Photocatalytic degradation of humic acid using a novel photocatalyst: Ce-doped ZnO†
N. C.
Birben
*a,
M. C.
Paganini
b,
P.
Calza
b and
M.
Bekbolet
a
aBogazici University, Institute of Environmental Sciences, 34342, Bebek Istanbul, Turkey. E-mail: cemre.birben@boun.edu.tr; Tel: +90 212 359 7145
bUniversity of Turin, Department of Chemistry, Via Giuria 7, 10125, Turin, Italy
First published on 22nd August 2016
Abstract
This study aimed at investigating the photocatalytic degradation of humic acid (HA) as a representative of natural organic matter (NOM) by using Ce-doped ZnO as a novel material. Following photocatalysis, HA degradation was characterized by specified UV-vis and fluorescence spectroscopic parameters as well as by the dissolved organic carbon (NPOC) content. Excitation–Emission Matrix (EEM) fluorescence features were also evaluated by using advanced techniques. Comparison of Ce-doped ZnO photocatalysis to TiO2 P-25 photocatalytic treatment of the HA samples was elucidated under similar experimental conditions. Kinetic modeling of the photocatalytic removal of HA expressed promising results indicating that Ce-doped ZnO could serve as an efficient catalyst for the degradation of NOM.
Introduction
A big effort has been done to orient scientific research towards the exploitation of solar light in various ways. This has occurred also within the catalytic community, which is currently more and more oriented towards studies directed to exploit light energy in various kinds of applications.1 Among photochemical applications in catalysis, one has to mention quite mature approaches like photocatalytic reactions for pollutant abatement.2 The basic component of a photocatalytic system is a semiconductor capable of generating a phenomenon of charge separation by absorbing light. The electron (excited in the conduction band, CB) and the hole (consequently formed in the valence band, VB) are potentially the agents of a reduction and of an oxidation reaction respectively.3 To achieve high performances of the whole photocatalytic system it is necessary to develop semiconductors having excellent electronic properties and, in parallel, co-catalysts having high efficiency. Recent interest has been directed to the use of novel photocatalysts as well as to the development of third generation photocatalysts (TGP) that are active both in the UV and in the visible light region. The third generation of photocatalysts tries to go beyond titanium dioxide and was initially a prediction proposed by Serpone and Emeline.4,5 This was based on the idea of a wide band gap semiconductor containing extra electronic levels at intermediate energy in the band gap capable of allowing the transition of electrons from the VB to the CB with a double excitation. This was based on the idea that a semiconductor containing extra electronic levels in the wide band gap could allow transition of electrons from VB to CB with a double excitation. These extra electronic levels can be obtained at the interfaces formed between crystallites of different oxides during the synthesis process.6 Though uncommon, the presence of rare earth ions in photocatalytic systems is not totally new. In recent years, for instance, Zaleska and coworkers have reported the photocatalytic properties of titanium dioxide doped with various rare earth ions.7 Very recently in the literature a growing number of papers based on doped ZnO with lanthanides and in particular with cerium, has occurred. In most of these cases Ce-doped ZnO showed interesting properties in abating organic pollutants.8–14 Calza and colleagues focused on obtaining different materials (i.e. ZnO doped with cerium) by changing both the precursors of the bare oxide and the synthesis process. The efficiency of the thus obtained photocatalysts was tested in the degradation of acesulfame K which is a persistent emerging contaminant under various conditions e.g. river water expressing the organic content as 4.8 mg L−1.15The presence of natural organic matter (NOM) in natural waters deserves prime importance to be investigated for maintaining safe drinking water. It is very well documented that TiO2 photocatalysis has been applied extensively for the degradation of NOM and NOM analogs as humic acids (HAs), and fulvic acids for decades.16–19 Application of the second generation of photocatalysts for the degradation of natural organic matter has received recent interest.20 Besides TiO2 photocatalysis, Oskoei and colleagues reported efficient removal of HA by ZnO nano-photocatalysis.21 Complementary to the reported findings, the role of TGP would be of interest to the researchers working in the field of photocatalysis primarily for effective removal of NOM and for elucidation of the role of NOM in the degradation of persistent pollutants. In this respect, the major aim of this work is devoted to use Ce-doped ZnO as a TGP for the photocatalytic treatment of humic acids selected as the model compound of NOM. Moreover, due to the polydispersity character of NOM, further interest was directed to the understanding of the molecular size fractionation of the humic matter displaying similar organic carbon contents. It should also be indicated that activity testing of a novel photocatalyst should also be extended to higher molecular weight recalcitrant organics for assessment of the real performance.22 As indicated above, TiO2 photocatalytic treatment of NOM had been systematically investigated by Bekbolet and colleagues, therefore TiO2 was also used for comparison purposes.
