets dispersed uniformly on g-C3N4. In a 1 wt% C60/g-C3N4 composite, the morphology of g-C3N4 was well maintained and appears like a flaky structure similar to that of neat-g-C3N4. In C60/g-C3N4 composites a 2D lamellar structure is present where the C60 particles are highly dispersed on the surface of g-C3N4. With an increase in the C60 content, the photocatalytic activity of the composite increases and the 1 wt% C60/g-C3N4 composite shows the maximum photocatalytic performance (Fig. 2).
 | ||
| Fig. 2 Mechanism of photogenerated charge separation of the C60/g-C3N4 composite under visible light irradiation. (Reprinted with permission from ref. 39, license number 3751320088021). | ||
The synthesis of graphene modified porous g-C3N4 (porous g-C3N4/graphene) was reported by Yu et al.44 without using templates and using the calcination method which shows efficient photocatalytic activity under visible light irradiation. The bubbles of ammonia gas produced during calcination makes the sample porous without using any template. The porous structure of the composite improves the optical absorption of materials. The mesoporous g-C3N4/graphene (MCN-G) and mesoporous g-C3N4/graphene oxide (MCN-GO) nanocomposites were synthesised by Li et al.45 by a facile sonochemical method. After sonication, a large number of MCN nanoparticles were found to be dispersed uniformly on the surface of graphene and graphene oxide sheets. Sun et al.47 reported the synthesis of layered graphene/g-C3N4 composites with increased conductivity and photocatalytic performance. Xiang et al.53 reported a simultaneous chemical reduction method using hydrazine hydrate as a reducing agent and involving polymerization of melamine in the presence of graphene oxide, followed by thermal treatment at 550 °C to synthesise graphene modified porous g-C3N4.56 C-PDA/g-C3N4 composites were synthesised by He et al. through in situ solution-based polymerization of dopamine (DA) and melamine, which results in the formation of a coating of dopamine on the surface after carbonization and condensation. Melamine is dispersed in a tris-buffer solution (pH, 8.5) along with DA; a coating of PDA is formed on the surface of melamine due to oxidative polymerization of DA. Afterwards, upon heating under an Ar atmosphere the C-PDA/g-C3N4 composite is obtained. The important biomolecule, dopamine, undergoes self-polymerization under a particular pH. By changing the dopamine (DA) concentration and the time of polymerization the thickness of the polydopamine (PDA) coating can be controlled. Ge et al. reported the fabrication of a polymeric nanocoating by the solution-based deposition method. Novel polyaniline–g-C3N4 (PANI–g-C3N4)57 composite photocatalysts were synthesized by in situ deposition oxidative polymerization with different PANI–g-C3N4 ratios. During synthesis, an aniline monomer is added gradually to g-C3N4 powder in an ice bath. Zhang et al.58 reported the synthesis of biopolymer-activated g-C3N4 by using an aqueous solution of dicyandiamide (DCDA, the precursor for g-C3N4) along with alginate or gelatin solution by the calcination method. The formation of P3HT–g-C3N4 heterojunction was reported by Bai et al.59via a ball milling method, where the surface –NH2 and –NH– groups of g-C3N4 are involved in polymer chemistry. Zhang et al.60 reported the formation of TCNQ–C3N4 hybrid photocatalysts by a liquid ultrasonic route in water by taking g-C3N4 and TCNQ–DMF solution. Formation of these nanocomposites facilitates visible light induced photocatalysis by tuning the band gaps which is important for the photocatalytic degradation of organic pollutants and water splitting for the production of H2 energy.
4. An in-depth analysis of composites through surface and bulk characterization
Furthermore an insight into the structure of g-C3N4 based composites is discussed by different types of structural analyses like XRD, SEM, TEM etc. X-ray diffraction studies indicate the crystal phase of the materials and the interlayer stacking. After modifying pure g-C3N4 with a low amount of carbonaceous materials, there was no significant change in the crystal structure of the g-C3N4. Therefore, the modification with various carbonaceous materials does not disturb the lattice structure, although a substantial broadening of the main peak confirms the formation of the nanocomposites. Generated strains at the interfaces of two integrated materials result in such broadening. The same two diffraction peaks, a sharp one at 27.4° indicates the graphite-like stacking of the conjugated aromatic units of carbon nitride, which corresponds to the (002) plane of g-C3N4, representing an interplanar distance of 0.325 nm. The other less intense diffraction peak at 13.1° corresponds to the (100) plane, representing an interplanar distance of 0.675 nm. This indicates that the introduction of CNT also hardly alters the in-plane and interlayer stacking of the g-C3N4. Chen et al. reported that after raising the CNT concentration even upto 0.5 wt% no significant variations can be detected between g-C3N4 and CNT/g-C3N4. Similarly Lei Shi et al.24 reported that in the OMC/g-C3N4 composites no extra peak is obtained in the XRD pattern of the composite (Fig. 3 and 4).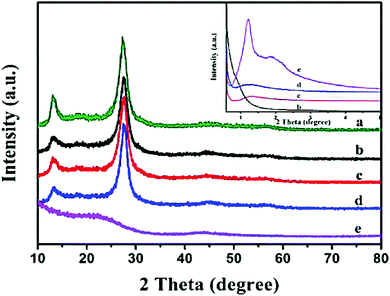 | ||
