Open Access Article
Open Access ArticleAllylboration as a versatile tool for the in situ post-polymerization functionalization of 1,4-cis-poly(butadiene)†
Hannes
Leicht
,
Steffen
Huber
,
Inigo
Göttker-Schnetmann
and
Stefan
Mecking
 *
*
Chair of Chemical Materials Science, Department of Chemistry, University of Konstanz, 78464 Konstanz, Germany. E-mail: stefan.mecking@uni-konstanz.de
First published on 23rd September 2016
Abstract
Allylboration is a versatile tool for the post-polymerization functionalization of poly(butadiene-co-[4,4,5,5-tetramethyl-2-(3-methyl-1,3-butadienyl)-1,3,2-dioxaborolane]). Polar functionalized aldehydes HC(O)C6H4(CH2)R (R = Br, NR2, PPh3, P(O)(OEt)2) react readily with the allyl boronic acid ester groups in the copolymer without interfering with the reactive double bonds in the polymer backbone. This provides access to stereoregular poly(butadiene) functionalized with a broad range of polar groups. Functionalization proceeds under polymerization conditions and therefore does not require a prior polymer work-up.
The synthesis of copolymers based on polar and nonpolar olefins via insertion polymerization is a challenging goal of polymer chemistry. Fundamental progress has been made recently in the polymerization of ethylene with polar vinyl monomers.1–4 The functionalization of poly(dienes) via direct copolymerization with polar monomers, however, is almost exclusively accomplished by free-radical or anionic methods.5–8 Polar functionalized poly(dienes) thus obtained can possess superior interactions with typical filler materials in tire applications such as silica or carbon black.9,10 However, the lack of microstructure control in these free-radical or anionic polymerizations is a major drawback because the properties of the poly(dienes), and hence their applicability, are strongly dependent on their polymer microstructure. Although progress has been made very recently towards copolymerizing 1,3-butadiene (BD) and isoprene (IP) with a broad variety of polar functionalized dienes to stereoregular copolymers, there are still functionalities that withdraw themselves from direct copolymerization.9,11 Post-polymerization functionalization is widely applied in poly(diene) chemistry. Vulcanization in the rubber industry is a prominent example. The reactivity of sulfur compounds with double bonds was also applied in the functionalization of 1,2-poly(butadiene) and poly(isoprene-co-3-methylenehepta-1,6-diene) via the thiol–ene reaction.10,12–14 Although the functionalizations were successful, they still require additional activation, namely UV-irradiation and/or the addition of radical initiators. However, this approach cannot be utilized for high 1,4-cis poly(butadiene) (PBD) as cross-linking occurs under the functionalization conditions.13 A mild method for a versatile post-polymerization functionalization of 1,4-cis-poly(butadiene) with a reactivity approach orthogonal to the backbone's double bonds is highly desirable.
For this purpose we explored the allylboration reaction of allyl boronic acid ester groups (Scheme 1) in poly(butadiene-co-[4,4,5,5-tetramethyl-2-(3-methyl-1,3-butadienyl)-1,3,2-dioxaborolane]) for post-polymerization reactions to introduce functional groups. High 1,4-cis-poly(butadiene-co-[4,4,5,5-tetramethyl-2-(3-methyl-1,3-butadienyl)-1,3,2-dioxaborolane]) was synthesized straightforwardly via direct insertion copolymerization of BD and 4,4,5,5-tetramethyl-2-(3-methyl-1,3-butadienyl)-1,3,2-dioxaborolane catalyzed by the cationic nickel complex [(allyl)Ni(mesitylene)](BArF4) Ni-1.11 4,4,5,5-Tetramethyl-2-(3-methyl-1,3-butadienyl)-1,3,2-dioxaborolane was chosen as a comonomer, because it is easily accessible through hydroboration of 2-methylbut-1-en-3-yne in excellent yields and high purity. Additionally, it readily generates the desired allyl boronic acid ester group in the polymer backbone upon insertion (Scheme 1).