Results and discussion
Characterization of the prepared materials
Physico-chemical properties of the prepared Ce-doped ZnO and of the TiO2 P-25 (used for comparison purposes) are presented in Table 1. Ce-doped ZnO displayed a mixed phase composed of CeO2 (1%) and ZnO (99%). The surface area was considerably smaller (<10 m2 g−1) than TiO2, (55 m2 g−1).HA solutions i.e. 0.45 μm ff HA and 100 kDa HA displayed almost similar organic carbon contents of NPOCi: 4.80 mg L−1 and 4.67 mg L−1 respectively that could be considered as resembling the natural water organic matter content investigated by Calza and colleagues.15 Molecular size distribution (fractions passing through 100 kDa, 30 kDa, 10 kDa and 3 kDa filters) profiles considered as NPOC expressed variations with respect to the prepared HA samples. The 0.45 μm ff HA sample contained the organic content with a molecular size greater than 100 kDa of 65.6%. Further fractionation displayed the 53.1% 30 kDa fraction, 18.1% 10 kDa fraction and 8.8% 3 kDa fraction. On the other hand, the 100 kDa HA sample displayed the 62.0% 30 kDa fraction, 31.7% 10 kDa fraction and 6.3% 3 kDa fraction. The UV-vis and fluorescence spectroscopic properties of the samples as well as NPOC and SUVA were also presented (Table 2).
| Color436 | UV365 | UV280 | UV254 | NPOCa | SUVAb | FI | |
|---|---|---|---|---|---|---|---|
| a NPOC: mg L−1. b SUVA: L m−1 mg−1. | |||||||
| 0.45 μm ff HA | |||||||
| 0.45 μm | 0.079 | 0.162 | 0.379 | 0.454 | 4.56 | 9.98 | 1.09 |
| 100 kDa | 0.025 | 0.051 | 0.129 | 0.156 | 1.41 | 11.1 | 1.25 |
| 30 kDa | 0.017 | 0.038 | 0.102 | 0.125 | 1.32 | 9.47 | 1.26 |
| 10 kDa | 0.003 | 0.007 | 0.025 | 0.031 | 1.08 | 2.87 | 1.22 |
| 3 kDa | 0.001 | 0.003 | 0.011 | 0.015 | 0.913 | 1.65 | 1.53 |
| 100 kDa HA | |||||||
| 100 kDa | 0.066 | 0.146 | 0.374 | 0.445 | 4.67 | 9.53 | 1.17 |
| 30 kDa | 0.035 | 0.085 | 0.236 | 0.287 | 2.97 | 9.66 | 1.21 |
| 10 kDa | 0.015 | 0.038 | 0.119 | 0.147 | 2.28 | 6.45 | 1.27 |
| 3 kDa | 0.004 | 0.008 | 0.023 | 0.029 | 1.14 | 2.54 | 1.44 |
The UV-vis spectroscopic parameters as well NPOC contents decreased in accordance with the decreasing molecular size for both of the HA samples. SUVA > 4 indicating aromaticity could be visualized for molecular size fractions greater than the 30 kDa fraction of the 0.45 μm ff HA sample and the 10 kDa fraction of the 100 kDa HA sample. EEM contour plots displayed the presence of humic-like and fulvic-like fluorophores in accordance with the SUVA values (Fig. 1a and b).