| Fig. 3 XRD patterns of (a) pure g-C3N4, (b) OMC/g-C3N4-1, (c) OMC/g-C3N4-2, (d) OMC/g-C3N4-3, and (e) OMC. (Reprinted with permission from ref. 24). | ||
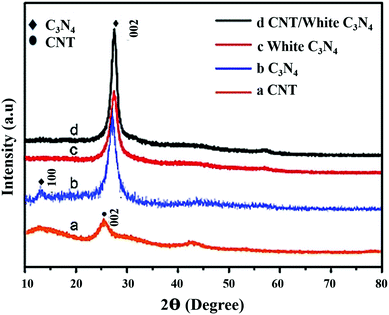 | ||
| Fig. 4 XRD patterns of CNT (a), g-C3N4 (b), the white g-C3N4 (c), CNT/white g-C3N4 (d). (Reprinted with permission from ref. 40). | ||
Therefore, the modification with different carbonaceous materials does not disturb the crystal structure of g-C3N4, which is a main advantage for the photocatalytic properties of the nanocomposites. Similarly the XRD pattern of TCNQ–g-C3N4 indicates no characteristic diffractions for crystalline TCNQ with low TCNQ loadings. The crystal phase of g-C3N4 did not change with increasing TCNQ content. But higher TCNQ loadings (>5%) show crystalline TCNQ peaks, and the peak intensities are also increased with the increasing TCNQ loading. Shi et al. reported that ordered mesoporous carbon,24–26 possesses a tubular structure with a large pore size, high surface area, and systematically arranged mono dispersed mesopores which provides a good opportunity for preparing nanocomposites with different semiconductor photocatalysts. The combination of ordered mesoporous carbon with g-C3N4 acts as an efficient photocatalyst for contaminant degradation. It was found that the addition of OMC slightly affects the graphitic layered structures of g-C3N4, but strongly enhances the specific surface area and pore volume of the synthesized OMC/g-C3N4 nanocomposites due to the presence of OMC with an extremely high surface area (1120.48 m2 g−1). In the OMC/g-C3N4 nanocomposite, OMC was dispersed and attached onto the surface of the g-C3N4 so that a clear interface was formed between the carbon particles and g-C3N4, which favours the transfer of photogenerated electrons from the surface of g-C3N4 to OMC. The retardation of the photogenerated electron–hole recombination is also evidenced from photoluminescence spectra as observed in Fig. 5. In the OMC/g-C3N4 composites, the decreased intensities of PL spectra with increased amounts of OMC confirm improvement in the photogenerated charge separation efficiency.
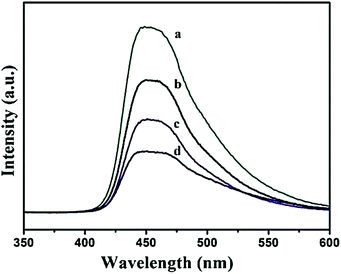 | ||
| Fig. 5 Photoluminescence emission spectra of (a) pure g-C3N4, (b) OMC/g-C3N4-1, (c) OMC/g-C3N4-2 and (d) OMC/g-C3N4-3. (Reprinted with permission from ref. 24). | ||
Chen et al.31 reported that due to the presence of inner mesopore channels CNTs have a large surface area. But by increasing the CNT content there is only a very slow-growth in the specific surface area of the CNT/g-C3N4 composite which is presented in Table 1.
| Sample | S BET in m2 g−1 | Pore diameter in nm |
|---|---|---|
| CNT | 10.4 | 4.24 |
| CNT/CN-0.1 | 10.7 | 4.30 |
| CNT/CN-0.2 | 11.2 | 4.28 |
| CNT/CN-0.5 | 17.6 | 4.32 |
Ge et al. reported in the neat g-C3N4 and pure PANI samples many smaller crystals are present in aggregated forms. After the introduction of the PANI polymer into the g-C3N4 network, the small particles of PANI get deposited on the surface of g-C3N4 forming an intimate interaction between g-C3N4 and PANI in the composite photocatalysts. But it is found from the SEM images of neat g-CN (a) and Alg-5-CN (b), and the TEM image of Alg-5-CN (c) in Fig. 6 that the structure and the morphology of g-C3N4 changes dramatically upon interaction with the biopolymers during calcination.
 | ||
| Fig. 6 SEM images of neat g-CN (a) and Alg-5-CN (b) and the TEM image of Alg-5-CN (c). (Reprinted from ref. 58, license number 3751340450109). | ||
With increase in the ratio of biopolymers to dicyandiamide, some wrinkled graphene like sheets were formed. The BET surface area for all of the biopolymer-activated g-C3N4 samples increases. For example, the surface area of alginate–5-g-C3N4 (5% biopolymer by mass) was found to be 42 m2 g−1 which was 4 times that of neat-g-C3N4 (10 m2 g−1), and that of alginate–10-g-C3N4 (5% biopolymer by mass) was 63 m2 g−1 even higher (6 times that of neat g-C3N4). When the ratio of the bio-polymers (alginate or gelatin) to DCDA is increased, the C/N ratio of the prepared samples alginate–g-C3N4 and gelatin-g-C3N4 also increased slightly in comparison with neat g-C3N4, which confirms the introduction of some more carbon species, preferably by replacing some of the N-atoms in the conjugated system of graphitic carbon nitride. Because the polysaccharides and polypeptides undergo decomposition fully during the calcination step whereas the g-C3N4 was almost unchanged due to its high thermal stability. The cathodic photo-current generation of g-C3N4 and alginate–g-C3N4 gradually increased when the biased potential became more negative and the anodic photocurrent of alginate–g-C3N4 was 2.6 times than that of neat-g-C3N4. Gelatin-g-C3N4 also shows the same enhanced photocurrent generation in the presence of sunlight. The TEM image of the P3HT–g-C3N4 composite indicates that g-C3N4 is closely packed on the surface of P3HT, forming dense thick sheets, in the p–n heterojunction. The results revealed that different types of structures ranging from lamellar assemblies to more disordered bundled conformations arise due to the adsorption of P3HT strongly on the surface of g-C3N4 through stacking forces (Fig. 7).
