Synthesis of acid-degradable hyperbranched polymers by chain-growth CuAAC polymerization of an AB3 monomer†
Abstract
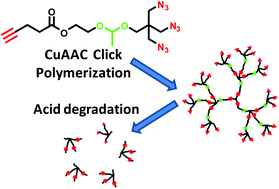
A tetrafunctional AB3 monomer that was composed of one alkynyl group, three azido groups and one acetal linker was used in the one-pot copper-catalyzed azide–alkyne cycloaddition (CuAAC) polymerization for producing acid-degradable hyperbranched polymers (HBPs). In various feed ratios of the AB3 monomer to a B3 core, the polymerizations demonstrated a chain-growth mechanism with a linear increase of molecular weight versus conversion, low polydispersity and a high degree of branching (DB). The large amount of terminal azido groups on the HBPs periphery were further modified via reaction with an alkynyl-terminated poly(ethylene glycol) (PEG) to produce water-soluble PEGylated HBPs. Under acidic conditions, both the HBPs and the PEGylated HBPs exhibited clean and fast degradation into low-molecular weight compounds, confirming the labile acetal linkers in the backbone of HBPs.

 Please wait while we load your content...
Please wait while we load your content...