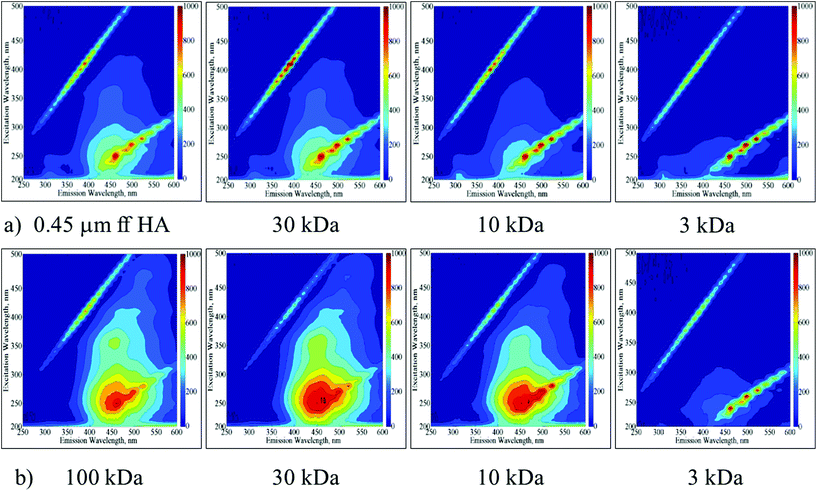 | ||
| Fig. 1 EEM fluorescence contour plots of molecular size fractions (30 kDa, 10 kDa and 3 kDa) of 0.45 μm ff HA (a) and 100 kDa HA (b) samples. | ||
Preliminary experiments
Preliminary experiments were conducted under conditions representing, i. adsorptive interactions: the absence of light and the presence of a photocatalyst, and ii. photolytic reactions: the absence of a photocatalyst and the presence of light.Photocatalytic degradation experiments and kinetics
Ce-doped ZnO photocatalytic degradation of HA samples was followed with respect to irradiation time (t) by using humic spectroscopic parameters and the mineralization extent was followed by NPOC. All of the parameters displayed an irradiation time dependent logarithmic decreasing profile irrespective of the photocatalyst type. Therefore, with respect to the prevailing degradation mechanism based on the non-selective oxidation via reactive oxygen species, a pseudo first order kinetic model was successfully applied to the degradation data expressed by all humic parameters (Table 3).| Photocatalyst | Rate constant k, min−1 | NPOC | ||||
|---|---|---|---|---|---|---|
| UV-vis spectroscopic parameters | ||||||
| Color436 | UV365 | UV280 | UV254 | k × 10−2, min−1 | Rate, mg L−1 min−1 | |
| 0.45 μm ff HA, NPOCi: 4.80 mg L−1 | ||||||
| Ce-doped ZnO | 5.06 × 10−2 | 6.18 × 10−2 | 6.54 × 10−2 | 5.79 × 10−2 | 1.80 | 0.0864 |
| TiO2 | 0.123 | 0.126 | 9.22 × 10−2 | 9.09 × 10−2 | 3.87 | 0.186 |
| 100 kDa HA, NPOCi: 4.67 mg L−1 | ||||||
| Ce-doped ZnO | 6.76 × 10−2 | 8.47 × 10−2 | 9.18 × 10−2 | 8.17 × 10−2 | 2.18 | 0.102 |
| TiO2 | 0.166 | 0.174 | 0.190 | 0.193 | 4.74 | 0.221 |
Photocatalytic degradation rates expressed as the reaction rate constants of 0.45 μm ff HA using Ce-doped ZnO displayed a decreasing sequence as UV280 > UV365 > UV254 > Color436. Decolorization of the humic fractions was mainly due to the removal of chromophoric groups composed of conjugated π systems and centers possessing a lone pair of electrons. On the other hand, the highly condensed core of humic acid fractions displayed a comparatively higher degradation rate constant due to the high aromatic character as expressed by the rate constants attained for UV280 and UV254. Although a similar trend of removal for UV-vis parameters was observed for 100 kDa HA with respect to 0.45 μm ff HA, considerably higher first order rate constants were attained. The reason could be attributed to the more uniform molecular size distribution profile of 100 kDa HA in comparison to 0.45 μm ff HA although both HA samples contained almost equal NPOC contents as presented previously (Table 2).
