Mechanochromic Cu(I) boron imidazolate frameworks with low-dimensional structures and reducing function†
De-Xiang
Zhang
ab,
Hai-Xia
Zhang
b,
Tian
Wen
*b,
Dong-Sheng
Li
a and
Jian
Zhang
*ab
aCollege of Materials & Chemical Engineering, China Three Gorges University, Yichang 443002, P. R. China
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Mater, Chinese Academy of Science, 350002, Fuzhou, P. R. China. E-mail: twen@fjirsm.ac.cn; zhj@fjirsm.ac.cn; Fax: +86-591-83715030; Tel: +86-591-83715030
First published on 2nd December 2015
Abstract
Two low-dimensional Cu(I) boron imidazolate frameworks (BIFs), [CuBH(bim)3]n (BIF-40; bim = benzimidazolate) and [CuBH(im)3]n (BIF-6, im = imidazolate), have been synthesized and both of them display unique mechanochromism. In addition, BIF-40 showed reducing activity due to the rich B–H bonds in the structure, and it was employed to load AuPd bimetal nanoparticles through a one-step process for further catalysis. The corresponding mechanism involving a grinding process between chains or layers is discussed in detail. This work not only provides compelling evidence for designing and applying coordination polymers as mechanochromic materials, but also develops an eco-friendly strategy for loading bimetallic NPs into BIFs.
Introduction
Porous coordination polymers (PCPs) have been popular because of their unique structures and potential applications in gas storage, photoluminescence, catalysis etc.1–3 Simple and effective design of PCPs with practical properties is still a great challenge. Recently, many catalytic reactions were realized by employing PCPs with loaded nanoparticles (NPs) as effective catalysts.4,5 In addition, PCPs may be easily changed in response to an external stimulus.6 However, PCPs with unusual luminescence mechanochromism have rarely been reported,7a,b though several discrete metal complexes and Cu(I) coordination polymers (CPs) have been reported to display distinct mechanoluminescence induced by mechanical grinding.8In recent years, our main research interest focused on the synthesis of zeolitic boron imidazolate frameworks (BIFs) by using tetrahedral Cu+/Li+ ions and pre-synthesized boron imidazolate ligands.7c Moreover, we are also interested in low dimensional BIFs, which not only have a close resemblance to CPs but can also serve as reducing agents to load noble metal nanoparticles directly.7b,8j,9,10 As a result, a series of new BIFs with zeolite topologies and unique redox properties were successfully obtained. However, only two multifunctional BIFs with unique luminescence and redox properties have been studied so far. One is BIF-38 with a three-dimensional entangled structure.8j The Cu⋯Cu interactions in BIF-38 played a vital role in the mechanochromism.7 Another is BIF-34 with a ladder-like chain structure,7b where the relative slide among the chains could tune the luminescence change. Inspired by these results, BIFs with 2D structures may be considered to act as potential luminescence tuning materials, because the slide between the layers may also be the force to change the luminescence. As a result, in order to explore more functional, economical, environmentally-friendly luminescent BIFs and enlarge the BIF mechanical-grinding-system, rational design of low dimensional Cu(I)-BIFs should be investigated in-depth.
Herein, we report two low dimensional Cu(I)-BIFs, [CuBH(bim)3]n (BIF-40; bim = benzimidazolate) and [CuBH(im)3]n (BIF-6, im = imidazolate),7c which displayed unique mechanochromism. Moreover, BIF-40 showed reducing activity and was firstly employed to load AuPd bimetal nanoparticles through a one step process for further catalysis.