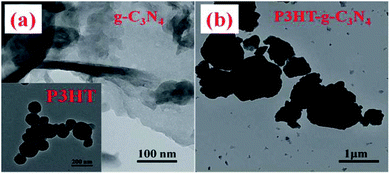 | ||
| Fig. 7 TEM image of g-C3N4, P3HT and the P3HT–g-C3N4 composite. (Reprinted with permission from ref. 59). | ||
5. An unique approach to evaluate the optical properties
Based upon the results of different groups of researchers, it can be predicted that the g-C3N4 based photocatalytic systems, show competitive efficiency as compared to that of neat g-C3N4. As an n-type semiconductor, the photoelectronic performance of g-C3N4 is significantly affected due to its tunable band gap which provides a controllable lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO). The investigation of wave function reveals that the valence and conduction bands are mainly formed by the 2pz orbitals of nitrogen and carbon respectively. When a photocatalyst absorbs either UV or visible light, electrons are excited from the valence band (2pz orbitals of nitrogen) to the conduction band (2pz orbitals of carbon) and a corresponding number of holes are generated in the valence band. This results in electron (e−), hole (h+) pairs and the semiconductor is in its photo-excited state, in which the photogenerated carriers are the preferred oxidation and reduction sites for splitting water and pollutant degradation. As no energy levels are available in the bandgap, it allows a delay in the recombination of electron hole pairs. Photocatalytic performance can be further improved in the case of the composites by promoting the interfacial electron transfer process and by enhancing the separation of photogenerated electron–hole pairs. Upon photoexcitation, the electrons and holes are separated and migrate to the surface of the redox catalysts. The nitrogen atoms are the preferred oxidation sites for H2O to form O2, whereas the carbon atoms provide the reduction sites for H2O to form H2. Similarly, photogenerated electrons and holes also produce free radicals like hydroxyl radicals (˙OH) and oxygen radicals (O2−) which are both strong oxidising agents for degrading organic pollutants.Suryawansi et al.30 reported that the absorption in the visible region is found to increase significantly with increase in the concentration of MWCNTs in the composite whereas the absorption edge is retained at the same wavelength as that for bulk g-C3N4 in the case of the 0.5% MWCNT/g-C3N4 composites. It is found that the introduction of CNT also remarkably enhances the optical properties of the composites. The absorption edge covers the whole visible range from 200 to 800 nm for all the CNT/g-C3N4, compared with the absorption spectrum of g-C3N4. However Bai et al.39 reported that the 1 wt% C60/g-C3N4 composite with a narrow band gap energy (2.85 eV) could easily generate the photo electrons and holes. The delocalized π structure of C60 favors the transfer of photoinduced electrons and holes, thus enhancing the photocatalytic activity. In the C60/g-C3N4 composite, the conduction band (CB) potential and valance band (VB) potential of g-C3N4 are −1.12 eV and 1.73 eV respectively, while the potential of C60/C60•− is −0.2 eV.38 Porous g-C3N4/graphene also extends the absorption edge between 450 and 800 nm.
Similarly, various composites of the g-C3N4 with carbon rich polymers undergo changes in their optical properties. He et al. reported that the absorption spectra of the C-PDA/g-C3N4 composites are slightly red-shifted and the composite shows enhanced absorption compared to that of neat g-C3N4. It reveals that the C-PDA/g-C3N4 composites could extend the absorption more towards the visible region to produce more number of photogenerated charge carriers. Ge et al. reported that neat g-C3N4 shows absorption in the visible range up to 460 nm and the pure PANI sample not only absorbs UV-Visible light, but also shows strong absorption near the infrared regions, due to transitions in the PANI molecules. But the PANI–g-C3N4 composite exhibits a red shift in the absorption edge and extends the absorption in the visible region at wavelengths longer than 400 nm.