Photocatalytic degradation extents expressed as the reaction rate constants of 0.45 μm ff HA using TiO2 displayed a different trend in a decreasing sequence as UV365 > Color436 > UV280 > UV254 with respect to the trend that was attained previously.20,24 On the other hand, 100 kDa HA expressed an increasing trend as Color436 < UV365 < UV280 < UV254. The prevailing mechanism could be visualized by electrostatic attractions between negatively charged deprotonated humic functional groups and positively charged photocatalyst surface groups as opposed by the repulsive interactions. Moreover, the denser aromatic rich inner core displayed considerably higher removal tendency through hydrophobic interactions. Therefore, it could be deduced that electrostatic attractions were suppressed over hydrophobic attractions as well as the van der Waals forces.
Photocatalytic mineralization (NPOC) extents could be expressed in terms of first order rates (R, mg L−1 min) of 0.0864 and 0.102 for 0.45 μm ff HA and 100 kDa HA respectively. On the other hand, the rates (R, mg L−1 min) of 0.186 and 0.221 were attained for TiO2 photocatalytic degradation of 0.45 μm ff HA and 100 kDa HA respectively. Ce-doped ZnO photocatalytic treatment of 0.45 μm ff HA, expressed half-life values in the range of 11–14 min for the UV-vis spectroscopic parameters, whereas the NPOC half-life was 39 min. In a similar manner, Ce-doped ZnO photocatalytic degradation of 100 kDa HA expressed half-life values for the UV-vis spectroscopic parameters in a range of 7–10 min whereas the NPOC half-life was 32 min. On the other hand, in accordance with the observed higher degradation rate constants, for the TiO2 photocatalytic degradation of 0.45 μm ff HA, half-life values for the UV-vis spectroscopic parameters were found to be in the range of 6–8 min whereas the NPOC half-life was 18 min. The TiO2 photocatalytic degradation of 100 kDa HA expressed half-life values for the UV-vis spectroscopic parameters as 3–4 min whereas the NPOC half-life was 15 min. NPOC removal rates were found to be in good agreement with the initial adsorption conditions of both of the photocatalysts onto 0.45 μm ff HA and 100 kDa HA respectively.
Based on the mineralization extent in comparison to UV254 removals, the SUVA (L m−1 mg−1) changes were found to be decreasing from 9.50 to 1.43 for Ce-doped ZnO and 8.47 to 1.34 for TiO2 representing significant elimination of aromaticity. Since SUVA > 4 indicates aromaticity and the presence of hydrophobic moieties, Ce-doped ZnO was found to be more effective on the removal of the aromatic core possibly occurring through a degradation pathway by the semi-selective action of reactive oxygen species. Further evaluation could be related to the diverse stability and aggregation properties of ZnO nanoparticles depending on the solution matrix conditions.25 On the other hand, the stabilizing effect of NOM significantly affected the stabilization and aggregation of TiO2 particles.26 Due to the differences in the primary particle sizes of the photocatalysts, through electrostatic attractions the role of the humic sub-fractions could be related to the disturbance of colloidal stability leading to possible aggregation conditions most probably excluding sweep co-precipitation through surface adsorption (Table 1). The resulting non-homogeneous reaction medium could possibly lead to the diminished light harvesting capacity of Ce-doped ZnO. The overall effect could be visualized by slower reaction rates in comparison to TiO2.