Experimental
Materials and instrumentation
All reagents were purchased commercially and used without further purification. All syntheses were carried out in a 20 mL vial under autogenous pressure. Diffraction data were collected by using an Oxford SuperNova diffractometer with graphite monochromated Mo Kα radiation (λ = 0.71073 Å) at 293 K. All powder X-ray diffraction (PXRD) analyses were recorded on a Rigaku Dmax2500 diffractometer with Cu Kα radiation (λ = 1.54056 Å) with a step size of 0.05°. Thermal stability studies were carried out on a NETZSCH STA-449C thermo analyzer with a heating rate of 10 °C min−1 under a nitrogen atmosphere. Fluorescence spectra were recorded with a HORIBA Jobin-Yvon FluoroMax-4 spectrometer.Synthesis of [CuBH(bim)3]n (BIF-40)
A mixture of CuI (0.018 g) and KBH(bim)3 (0.027 g) was added in a mixed solvent (2 mL DMF, 1 mL (±)-2-amino-1-ethanol and 1.5 mL cyclohexane and 1.5 ml acetonitrile) and stirred for 30 min. The mixture was sealed in a 20 mL vial and transferred into an oven, which was heated at 100 °C for 72 h and then cooled to room temperature. Pale yellow block crystals were obtained as the main product (71% yield).Synthesis of Au/Pd bimetallic NPs
At room temperature, fresh BIF-40 crystals (110 mg) were immersed in a mixed ethanol solution of HAuCl4 (20 mmol L−1, 4 ml) and HPdCl4 (20 mmol L−1, 4 ml) for 5 hours at room temperature to produce AuPd@BIF-40.Catalytic reduction of 4-nitrophenol
The catalytic reduction of 4-nitrophenol was investigated using a standard quartz cuvette by adding 15 mL of NaBH4 (0.2 M) to 5 mL of 4-nitrophenol (0.101 mM). Then 4.5 mg of sample was added into the solution and time-dependent absorption spectra were recorded with a range of 200 to 550 nm. The pale yellow solution became colorless under continuous stirring. The total reaction progress and UV-vis spectra of the starting material, 4-nitrophenol and the product 4-aminophenol were recorded using a Perkin-Elmer Lambda 950 UV/vis spectrophotometer, exhibiting different absorptions in the UV-visible region.Crystal data for BIF-40
M = 426.75, triclinic, a = 10.4075(8) Å, b = 11.0056(11) Å, c = 18.3138(11) Å, α = 100.009(3)°, β = 105.5440(10)°, γ = 108.045(3)°, V = 1939.7(3) Å3, T = 293 K, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, 7026 reflections measured, 3659 independent reflections (Rint = 0.0243). The final R1 value was 0.0288 (I > 2σ(I)). The final wR(F2) value was 0.0865 (I > 2σ(I)). The final R1 value was 0.0618 (all data). Nref values are 7033. The goodness of fit on F2 was 0.856.
, Z = 2, 7026 reflections measured, 3659 independent reflections (Rint = 0.0243). The final R1 value was 0.0288 (I > 2σ(I)). The final wR(F2) value was 0.0865 (I > 2σ(I)). The final R1 value was 0.0618 (all data). Nref values are 7033. The goodness of fit on F2 was 0.856.
Results and discussion
Pale yellow crystals of BIF-40 were synthesized under solvothermal conditions. Single-crystal X-ray diffraction analysis shows that BIF-40 crystallizes in a triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The phase purity of BIF-40 has been characterized by powder X-ray diffraction (PXRD) (Fig. S2†). The crystalline sample of BIF-40 is insoluble in common organic solvents (e.g. DMF, ethanol and acetonitrile). The thermogravimetric analysis (TGA) displays that there is no clear weight loss before 400 °C (Fig. S3†).
. The phase purity of BIF-40 has been characterized by powder X-ray diffraction (PXRD) (Fig. S2†). The crystalline sample of BIF-40 is insoluble in common organic solvents (e.g. DMF, ethanol and acetonitrile). The thermogravimetric analysis (TGA) displays that there is no clear weight loss before 400 °C (Fig. S3†).