In the case of the composites of the g-C3N4 the photocurrent is significantly enhanced and the enhanced photocurrent is due to both the porosity and the concentration of dopants, but still the exact reason to quantify their contribution towards photocurrent activity is uncertain. It is observed that although the BET surface area of alginate–g-C3N4 (42 m2 g−1) and gelatin–g-C3N4 (34 m2 g−1) are different and the concentration of biopolymers for gelatin (5%) g-C3N4 having a C/N ratio of 1.1 is higher than that of alginate (5%) g-C3N4 having a C/N ratio of 1.08, they show a similar photocurrent activity in the presence of visible light. The enhanced photocurrent generation in the presence of visible light is due to the synergistic effect between the biopolymer and the precursor of carbon nitride to create a spongy material with an increased number of absorption sites. Furthermore, a suitable concentration of the biopolymer content regulates the number of absorption sites in the network and enhances the mobility of the charge carriers. As a result, the application of biopolymer-activated carbon nitrides towards the photocathodic performance improved nearly about 300% under visible light irradiation as shown in Fig. 8.
 | ||
| Fig. 8 Photoelectrochemical properties of carbon nitrides in 0.1 M (aq.) KCl (reprinted with permission from ref. 58, license number 3751340450109). | ||
Furthermore, in the P3HT–g-C3N4 composite, with an increase in the amount of P3HT, the absorption intensity of the composites increases and its absorption edge shifts to 704 nm. g-C3N4 shows weak Raman signals but P3HT and P3HT–g-C3N4 samples show various Raman signals within 400–2000 cm−1 as Raman spectroscopy is mainly used to study the properties of different carbon rich materials. The signal at 1455 cm−1 represents the symmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration and at 1376 cm−1 represents C–C stretching vibration which is intra-ring, whereas C–C stretching vibration at 1208 cm−1 is inter-ring. Along with that the C–H bending vibration at 1180 cm−1 and the C–S–C deformation signal at 728 cm−1 give the supporting information about the P3HT–g-C3N4 system. Under 514 nm excitation the intensity of C
C stretching vibration and at 1376 cm−1 represents C–C stretching vibration which is intra-ring, whereas C–C stretching vibration at 1208 cm−1 is inter-ring. Along with that the C–H bending vibration at 1180 cm−1 and the C–S–C deformation signal at 728 cm−1 give the supporting information about the P3HT–g-C3N4 system. Under 514 nm excitation the intensity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration for the P3HT–g-C3N4 (0.7 wt%) composite increased about 3 times in comparison with that of pure P3HT, due to the pre-resonance Raman effect. The increased intensity of the C
C stretching vibration for the P3HT–g-C3N4 (0.7 wt%) composite increased about 3 times in comparison with that of pure P3HT, due to the pre-resonance Raman effect. The increased intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching mode in Raman spectra, indicates more ordered and longer range conjugation in the P3HT–g-C3N4 system. This high degree of order arrangement in the composite shifts the absorption towards the longer wavelength region and increases the mobility of charge carriers in comparison with its disordered form. Therefore, the photocatalytic activity of P3HT–g-C3N4 in the system is clearly dependent on the degree of molecular order (Fig. 9).
C stretching mode in Raman spectra, indicates more ordered and longer range conjugation in the P3HT–g-C3N4 system. This high degree of order arrangement in the composite shifts the absorption towards the longer wavelength region and increases the mobility of charge carriers in comparison with its disordered form. Therefore, the photocatalytic activity of P3HT–g-C3N4 in the system is clearly dependent on the degree of molecular order (Fig. 9).
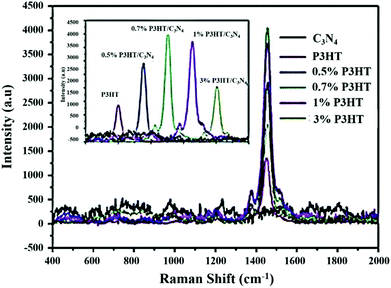 | ||
| Fig. 9 Raman spectra of the P3HT–g-C3N4 composite under 514 nm excitation. (Reprinted with permission from ref. 59). | ||
With the increase in TCNQ content, the photocurrent of the TCNQ–g-C3N4 composite was found to be remarkably enhanced under visible light irradiation. The photocurrent of 10%-TCNQ–g-C3N4 was about 23 times as high as that of the neat g-C3N4, which indicates that the improved photogenerated charge separation is due to the strong interaction between TCNQ and g-C3N4 (Fig. 10).
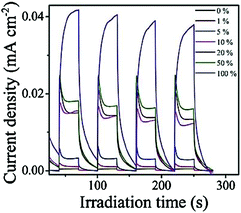 | ||
| Fig. 10 Photo-current density of the pure g-C3N4 and TCNQ–g-C3N4 composites with different TCNQ wt% under visible light irradiation (λ > 420 nm, [Na2SO4] ¼ 0.1 M). (Reprinted with permission from ref. 60). | ||
6. Evaluation of photocatalytic activity
The photocatalytic activity mainly depends upon the concentration of the electron and holes and their mobility. The development of visible-light responsive photocatalysts with a delayed rate of recombination of charge carriers is a great challenge in the field of photocatalysis. The composites of the g-C3N4 and carbonaceous material/polymer have a significant role over photocatalytic reactions like H2 evolution and organic pollutant degradation. Shi et al.24 reported the photocatalytic activity of OMC/g-C3N4 nano-composites under visible light irradiation was towards the photodegradation of rhodamine B (RhB) and 2,4-dichlorophenol (2,4-DCP). The photocatalytic efficiencies of the OMC/g-C3N4 composite over 2,4-DCP were found to be 3.68 times more than that of g-C3N4 whereas the degradation rate of the OMC/g-C3N4 photocatalyst over rhodamine B (RhB) was almost 10 times more than that of the neat g-C3N4, which indicated that OMC played a vital role in the remarkable improvement of photocatalytic activity. The mechanism of the photodegradation process based on the detection of active species and characterization of the photocatalyst indicates the presence of a synergetic effect between photoactive