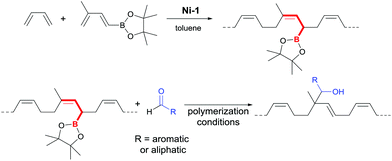 | ||
| Scheme 1 Formation of an allyl boronic acid ester group in the copolymer employed and conversion in an allylboration reaction. | ||
Initial experiments with an allylboronic acid pinacol ester as a small molecule model compound were designed to investigate the behavior of the reaction under conditions comparable to those of the desired application. All model reactions with the allylboronic acid pinacol ester and different aldehydes (pentanal, benzaldehyde, p-NO2-benzaldehyde, p-dimethylaminobenzaldehyde, and 4-(1-pyrrolidinyl)benzaldehyde) showed the allylboration reaction to be a potentially robust and easy method for the introduction of functional groups into the backbone of poly(dienes). The expected products were formed in good (e.g. 73% with p-dimethylaminobenzaldehyde) to high yields (e.g. 89% with p-NO2-benzaldehyde) as observed by means of 1H NMR.
To assess the general reactivity of aldehydes towards the allyl boronic acid ester groups in the polymer backbone, 500 mg copolymer were reacted with 10 equiv. benzaldehyde (60 °C, 2–3 days in 5 mL toluene). The excess of benzaldehyde was removed subsequent to the reaction by precipitation of the polymer in methanol. After drying under reduced pressure, the polymer was characterized comprehensively by NMR spectroscopy.
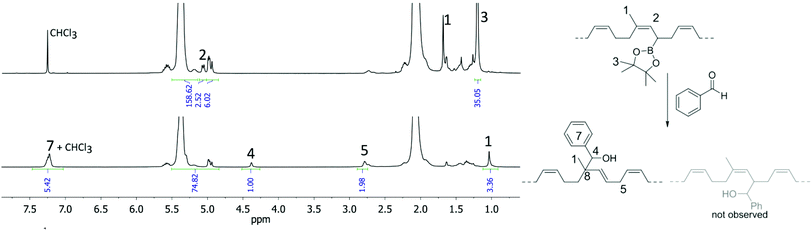
The full conversion of the allylboronic acid pinacol ester groups in the copolymer to the desired secondary alcohol is evident by comparing key signals in the copolymer before and after the allylboration reaction (Fig. 1). While the signals for the vinylic CH3 group 1, the olefinic proton 2, and the CH3 groups of the pinacol ester 3 disappear, a set of new key resonances can be found in the product. These key resonances include the OH-substituted CH group 4 with a distinctive shift in 1H (4.38 ppm) as well as 13C NMR (80.8 ppm). In addition, a signal for the newly formed biallylic CH2 group 5 resonates at δ = 2.79 ppm (13C: 30.8 ppm), the peak of the CH3 group 1 shifts from 1.69 ppm to 1.04 ppm, and the signals of the aromatic protons of the aryl ring 7 appear between 7.15 and 7.32 ppm. These observations and the chemical shift of the quaternary carbon atom 8 (44.5 ppm) prove the nucleophilic substitution to proceed highly selectively in an SN2′ fashion. Signals indicating the formation of the product formed in the substitution following an SN2 mechanism or other side-reactions were not observed.
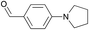
To enlarge the scope of this functionalization approach, further different aldehydes were reacted with the copolymer: the full conversion of the allylboronic acid pinacol ester groups of the copolymer with pentanal under otherwise identical conditions showed that the reactivity is not limited to aromatic aldehydes and also that alkyl aldehydes are suitable reagents for functionalization of diene copolymers via an allylboration reaction. Quantitative conversions are also observed when commercially available, N-functionalized, aromatic aldehydes are used. Both, 4-(1-pyrrolidinyl)benzaldehyde and p-dimethylaminobenzaldehyde were individually reacted for two days with the copolymer under otherwise identical conditions as the reaction with benzaldehyde. NMR analyses after repeated precipitation of the polymers showed in both cases additional signals compared to the polymer functionalized with benzaldehyde: The polymer functionalized with 4-(1-pyrrolidinyl)benzaldehyde exhibits both 1H and 13C signals for the pyrrolidine moiety at δ = 3.28 ppm (47.7 ppm) and 2.00 ppm (25.6 ppm). In the case of p-dimethylaminobenzaldehyde, both methyl groups resonate at 2.94 ppm as a key signal in the proton NMR spectrum.