Fluorescence spectroscopic elucidation of the photocatalytic degradation of HA
EEM fluorescence contour plots were presented for the photocatalytic degradation of 0.45 μm ff HA and 100 kDa HA using Ce-doped ZnO (Fig. 2a and b respectively).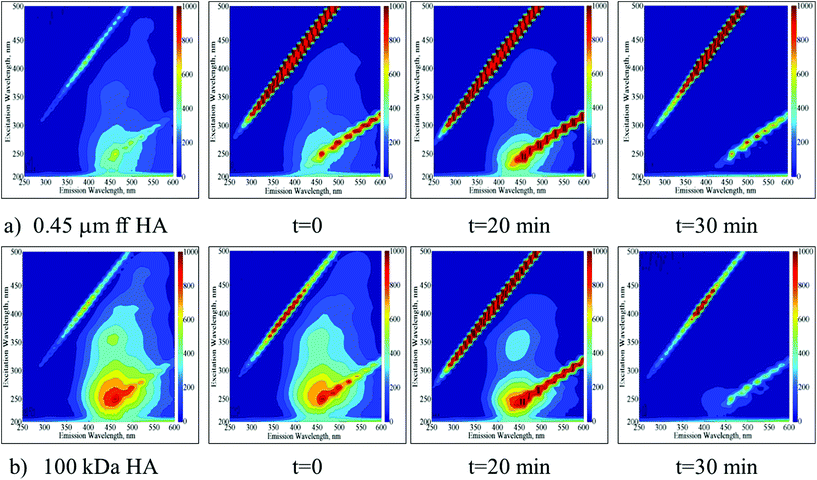 | ||
| Fig. 2 Time dependent EEM fluorescence contour plots of 0.45 μm ff HA (a) and 100 kDa HA (b) upon photocatalytic treatment by using Ce-doped ZnO. | ||
Following the initial adsorption (t = 0) of 0.45 μm ff HA onto Ce-doped ZnO, the presence of humic-like and fulvic-like fluorophores was still evident up to an irradiation period of 20 min. Upon further irradiation conditions, the EEM contour plots were found to be devoid of any fluorescence signatures. In a similar manner, following the initial adsorption (t = 0) of 100 kDa HA onto Ce-doped ZnO, the presence of humic-like and fulvic-like fluorophores was also still evident up to an irradiation period of 20 min. However, the presence of fulvic-like fluorophores displayed considerably lower fluorescence intensities in comparison to 0.45 μm ff HA. Upon further irradiation conditions, the EEM contour plots were also found to be devoid of any fluorescence signatures.
EEM fluorescence contour plots were presented for the photocatalytic degradation of 0.45 μm ff HA and 100 kDa HA using TiO2 (Fig. 3a and b respectively).
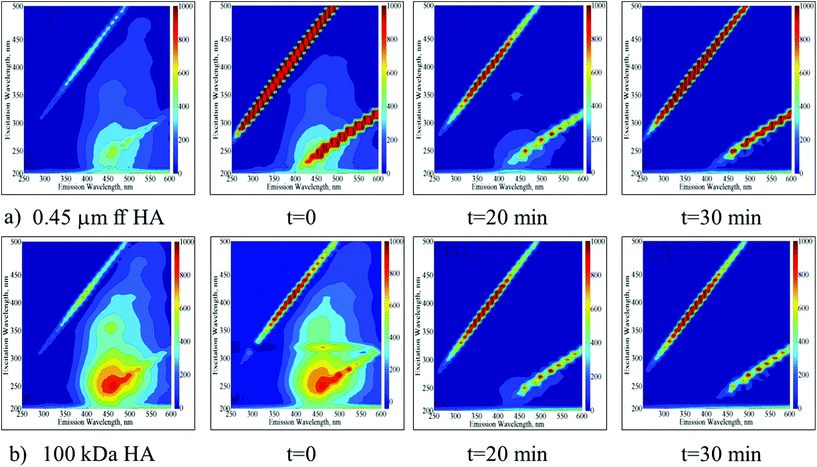 | ||
| Fig. 3 Time dependent EEM fluorescence contour plots of 0.45 μm ff HA (a) and 100 kDa HA (b) upon photocatalytic treatment by using TIO2. | ||
Following the initial adsorption (t = 0) of 0.45 μm ff HA onto TiO2, the presence of humic-like and fulvic-like fluorophores was still obvious up to an irradiation period of 20 min. Upon further irradiation conditions, the EEM contour plots were devoid of any fluorescence signatures. Following the initial adsorption (t = 0) of 100 kDa HA onto TiO2, humic-like and fulvic-like fluorophores were still present up to an irradiation period of 20 min. However, humic-like fluorophores displayed considerably higher fluorescence intensities in comparison to 0.45 μm ff HA. Upon further irradiation conditions (t = 30 min) the EEM contour plots were also devoid of any fluorescence signatures.