g-C3N4 and OMC. It is reported that the cause of enhanced activity is due to the addition of ammonium oxalate (AO) as a hole scavenger. AO not only acts as a hole scavenger but also accelerates the photogenerated charge separation. Moreover, the electron transfer from the surface of photoactive g-C3N4 to OMC is facilitated by the interfacial contact formed between OMC and g-C3N4 and thus hinders the recombination of the electron–hole pairs. Hence, in the OMC/g-C3N4 composite, the generated photoactive species such as e−, ˙O2− and ˙OH reacted with RhB and the holes are not responsible for reacting with the organic dye to form the degradation products. Moreover, with increasing the content of OMC in the composite the specific surface area of the composites increased which helps to enhance the photocatalytic activity of the composites. The enhanced photocatalytic activities are due to a combined effect of stronger absorption in the visible region than g-C3N4, larger specific surface area, increased adsorption capacity towards the dye and the reduced rate of photogenerated electron–hole recombination due to effective interfacial contact between OMC and g-C3N4. Similarly Chai et al.38 reported that the degradation percentage of RhB by neat g-C3N4 is only 54%, the photocatalytic degradation of RhB by 1 wt% C60/g-C3N4 composites was about 97% after 60 min visible light irradiation. However with further increase in the amount of C60, the photocatalytic performance decreases. The 1 wt% C60/g-C3N4 composite with a narrow band gap energy (2.85 eV) could easily generate the photo electrons and holes. The delocalized π structure of C60 favours the transfer of photoinduced electrons and holes, thus enhancing the degradation of RhB as an excellent electron accepter. The enhanced photocatalytic performance of the C60/g-C3N4 composite is due to the synergetic effect between graphitic-C3N4 and fullerene (C60), which favours the separation of photogenerated electrons and holes. The composites of the g-C3N4-polymer/carbonaceous materials work actively in the degradation of methylene blue. Xu et al.32 reported the photocatalytic activity of the CNT/g-C3N4 catalysts towards the degradation of methylene blue. The results showed that the degradation rate of the CNT/white g-C3N4 composite was nearly 8.1 times more than that of white g-C3N4. Similarly Ge et al.57 reported the photocatalytic activity of the PANI–g-C3N4 composite towards the photo-degradation of MB. The pure PANI sample shows almost no photodegradation of MB, neat g-C3N4 under visible light only degrades 41.2% MB in 120 min. But, in the PANI–g-C3N4 composite photocatalytic performance is enhanced significantly. The MB degradation rate of the CNP1 sample was found to be 46.7% whereas the photocatalytic MB degradation rate of the composite increases with increasing the amount of PANI from 1 wt% to 5 wt%. The PANI (5 wt%)–g-C3N4 composite shows the highest activity with a MB degrading rate of 92.8% (Scheme 1).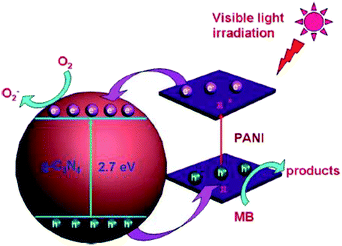 | ||
| Scheme 1 Schematic separation and migration of photogenerated charge-carriers in the PANI–g-C3N4 composite. (Reprinted with permission from ref. 57). | ||
The photocatalytic activity of the porous g-C3N4/graphene composite was evaluated by Yu et al. for the degradation of MB. Under visible light irradiation for 51 min, 58% of MB is degraded by using neat-g-C3N4 as the photocatalyst, whereas 87% of MB is degraded by using the porous g-C3N4/graphene hybrid photocatalyst. However, in the case of g-C3N4/graphene and porous g-C3N4, the degradation rate of MB reaches 60% and 77% respectively which indicates that the g-C3N4/graphene hybrid photocatalyst possesses better photocatalytic activity in comparison with neat g-C3N4 and porous g-C3N4. Bai et al. reported that the photocatalytic activity of the P3HT–g-C3N4 heterojunction for degradation of MB was 2 times greater than that of neat g-C3N4. The increase in photocatalytic activity is due to the high separation efficiency of photogenerated electron–hole pairs across the interface of P3HT–g-C3N4 photocatalysts. Diffusion of the charge carriers in the opposite direction at the p–n heterojunction forms an internal electric field. At the heterojunction interface the photo-generated charge carriers move from n-type g-C3N4 to p-type P3HT. According to the band edge position, the photo-excited electrons generated by P3HT were transferred to the conduction band of g-C3N4, whereas the photo-generated holes were collected in the valence band of P3HT. The mobility of the photogenerated charge carriers was enhanced due to the creation of an internal electric field. To increase the photocatalytic activity, polymeric P3HT plays a significant role in extending the π-conjugation and electron delocalisation in the system because of the π–π stacking interaction at the interface.
The photocatalytic rate constant (for phenol degradation) of the TCNQ–g-C3N4 composite is enhanced significantly with the increasing TCNQ content. When the TCNQ wt percentage is 10%, the rate constant k is almost 9.4 times as high as that of neat g-C3N4. But on further increasing the TCNQ amount, the phenol degradation rate decreases although it remains higher than that of neat g-C3N4. This increase in photocatalytic activity of TCNQ–g-C3N4 may be due to greater light harvesting capacity and enhanced charge separation. The increased rate of phenol degradation is observed by using the 10%-TCNQ–g-C3N4 composite throughout the visible spectrum. This wavelength-independent photocatalytic activity indicates the presence of some interaction between TCNQ and g-C3N4 that is responsible for enhancing the photocatalytic activity. In addition, the photocatalytic degradation of 2,4-dichlorophenol and bisphenol by TCNQ–g-C3N4 is also enhanced and the photocatalytic rate constants of TCNQ–g-C3N4 are 3.4 and 2.3 times more than that of neat g-C3N4 for 2,4-dichlorophenol and bisphenol A respectively (Scheme 2).