The aforementioned studies were all conducted with a separately synthesized copolymer. However, functionalization is desirable without an additional step (i.e. work-up of the polymer) directly after the copolymerization of butadiene with 4,4,5,5-tetramethyl-2-(3-methyl-1,3-butadienyl)-1,3,2-dioxaborolane. For this purpose, we ran two copolymerizations under standard polymerization conditions.15 At the end of both polymerizations, benzaldehyde (42 equiv. to comonomer) or 4-(1-pyrrolidinyl)benzaldehyde (10 equiv. to comonomer) was added. Both reaction mixtures were then stirred at 50 °C and the conversion was followed by 1H NMR taking aliquots. The reaction with benzaldehyde showed a degree of functionalization of ca. 50% after 48 min and complete functionalization after two hours. The reaction using 4-(1-pyrrolidinyl)benzaldehyde, however, proceeded significantly slower. After 2.5 h only 25% of the allylboronic acid ester groups in the copolymer were converted. A conversion of 50% was reached after 28 h and full functionalization was reached after two days. This difference in the reaction rates is possibly due to the different amounts of aldehyde compared to that of the comonomer, or due to a deactivating influence of the amine group on the para-position to the aldehyde moiety.
This outcome prompted us to engage in the synthesis of aromatic aldehydes with a methylene group as a spacer between the aromatic ring and the functional group.
Additionally, this approach should generate a platform to synthesize aromatic aldehydes with different functional groups.
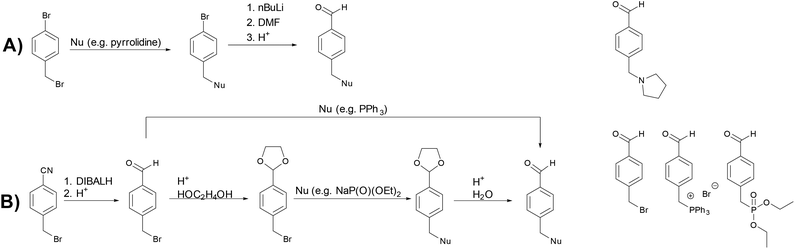
Syntheses of differently functionalized aromatic aldehydes were accomplished via two different routes (Scheme 2). Route (A) encompasses the functionalization of 1-bromo-4-(bromomethyl)benzene with a nucleophile (e.g. pyrrolidine) followed by conversion to the desired aromatic aldehyde by reaction with n-BuLi and DMF followed by an acidic aqueous work-up. Both steps give the desired product in high yields and purity, making purification steps like distillation or column chromatography unnecessary. However, not all functional groups that can be introduced this way are stable towards the conditions in the second step. Therefore, we also used a second route to synthesize functionalized aromatic aldehydes. Route (B) starts with the synthesis of 4-(bromomethyl)benzaldehyde which can be directly reacted with a nucleophile (e.g. PPh3). If the nucleophile is reactive towards the aldehyde group (e.g. NaP(O)(OEt)2), the application of well-known protecting group chemistry enables the successful synthesis of the desired product. Except for the first step, the synthesis of 4-(bromomethyl)benzaldehyde, no further purification was necessary for the obtained products.
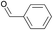
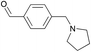
To gain more information about the reaction's actual progress over time influenced by the para-substituents, we followed the reaction of the copolymer with benzaldehyde, 4-(1-pyrrolidinyl)benzaldehyde, and 4-(pyrrolidinylmethyl)benzaldehyde by 1H NMR (12 equiv. of aldehyde to comonomer units, 60 °C, Table 1 entries 1–3). The reactivity differences of the compared aldehydes are significant: The conversion with benzaldehyde reached 60% after 10 min and full conversion after 75 min (Table 1, entry 1). In comparison, the reaction with 4-(1-pyrrolidinyl)benzaldehyde is much slower as 38% conversion is reached after 235 min and full conversion requires heating overnight (Table 1, entry 2).
| Entry | Aldehyde | Equiv.a | Conversion of the polymers’ functn. groups (time)b |
|---|---|---|---|
| Reaction conditions: 65 mg polymer, 0.6 mL CDCl3, 60 °C.a Equivalents of aldehyde compared to functional groups present in the polymer.b Conversion of the functional groups in the polymer, determined from 1H NMR spectra.c Additional 0.5 equiv. of aldehyde were added after 22.5 h, full conversion was observed after additional 2.3 h. | |||
| 1 |
|
12 | 99% (75 min) |
| 2 |
|
12 | 38% (235 min) |
| 99% (21 h) | |||
| 3 |
|
12 | 99% (95 min) |
| 1 | 99% (18.5 h) | ||
| 4 |
|
1 | 99% (18.75 h) |
| 5 |
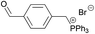
|
1 | 99% (20.3 h) |
| 6 |
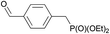
|
0.7 | 70% (22.5 h) |
| +0.5c | 99% (+2.3 h) | ||
The reaction with 4-(pyrrolidinylmethyl)benzaldehyde is again significantly faster and proceeds with rates comparable to those observed for the allylboration reaction using benzaldehyde (full conversion after 95 min, Table 1, entry 3).