EEM contour plots of the treated HA samples displayed close similarities to the 3 kDa fraction of untreated HA being more apparent for 100 kDa HA upon 30 minutes of Ce-doped ZnO photocatalytic treatment. On the other hand, a similar result could be addressed upon TiO2 photocatalysis for 20 minutes. These results significantly demonstrated the transformation of higher molecular weight fractions to lower molecular weight fractions via successful mineralization of the total organic carbon contents irrespective of the starting humic material composition.
Since Ce doped ZnO exhibited buffer properties at pH 7.4–7.6, dissolution of the photocatalyst was prevented. However, in the presence of HA, through chelation by ligand–metal attractions, leaching of metal ions could be expected. It was also reported by Jiang and colleagues that in the presence of HA, ZnO dissolution kinetics were positively correlated with SUVA and the molecular weight of the studied NOM and NOM analogs.23 However, neither Ce nor Zn leaching in considerable amounts could be determined under the specified experimental conditions. On the other hand, the possible fluorescence quenching conditions could be attributed to the nonexistence of humic-like and fulvic-like fluorophores rather than achievement of complete mineralization.
Experimental
Preparation and characterization of Ce-doped ZnO photocatalysts
All reactants were purchased from Aldrich and have purity higher than 99.9%. All reactants were used without any further purification treatment. The bare ZnO sample was synthesized starting from a 1 M water solution of Zn(NO3)2·6H2O. Then a 4 M NaOH solution was added dropwise until the pH was 10–11, and finally the solution was transferred into a Teflon lined stainless steel 100 mL autoclave (filling 70%), and then treated at 175 °C for 16 hours. The Ce-doped ZnO sample (Ce molar concentration 1%) was prepared by adding a stoichiometric amount of CeCl3·7H2O in the starting solution, and then the same procedure was followed. The sample was labelled as Ce-doped ZnO. Detailed information regarding the properties of the thus prepared Ce-doped ZnO is presented in comparison to TiO2 P-25 (Table 1).Powder X-ray diffraction (XRD) patterns were recorded with a PANalytical PW3040/60 X'Pert PRO MPD using a copper Kα radiation source (0.15418 nm). The intensities were obtained in the 2θ range between 20° and 70°. The X'Pert High-Score software was used for data handling. Rietveld refinement was performed on the diffraction patterns to determine the crystallite size and relative abundance of phases, using the MAUD 2.26 software and a NIST Si powder to determine the instrumental function.27 The UV-Vis absorption spectra were recorded using a Varian Cary 5000 spectrometer, coupled with an integration sphere for diffuse reflectance studies (DRS), using Carywin-UV/scan software. A sample of PTFE with 100% reflectance was used as the reference. Surface area measurements were carried out on a Micromeritics ASAP 2020/2010 using the Brunauer–Emmett–Teller (B.E.T.) model on the N2 adsorption measurement. Prior to the adsorption run, all the samples were outgassed at 160 °C for 3 hours.