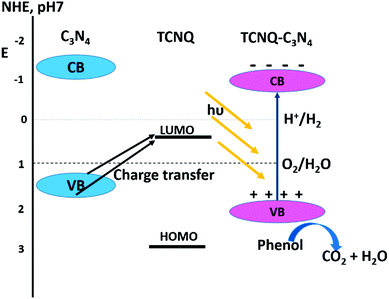 | ||
| Scheme 2 Schematic of the band structure of the TCNQ–g-C3N4. (Reprinted with permission from ref. 60). | ||
Li et al.45 proposed the improved photocatalytic activity of the g-C3N4/graphene composite towards the removal of NO, (found to be 64.9% and 60.7%). The visible light-harnessing capacity of g-C3N4/graphene and g-C3N4/graphene-oxide hybrids was enhanced and the conduction bands were negatively shifted when 1.0 wt% graphene/graphene oxide was incorporated.46 The enhanced activity of g-C3N4/graphene and g-C3N4/graphene-oxide hybrids is due to the large surface area and pore volume, significantly enhanced light-harnessing power, increased reduction capacity of photogenerated electrons and greater charge separation. The composites of the g-C3N4-polymer/carbonaceous materials are highly useful for water splitting and H2 evolution. Xiang et al.53 reported that the g-C3N4/graphene hybrid with an optimum graphene content (1.0 wt%) shows a H2-evolution rate of 451 μmol h−1 g−1, which was 3.07 times more than that of pure g-C3N4. It was also found from Fig. 11 that the graphene/g-C3N4 nanocomposite was extraordinarily stable. Even after the use of four cycles, there was no change in the H2 evolution rate, indicating that the material can be reused.
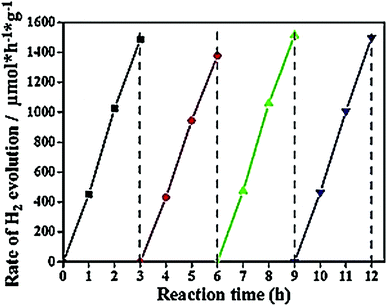 | ||
| Fig. 11 Cyclic H2-evolution curve for the g-C3N4/graphene hybrid sample. Reprinted with permission from ref. 53. Copy right American Chemical Society 2011. | ||
The visible light induced photocatalytic activity of the composite shows a hydrogen evolution rate of 42 mmol g−1 for the composite (0.5% MWCNT/g-C3N4).32 Thus the photocatalytic activity of the MWCNT/g-C3N4 composite metal oxides is effectively enhanced. The nanocomposite of graphitic carbon nitride with multiwall carbon nanotubes shows 100% enhancement in its photocatalytic activity towards water splitting. This improvement in visible light induced photocatalytic activity is due to morphological changes in g-C3N4 by introducing MWCNTs and the charge transfer effects.30 The pi-conjugated network mobilises the accepting, transporting and storing of electrons, and thus delays the process of recombination of photogenerated electron–hole pairs on g-C3N4. Sun et al.47 reported that graphene sheets also favour electron channelization to enhance the photogenerated electron–hole separation and consequently enhance the visible light driven photocatalytic H2-evolution of g-C3N4. The photocatalytic activity of pure g-C3N4 and C-PDA/g-C3N4 composites was evaluated by H2 evolution by the splitting of water under visible light irradiation (400 nm). Neat g-C3N4 shows the photocatalytic H2 evolution of 9 μmol h−1, whereas C-PDA shows no H2 evolution, which indicates that C-PDA is not photocatalytically active for H2 evolution. But, after introducing the C-PDA layer, the C-PDA/g-C3N4 composite shows an enhanced H2 evolution rate compared to that of neat g-C3N4. The 1.5 wt% C-PDA/g-C3N4 composite shows an optimum photocatalytic H2 evolution rate. The H2 evolution rate of the PDA/g-C3N4 composite is 81.1 μmol h−1, which is about 9 times more than that of neat g-C3N4. The photocatalytic H2 evolution rate of the 1.5 wt% C-PDA/g-C3N4 composite irradiated with visible light at 420 nm is 20.6 μmol h−1. Thus electronic integration of g-C3N4 with different carbon species having unpaired electrons in the lattice and conjugated π-electron systems significantly enhance the delocalisation, hinders the recombination process of the electron–hole pairs and stabilizes charge separation. An optimum amount of the dopants favours the electron transmission of the composite material and enhances the photocatalytic ability.