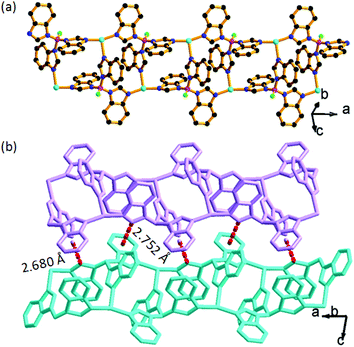
In the structure of BIF-40, each Cu+ center is coordinated by three N atoms from three BH(Bim)3− ligands, displaying a planar trigonal coordination geometry. The BH(bim)3− ligands connect the Cu+ centers into a ladder-like chain (Fig. 1a). These chains are further packed along the b-axis. BIF-40 has a similar ladder-like chain structure to BIF-34,7b but its packing modes are distinctively different. In BIF-40, most of the phenyl rings and imidazolate rings take on the edge-to-face mode, and there are obvious C–H⋯π interactions between the adjacent chains (Fig. 1b). However, no obvious interactions are observed between the adjacent chains in BIF-34. The different packing modes and similar chains suggested that BIF-40 may also exhibit charming mechanochromic properties.
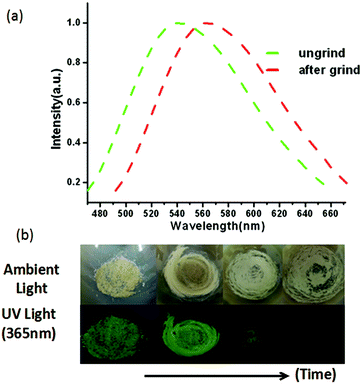
The solid-state photoluminescent properties of BIF-40 were explored at room temperature. Upon the irradiation of ultraviolet light at 360 nm, the emission maximum wavelength for BIF-40 is 548 nm (Fig. 2a). The emission of BIF-40 can be tentatively assigned to the [Cu → π*BH(bim)3−] metal-to-ligand charge transfer (MLCT), which is similar to those observed in some reported Cu(I) compounds.12
In addition, the mechanical-grinding-triggered-luminescence change of BIF-40 was further investigated. The emission spectrum shows that the ground sample of BIF-40 exhibits an obvious red shift about 20 nm (λem = 568 nm, Fig. 2a). This phenomenon indicates that BIF-40 shows excellent behavior of pressure-induced luminescence change. In order to investigate the structure of the ground sample, its PXRD was measured. The result showed that there are only very weak and ambiguous reflections, indicating that the crystal lattice is significantly disrupted and there is a crystal-to-amorphous phase conversion caused by the strong grinding. Such an unusual luminescent red shift change should be related to the structural modification. Since BIF-40 is a double chain structure, the disordered slide between chains, accompanying the alteration of Cu⋯Cu interactions, C–H⋯π interactions, even π⋯π interactions among the chains, may respond to this luminescence change.7 The crystalline sample of BIF-40 was recovered over a month from a mixed chloroform and acetonitrile solvent, which was proved by the PXRD studies (Fig. S2†). These results also demonstrate the inherent self-restoring function of CPs.
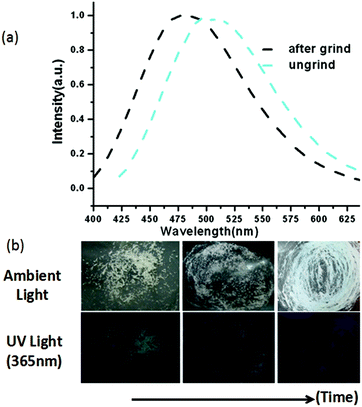
It has been demonstrated that Cu⋯Cu interactions and π–π interactions play key roles in influencing the luminescence properties of Cu(I) complexes.7a,8i In addition, several mechanochromic luminescent metal copper(I) boron imidazolate frameworks have been studied by our group such as the BIF-34 and BIF-38.7b,8j In order to expand the mechanical-grinding-system, 2D Cu(I)-BIFs are considered for grinding as new functional CPs. We anticipate that if there are Cu⋯Cu interactions between adjacent layers in the BIF structures, which should also change upon external force stimuli such as mechanical-grinding. In the BIF families, we notice that BIF-6 has a ground-like layered structure. The Cu–Cu distances (average 3.02 Å) in BIF-6 (Fig. 3) are slightly shorter than those in BIF-38 (average 3.3 Å). Thus, the mechanical-grinding-triggered-luminescence change of BIF-6 was also investigated.