Preparation and characterization of HA samples
HA (20 mg L−1) was prepared upon dilution of the stock solution (1.0 g L−1) and filtered through a 0.45 μm membrane filter (designated as 0.45 μm ff HA). HA solution (50 mg L−1) was subjected to ultrafiltration through a 100 kDa membrane filter and thus the obtained sample was designated as 100 kDa HA. The molecular size distribution of the prepared HA solutions was performed by a stirred cell ultrafiltration system applied in sequential mode using membrane filters with nominal molecular weight cut-offs of 100 kDa, 30 kDa, 10 kDa and 3 kDa. The NPOC mass balance was 95%. The reproducibility of molecular size fractionation experiments was reported previously.24 Millipore MilliQ water, resistivity of 18.2 MΩ cm at 25 °C was used in the preparation of all solutions.Following photocatalytic treatment, all samples were immediately filtered through 0.45 μm membrane filters to remove the photocatalyst from the reaction medium. Thus, the prepared clear solutions were subjected to further analysis. NPOC was determined by using a Shimadzu TOC-VWP Total Organic Carbon Analyzer. UV-vis and fluorescence spectroscopic measurements were performed by using a Perkin Elmer lambda 35 UV-vis Spectrophotometer and a Perkin Elmer LS 55 Luminescence Spectrometer respectively.
Photocatalytic degradation experiments
Photocatalytic degradation experiments were performed by using an Atlas Suntest CPS+ simulator equipped with an air cooled Xenon Lamp (wavelength range: 300–800 nm and intensity, I: 250 W m−2). Irradiation periods were 0–60 min with 10 min intervals. Related information on the reaction conditions was reported in detail by Birben and colleagues.20 The photocatalyst dose was 0.25 mg mL−1 for Ce-doped ZnO as well as TiO2. Furthermore, ZnO was also used as a photocatalyst and the attained results are presented in S1 ESI.†Conclusions
Based on the presented data, photocatalytic degradation kinetics indicated the possible use of Ce-doped ZnO for the degradation of humic acid. The initial adsorption extent affected the Ce doped ZnO photocatalytic degradation efficiency of the HA samples in comparison to TiO2. The humic molecular size distribution profiles as well as the EEM fluorescence features displayed the role of a denser aromatic core. Further assessment on the influence of operational parameters, such as catalyst loading, light intensity and aqueous medium constituents should also be performed. Moreover, Ce leaching into aqueous medium and possible complexation with oxidized and non-oxidized humic molecular fractions should also be investigated in detail. With respect to the attained results, further studies should be designed for the elucidation of the photocatalytic reaction mechanism using ZnO and/or doped ZnO specimens. Based on the above given information as well as the presented ESI,† it could be deduced that using humic acid and its sub molecular size fractions as representatives of NOM should be cautiously interpreted in the elucidation of the photocatalytic activity of a novel photocatalyst. Furthermore, direct evaluation of the experimental results could lead to erroneous interpretation of the photocatalytic activities of the novel materials in comparison to TiO2.Acknowledgements
Financial support provided by the Research Fund of Bogazici University through Project Number 11081 is gratefully acknowledged.References
- K. Maeda and K. Domen, J. Phys. Chem. Lett., 2010, 1, 2655–2661 CrossRef CAS.
- S. L. Suib, New and Future Developments in Catalysis: Solar Photocatalysis, Elsevier, Amsterdam, The Netherlands, 2013 Search PubMed.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS.
- A. V. Emeline, V. N. Kuznetsov, V. K. Ryabchuk and N. Serpone, Environ. Sci. Pollut. Res., 2012, 19(9), 3666–3675 CrossRef CAS PubMed.
- N. Serpone and A. V. Emeline, J. Phys. Chem. Lett., 2012, 3(5), 673–677 CrossRef CAS PubMed.
- C. Gionco, M. C. Paganini, E. Giamello, R. Burgess, C. Di Valentin and G. J. Pacchioni, J. Phys. Chem. Lett., 2014, 5(3), 447–451 CrossRef CAS PubMed.
- J. Reszczynska, T. Grzyb, J. W. Sobczak, W. Lisowski, M. Gazda, B. Ohtani and A. Zaleska, Appl. Catal., B, 2015, 163, 40–49 CrossRef CAS.
- M. Ahmad, E. Ahmed, F. Zafar, N. R. Khalid, N. A. Niaz, A. Hafeez, M. Ikram, A. Khan and Z. Hong, J. Rare Earths, 2015, 33(3), 255–262 CrossRef CAS.