7. Summary and outlook
The present review summarizes the recent developments in g-C3N4-based photocatalysts coupled with different carbon containing materials for hydrogen generation and organic contaminant degradation under visible-light irradiation. The unique optical and electronic properties of g-C3N4 based carbonaceous materials have been well explained by their surface and bulk characterization like XRD, SEM, TEM, photoluminescence spectra and photocurrent measurements. It is concluded that the modification of g-C3N4 with sustainable carbonaceous materials and high carbon content polymers significantly improves its optical and electronic properties to achieve significant quantum yield for hydrogen production. The electronic integration of g-C3N4 with different carbon species having unpaired electrons in the lattice and conjugated π-electron systems significantly enhance the delocalisation, inhibits the recombination process of the electron–hole pairs, and stabilizes charge separation which enhances the photocatalytic activity. The union of two π-conjugated systems stabilizes the hybrid structure and significantly enhances the utilization of solar spectra by extending the optical absorption to a longer wavelength region. Coupling of g-C3N4 with various carbon rich materials not only compensates for the disadvantages of individual semiconductor materials but also induces synergetic effects like visible light harnessing abilities, enhanced electron hole separation ability and improved photo stability which ultimately enhances photocatalytic activity. It is expected that the inexpensive, environmentally friendly carbon based material modified g-C3N4 will play an important role in hydrogen production and contribute much to the coming hydrogen based economy. Due to the special nanostructure of the modified g-C3N4, it is not only helpful for hydrogen economy but also extends its application towards fuel cell application, lithium ion batteries, sensors and solar cells. This review gives a pathway to develop a sustainable and cost effective way for modification of g-C3N4 for hydrogen production and organic contaminant degradation.Acknowledgements
The authors are very much thankful to the SOA university management for their support and encouragement.References
- S. Yan, Z. Li and Z. Zou, Langmuir, 2009, 25, 10397 CrossRef CAS PubMed.
- Y. Wang, J. Zhang, X. Wang, M. Antonietti and H. Li, Angew. Chem., Int. Ed., 2010, 49, 3356 CrossRef CAS PubMed.
- H. Yan, Chem. Commun., 2012, 48, 3430 RSC.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Adv. Mater., 2015, 27, 2150 CrossRef CAS PubMed.
- S. Nayak, L. Mohapatra and K. M. Parida, J. Mater. Chem. A, 2015, 3, 18622 CAS.
- J. J. Zhu, Y. C. Wei, W. K. Chen, Z. Zhao and A. Thomas, Chem. Commun., 2010, 46, 6965 RSC.
- M. Zhang, W. Jiang, D. Liu, J. Wang, Y. Liu and Y. Zhu, Appl. Catal., B, 2016, 183, 263 CrossRef CAS.
- Y. Chen, B. Lin, H. Wang, Y. Yang, H. Zhu and W. Yu, Chem. Eng., 2016, 286, 339 CrossRef CAS.
- L. Pi, R. Jiang, W. Zhou, H. Zhu, W. Xiao and D. Wang, Appl. Surf. Sci., 2015, 358, 231 CrossRef CAS.
- M. G. Walter, E. L. Warren, J. R. McK, S. W. Boettcher, Q. X. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446 CrossRef CAS PubMed.
- S. Martha, A. Nasim and K. M. Parida, J. Mater. Chem. A, 2013, 1, 7816 CAS.
- Y. Zheng, J. Liu, J. Liang, M. Jaroniec and S. Z. Qiao, Energy Environ. Sci., 2012, 5, 6717 CAS.
- Y. Wang, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68 CrossRef CAS PubMed.
- F. E. Osterloh, J. Phys. Chem. Lett., 2014, 5, 2510 CrossRef CAS PubMed.
- X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680 CrossRef CAS PubMed.
- Y. Wang, X. Wang, M. Antonietti and Y. Zhang, ChemSusChem, 2010, 3, 435 CrossRef CAS PubMed.
- X. Chen, J. Zhang, X. Fu, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 11658 CrossRef CAS PubMed.
- G. Liu, P. Niu, C. Sun, S. C. Smith, Z. Chen, G. Q. Lu and H.-M. Cheng, J. Am. Chem. Soc., 2010, 132, 11642 CrossRef CAS PubMed.
- J. Zhang, G. Zhang, X. Chen, S. Lin, L. Möhlmann, G. Dołega, G. Lipner, M. Antonietti, S. Blechert and X. Wang, Angew. Chem., Int. Ed., 2012, 124, 3237 CrossRef.
- D. P. Sahoo, D. Rath, B. Nanda and K. M. Parida, RSC Adv., 2015, 5, 83707 RSC.
- S. Martha, P. C. Sahoo and K. M. Parida, RSC Adv., 2015, 5, 61535 RSC.
- S. Acharya, S. Martha, P. C. Sahoo and K. M. Parida, Inorg. Chem. Front., 2015, 2, 807 RSC.
- S. Ye, R. Wang, M. Z. Wu and Y. P. Yuan, Appl. Surf. Sci., 2015, 358, 15 CrossRef CAS.
- L. Shi, L. Liang, J. Ma, F. Wanga and J. Sun, Dalton Trans., 2014, 4, 7236 RSC.
- S. Yang, X. Feng, X. Wang and K. Mullen, Angew. Chem., Int. Ed., 2011, 50, 5339 CrossRef CAS PubMed.
- R. Liu, D. Wu, X. Feng and K. Mullen, Angew. Chem., Int. Ed., 2010, 49, 2565 CrossRef CAS PubMed.
- J. Wen, X. Li, H. Li, S. Ma, K. He, Y. Xu, Y. Fang and W. Liu, Appl. Surf. Sci., 2015, 358, 204 CrossRef CAS.