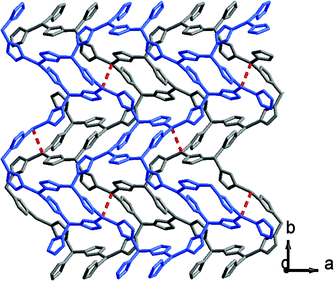 | ||
| Fig. 3 View of the layers in BIF-6 (two independent layers are shown in gray and blue), showing the Cu⋯Cu distance (red dashed line). | ||
When the crystalline sample of BIF-6 was ground gently in an agate mortar with a pestle, the color of BIF-6 under ambient light remained almost unchanged. However, upon irradiation under a UV lamp (365 nm), the ground sample of BIF-6 emitted in green. The ground BIF-36 sample was treated with time, the luminescence of the sample converted into relatively weak blue emission (Fig. 4b). The solid sample of BIF-6 is emissive at room temperature with emission bands centred at 506 nm. The emission spectrum shows that the ground sample of BIF-6 displays an evident blue shift about 26 nm (λem = 480 nm, Fig. 4a). To investigate the structure of the ground sample, its PXRD was measured. The prominent peaks decrease when the BIF-36 sample was treated with time, suggesting the loss of the crystalline form to an amorphous state. The resulting amorphous state by grinding tends to be caused by the disordered sliding between the CuBH(im)3 layers in the structure. Since BIF-6 is a layered structure, the disordered slide between layers, accompanying the alteration of Cu⋯Cu interactions and π⋯π interactions among the layers, may be responsible for this luminescence change. PXRD showed that BIF-6 was recovered in crystalline form from a mixed organic solvent such as chloroform and acetonitrile after a month (Fig. S4†). BIF-6 stands for the first mechanochromic luminescent material with a two dimensional structure in the BIF family. This satisfactory result shows that we have realized luminescence mechanochromism from the 1D to 3D structures of BIFs.
Inspired by our previous studies,7b,8i,j,9–11BIF-40 could act as the reducing agent to load noble metal nanoparticles due to the presence of active B–H bonds from BH(bim)3− ligands. Here, we have obtained AuPd@BIF-40 through a one-step process. Fresh BIF-40 crystals (110 mg) were immersed in a mixed ethanol solution of HAuCl4 (20 mmol L−1, 4 ml) and HPdCl4 (20 mmol L−1, 4 ml) for 5 hours at room temperature to produce AuPd@BIF-40. The color of BIF-40 crystals changed after loading different noble metals, which is related to the surface plasmons of spherical noble metal NPs (Fig. S5†). After the loading, inductively coupled plasma atomic emission spectroscopy (ICP-AES) demonstrated that the weight percentage of Au NPs and Pd NPs in BIF-40 is 1.09% and 0.65%, respectively. The field-emission (FE) TEM image of the resulting AuPd@BIF-40 sample indicated the different size formation of ca. 5 nm, 20 nm and 100 nm AuPd bimetal NPs in the crystals (Fig. 5a–c). The lattice fringe spacing of the bimetal AuPd NPs was measured to be 0.221 nm and 0.235 nm, which match the d value for Pd (111) and Au (111), respectively (Fig. 5d). Moreover, the X-ray photoelectron spectra (XPS) (Fig. S6†), the scanning electron microscopy (SEM) images and the corresponding EDS elemental mapping images of AuPd@BIF-40 (Fig. S7–S9†) as well as the energy-dispersive X-ray (EDX) spectroscopy data indicate that Au(0) (84 eV and 86 eV, 4f7/2 and 4f5/2, respectively) and Pd(0) (340 eV and 335 eV, 3d3/2 and 3d5/2, respectively) coexist in the solid state (Fig. 5e–h). The PXRD patterns further showed that the framework of BIF-40 is retained after the loading of AuPd NPs. These results suggested that the BIF-40 crystals directly loaded Au and Pd NPs without external reducing agents or photochemical reactions.