- O. Bechambi, L. Jlaiel, W. Najjar and S. Sayadi, Mater. Chem. Phys., 2016, 173, 95–105 CrossRef CAS.
- S. G. Kumar and K. S. R. K. Rao, RSC Adv., 2015, 5, 3306–3351 RSC.
- J.-C. Sin, S. M. Lamb, K. T. Lee and A. R. Mohamed, J. Mol. Catal. A: Chem., 2015, 409, 1–10 CrossRef CAS.
- C. Yu, K. Yang, Y. Xie, Q. Fan, J. J. Yu, Q. Shu and C. Wang, Nanoscale, 2013a, 5, 2142–2151 Search PubMed.
- C. Yu, K. Yang, W. Zhou, Q. Fan, L. Wei and J. C. Yu, J. Phys. Chem. Solids, 2013b, 74, 1714–1720 Search PubMed.
- C. Yu, W. Zhou, H. Liu, Y. Liu and D. D. Dionysiou, Chem. Eng. J., 2016, 287, 117–129 CrossRef CAS.
- P. Calza, C. Gionco, M. Giletta, M. Kalaboka, V. A. Sakkas, T. Albanis and M. C. Paganini, J. Hazard. Mater., 2016 DOI:10.1016/j.jhazmat.2016.03.093.
- M. Bekbolet and G. Ozkosemen, Water Sci. Technol., 1996, 33(6), 189–196 CrossRef CAS.
- M. Bekbolet, A. S. Suphandag and C. S. Uyguner, J. Photochem. Photobiol., A, 2002, 148, 121–128 CrossRef CAS.
- C. S. Uyguner and M. Bekbolet, Desalination, 2005a, 176, 167–176 Search PubMed.
- C. S. Uyguner-Demirel and M. Bekbolet, Chemosphere, 2011, 84(8), 1009–1031 CrossRef CAS PubMed.
- N. C. Birben, C. S. Uyguner-Demirel, S. Sen-Kavurmaci, Y. Y. Gurkan, N. Turkten, Z. Cinar and M. Bekbolet, Catal. Today, 2015, 240, 125–131 CrossRef CAS.
- V. Oskoei, M. H. Dehghani, S. Nazmara, B. Heibati, M. Asif, I. Tyagi, S. Agarwal and V. K. Gupta, J. Mol. Liq., 2016, 213, 374–380 CrossRef CAS.
- W. Y. Teoh, J. A. Scott and R. Amal, J. Phys. Chem. Lett., 2012, 3, 629–639 CrossRef CAS PubMed.
- C. Jiang, G. R. Aiken and H. Hsu-Kim, Environ. Sci. Technol., 2015, 49, 11476–11484 CrossRef CAS PubMed.
- C. S. Uyguner and M. Bekbolet, Catal. Today, 2005b, 101(3), 267–274 Search PubMed.
- D. Zhou and A. A. Keller, Water Res., 2010, 44, 2948–2956 CrossRef CAS PubMed.
- I. Chowdhury, D. M. Cwiertny and S. L. Walker, Environ. Sci. Technol., 2012, 46, 6968–6976 CrossRef CAS PubMed.
- L. Lutterotti, Nucl. Instrum. Methods Phys. Res., Sect. B, 2010, 268, 334–340 CrossRef CAS.
- J. K. Edzwald, W. C. Becker and K. L. Wattier, J. AWWA, 1985, 77, 122–132 CAS.
- M. Bekbolet and S. Sen Kavurmaci, Photochem. Photobiol. Sci., 2015, 14, 576–582 CAS.
- P. G. Coble, Mar. Chem., 1996, 51, 325–346 CrossRef CAS.
- APHA, AWWA and WPCF, Standard Methods for the Examination of Water and Wastewater, ed. E. W. Rice, R. B. Baird, A. D. Eaton and L. S. Cercei, American Water Works Association, Washington, DC, USA, 22nd edn, 2012 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6pp00216a |
| This journal is © The Royal Society of Chemistry and Owner Societies 2017 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)