- J. Zhang and F. Huang, Appl. Surf. Sci., 2015, 358, 287 CrossRef CAS.
- S. Fang, Y. Xia, K. Lv, Q. Li, J. Sun and M. Li, Appl. Catal., B, 2016, 185, 225 CrossRef CAS.
- A. Suryawanshi, P. Dhanasekaran, D. Mhamane, S. Kelkar, S. Patil, N. S. Gupta and S. Ogale, Int. J. Hydrogen Energy, 2012, 37, 9584 CrossRef CAS.
- Y. Chen, J. Li, Z. Hong, B. Shen, B. Lin and B. Gao, Phys. Chem. Chem. Phys., 2014, 16, 8106 RSC.
- Y. Xu, H. Xu, L. Wang, J. Yan, H. Li, Y. Song, L. Huang and G. Cai, Dalton Trans., 2013, 42, 7604 RSC.
- J. Ming, Y. Q. Wu, Y. C. Yu and F. Y. Zhao, Chem. Commun., 2011, 47, 5223 RSC.
- Q. F. Liu, W. C. Ren, Z. G. Chen, B. L. Liu, B. Yu, F. Li, H. T. Cong and H. M. Cheng, Carbon, 2008, 46, 1417 CrossRef CAS.
- H. Q. Wu, Q. Y. Wang, Y. Z. Yao, C. Qian, X. J. Zhang and X. W. Wei, J. Phys. Chem. C, 2008, 112, 16779 CAS.
- K. Dai, T. Y. Peng, D. N. Ke and B. Q. Wei, Nanotechnology, 2009, 20, 125603 CrossRef PubMed.
- L. Ge and C. C. Han, Appl. Catal., B, 2012, 268, 117 Search PubMed.
- B. Chai, X. Liao, F. Song and H. Zhou, Dalton Trans., 2014, 43, 982 RSC.
- X. Bai, L. Wang, Y. Wang, W. Yao and Y. Zhu, Appl. Catal., B, 2014, 152, 262 CrossRef.
- H. Wang, M. Xie, L. Thia, A. Fisher and X. Wang, J. Phys. Chem. Lett., 2014, 5, 119 CrossRef CAS PubMed.
- S. M. Unni, R. Illathvalappil, P. K. Gangadharan, S. N. Bhange and S. Kurungot, Chem. Commun., 2014, 50, 13769 RSC.
- B. Yuan, J. Wei, T. Hu, H. Yao, Z. Jiang and Z. Fang, Chin. J. Catal., 2015, 36, 1009 CrossRef CAS.
- W. J. Ong, L. L. Tan, S. P. Chai, S. T. Yong and A. R. Mohamed, Nano Energy, 2015, 13, 757 CrossRef CAS.
- Q. Yu, S. Guo, X. Li and M. Zhang, Mater. Technol.: Adv. Perform. Mater., 2014, 29, 172 CAS.
- D. Li, M. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101 CrossRef CAS PubMed.
- Y. Li, Y. Sun, F. Dong and W. K. Ho, J. Colloid Interface Sci., 2014, 436, 29 CrossRef CAS PubMed.
- Y. Q. Sun, C. Li, Y. X. Xu, H. Bai, Z. Y. Yao and G. Q. Shi, Chem. Commun., 2010, 46, 4740 RSC.
- X. Wang, L. Wang, F. Zhao, C. Hu, Y. Zhao, Z. Zhang, S. Chen, G. Shib and Q. Liangti, Nanoscale, 2015, 7, 3035 RSC.
- J. Yin, G. Liao, D. Zhu, P. Lu and L. Li, J. Photochem. Photobiol., A, 2016, 315, 138 CrossRef CAS.
- J. Q. Tian, R. Ning, Q. Liu, A. M. Asiri, A. O. Al-Youbi and X. P. Sun, ACS Appl. Mater. Interfaces, 2014, 6, 1011 CAS.
- C. G. Hu, L. Song and Z. P. Zhang, Energy Environ. Sci., 2015, 8, 31 CAS.
- H. Gu, T. Zhou and G. Shi, Talanta, 2015, 132, 871 CrossRef CAS PubMed.
- Q. Xiang, J. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355 CAS.
- W. K. Jo and N. C. S. Selvam, J. Hazard. Mater., 2015, 299, 462 CrossRef CAS PubMed.
- Q. Liu, Y. Guo, Z. Chen, Z. Zhang and X. Fang, Appl. Catal., B, 2016, 183, 231 CrossRef CAS.
- F. He, G. Chen, Y. Yu, Y. Zhou, Y. Zheng and S. Hao, Chem. Commun., 2015, 51, 6824 RSC.
- L. Ge, C. Hanb and J. Liub, J. Mater. Chem., 2012, 22, 11843 RSC.
- Y. Zhang, Z. Schnepp, J. Cao, S. Ouyang, Y. Li, J. Ye and S. Liu, Sci. Rep., 2013, 3, 2163 Search PubMed.
- X. Bai, C. Sun, S. Wu and Y. Zhu, J. Mater. Chem. A, 2015, 3, 2741 CAS.
- M. Zhang, W. Yao, Y. Lv, X. Bai, Y. Liu, W. Jiang and Y. Zhu, J. Mater. Chem. A, 2014, 2, 11432 CAS.
| This journal is © the Partner Organisations 2016 |