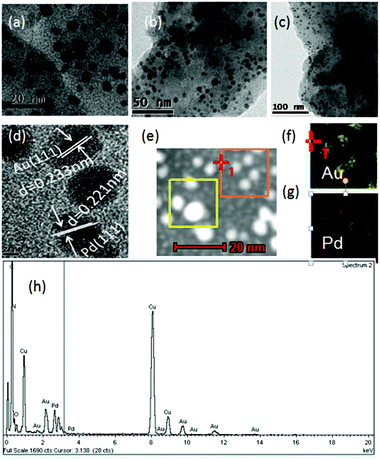 | ||
| Fig. 5 (a–c) Typical TEM images of the prepared AuPd@BIF-40 with different magnifications; (d) HRTEM image of AuPd@BIF-40; (e–g) mapping images of Au and Pd; (h) EDX spectrum of AuPd@BIF-40. | ||
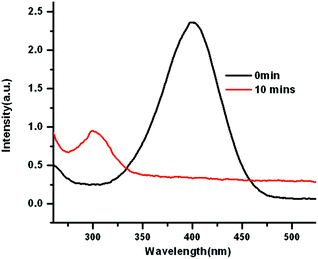
To explore the catalytic activities of AuPd@BIF-40, the reduction of 4-nitrophenol (4-NP) was chosen as a model reaction. The reaction was monitored by UV-vis absorption spectroscopy. The absorption of 4-NP at 400 nm significantly decreases along with a concomitant increase of the ∼300 nm peak of 4-aminophenol (4-AP) (Fig. 6). In order to study the reusability, three recycles of the catalytic reaction were tested for the same AuPd@BIF-40 catalyst. The catalyst exhibits similar catalytic performance without obvious reduction in the conversion for the same reaction time (Fig. S10†), showing the stability of the catalysts. Moreover, TEM measurements of the catalysts indicate that the size of the AuPd NPs almost remained the same after the reaction, further suggesting good stability and long life (Fig. S11†).
Conclusions
In summary, a new BIF-40 was successfully synthesized and characterized, which showed interesting mechanochromic and reducing functions. As a result, BIF-40 will be an excellent candidate for functional luminescence materials and a potential catalyst reducing 4-nitrophenol functions. In addition, BIF-6 with a layered structure exhibiting unusual luminescence mechanochromism was firstly explored, which enlarged the mechanochromic research field. We have realized the luminescence mechanochromism from 1D to 3D structures in the BIF family. The excellent results not only show that the mechanochromic properties are related to the weak interactions of cuprophilicity and C–H⋯π interactions, but also provide convincing examples for designing and applying CPs as mechanochromic materials.Acknowledgements
We acknowledge the support of this work by the 973 program (2012CB821705), NSFC (21221001, 21203196, 21425102) and CAS (XDA07070200).Notes and references
- (a) T. A. Makal, J. R. Li, W. Lu and H. C. Zhou, Chem. Soc. Rev., 2012, 41, 7761–7779 RSC; (b) H. Wu, Q. Gong, H. Olson D and J. Li, Chem. Rev., 2012, 112, 836–868 CrossRef CAS PubMed; (c) Z. Zhang, Z. Z. Yao, S. Xiang and B. Chen, Energy Environ. Sci., 2014, 7, 2868–2899 RSC; (d) C. Wang, D. Liu and W. B. Lin, J. Am. Chem. Soc., 2013, 135, 13222–13234 CrossRef CAS PubMed.
- (a) Z. C. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC; (b) J. Lin, Q. Zhang, L. Wang, X. Liu, W. Yan, T. Wu, X. Bu and P. Feng, J. Am. Chem. Soc., 2014, 136, 4769–4779 CrossRef CAS PubMed; (c) J. Lin, Y. Dong, Q. Zhang, D. Hu, N. Li, L. Wang, Y. Liu and T. Wu, Angew. Chem., Int. Ed., 2015, 54, 5103–5107 CrossRef CAS PubMed; (d) Y. J. Cui, Y. F. Yue, G. D. Qian and B. L. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS PubMed.
- (a) J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C. Y. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC; (b) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS PubMed.