The high reactivities of the synthesized functionalized aromatic aldehydes and the clean and selective formation of the desired target structure allows the use of equimolar or even substoichiometric amounts of aldehyde (Table 1, entries 3–6). Although longer reaction times are required for equimolar reactions, a waste of reagents is avoided. Allylboration reactions with all synthesized aromatic aldehydes were successfully performed and the obtained functionalized polymers were scrutinized by NMR-spectroscopy to prove the complete functionalization of 1,4-cis-poly(butadiene) (for NMR spectra and assignments cf. the ESI†).
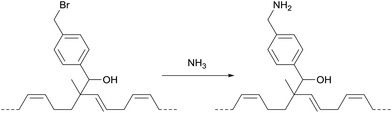
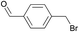
The polymer obtained with 4-(bromomethyl)benzaldehyde (entry 4) can be functionalized further by virtue of the electrophilic nature of the bromobenzyl-moiety. Notably, this allowed the introduction of primary amine groups by reaction with ammonia (Scheme 3; for details see the ESI†).
In conclusion, we have shown that the allylboration reaction is a versatile and robust tool for the post-polymerization functionalization of 1,4-cis-poly(butadiene). Allyl boronic acid ester groups in poly(butadiene-co-[4,4,5,5-tetramethyl-2-(3-methyl-1,3-butadienyl)-1,3,2-dioxaborolane]) react readily with para-functionalized aromatic aldehydes to give access to functionalized high 1,4-cis-poly(butadienes) that are not accessible via a direct copolymerization approach. The allylboration reaction can be performed directly after polymerization without the need for a prior work-up of the polymer and proceeds quantitatively in an SN2′ fashion without the occurrence of side-reactions.
Acknowledgements
We thank Karen Burke, Maggie Flook, and Stephan Rodewald for fruitful discussions. The Goodyear Tire & Rubber Company is gratefully acknowledged for financial support.Notes and references
- E. Y. X. Chen, Chem. Rev., 2009, 109, 5157–5214 CrossRef CAS PubMed.
- E. Drent, R. van Dijk, R. van Ginkel, B. van Oort and R. I. Pugh, Chem. Commun., 2002, 744–745 RSC.
- L. K. Johnson, S. Mecking and M. Brookhart, J. Am. Chem. Soc., 1996, 118, 267–268 CrossRef CAS.
- A. Nakamura, S. Ito and K. Nozaki, Chem. Rev., 2009, 109, 5215–5244 CrossRef CAS PubMed.
- V. V. Sheares, L. Wu, Y. Li and T. K. Emmick, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 4070–4080 CrossRef CAS.
- K. Sunada, K. Takenaka and T. Shiomi, J. Appl. Polym. Sci., 2005, 97, 1545–1552 CrossRef CAS.
- Y. Yang, J. Lee, M. Cho and V. V. Sheares, Macromolecules, 2006, 39, 8625–8631 CrossRef CAS.
- Y. Yang and V. V. Sheares, Polymer, 2007, 48, 105–109 CrossRef CAS.
- C. Yao, N. Liu, S. Long, C. Wu and D. Cui, Polym. Chem., 2016, 7, 1264–1270 RSC and references cited therein.
- L. Li, S. H. Li and D. M. Cui, Macromolecules, 2016, 49, 1242–1251 CrossRef CAS and references cited therein.
- H. Leicht, I. Göttker-Schnetmann and S. Mecking, ACS Macro Lett., 2016, 5, 777–780 CrossRef CAS.
- R. L. A. David and J. A. Kornfield, Macromolecules, 2008, 41, 1151–1161 CrossRef CAS.
- J. Justynska, Z. Hordyjewicz and H. Schlaad, Polymer, 2005, 46, 12057–12064 CrossRef CAS.
- N. Ten Brummelhuis, C. Diehl and H. Schlaad, Macromolecules, 2008, 41, 9946–9947 CrossRef CAS.
- 20 μmol Ni-1, 20 mL toluene, 1.05 bar BD, 0.7–0.8 mmol comonomer, 25 °C, 30 min.
Footnote |
| † Electronic supplementary information (ESI) available: Selected NMR-spectra and synthetic procedures. See DOI: 10.1039/c6py01388k |
| This journal is © The Royal Society of Chemistry 2016 |