- Q. L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC.
- (a) H. L. Jiang, T. Akita, T. Ishida, M. Haruta and Q. Xu, J. Am. Chem. Soc., 2011, 133, 1304–1306 CrossRef CAS PubMed; (b) Y. Z. Chen, Q. Xu, S. H. Yu and H. L. Jiang, Small, 2015, 11, 71–77 CrossRef CAS PubMed.
- J. M. Falkowski, C. Wang, S. Liu and W. B. Lin, Angew. Chem., Int. Ed., 2011, 50, 8674–8678 CrossRef CAS PubMed.
- (a) T. Wen, X. P. Zhou, D. X. Zhang and D. Li, Chem. – Eur. J., 2014, 20, 644–648 CrossRef CAS PubMed; (b) T. Wen, D. X. Zhang, J. Liu, H. X. Zhang and J. Zhang, Chem. Commun., 2015, 51, 1353–1355 RSC; (c) J. Zhang, T. Wu, C. Zhou, S. Chen, P. Feng and X. Bu, Angew. Chem., Int. Ed., 2009, 48, 2542–2545 CrossRef CAS PubMed.
- (a) T. Seki, K. Sakurada and H. Ito, Angew. Chem., Int. Ed., 2013, 52, 12828–12832 CrossRef CAS PubMed; (b) T. Lasanta, M. E. Olmos, A. Laguna, J. M. López-de-Luzuriaga and P. Naumov, J. Am. Chem. Soc., 2011, 133, 16358–16361 CrossRef CAS PubMed; (c) V. N. Kozhevnikov, B. Donnio and D. W. Bruce, Angew. Chem., Int. Ed., 2008, 47, 6286–6289 CrossRef CAS PubMed; (d) S. Perruchas, X. F. Le Goff, S. Maron, I. Maurin, F. Guillen, A. Garcia, T. Gacoin and J. P. Boilot, J. Am. Chem. Soc., 2010, 132, 10967–10969 CrossRef CAS PubMed; (e) Q. Benito, X. F. Le Goff, S. Maron, A. Fargues, A. Garcia, C. Martineau, F. Taulelle, S. Kahlal, T. Gacoin, J. P. Boilot and S. Perruchas, J. Am. Chem. Soc., 2014, 136, 11311–11320 CrossRef CAS PubMed; (f) X. Zhang, J. Y. Wang, J. Ni, L. Y. Zhang and Z. N. Chen, Inorg. Chem., 2012, 51, 5569–5579 CrossRef CAS PubMed; (g) J. Ni, X. Zhang, Y. H. Wu, L. Y. Zhang and Z. N. Chen, Chem. – Eur. J., 2011, 17, 1171–1183 CrossRef CAS PubMed; (h) J. Ni, X. Zhang, N. Qiu, Y. H. Wu, L. Y. Zhang, J. Zhang and Z. N. Chen, Inorg. Chem., 2011, 50, 9090–9096 CrossRef CAS PubMed; (i) T. Wen, D. X. Zhang, J. Liu, R. Lin and J. Zhang, Chem. Commun., 2013, 49, 5660–5662 RSC; (j) T. Wen, D. X. Zhang, H. X. Zhang, H. B. Zhang, J. Zhang and D. S. Li, Chem. Commun., 2014, 50, 8754–8756 RSC.
- D. X. Zhang, H. X. Zhang, H. Y. Li, T. Wen and J. Zhang, Cryst. Growth Des., 2015, 15, 2433–2436 CAS.
- D. X. Zhang, H. X. Zhang, T. Wen and J. Zhang, Dalton Trans., 2015, 44, 9367–9369 RSC.
- H. X. Zhang, M. Liu, X. Bu and J. Zhang, Sci. Rep., 2014, 4, 3923 Search PubMed.
- V. W. W. Yam and K. K. W. Lo, Chem. Soc. Rev., 1999, 28, 323–334 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures, TGA, powder X-ray diffraction patterns and CIF file. CCDC 1057287. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qi00155b |
| This journal is © the Partner Organisations 2016 |