Open Access Article
Open Access ArticleStrain-promoted azide–alkyne cycloaddition for protein–protein coupling in the formation of a bis-hemoglobin as a copper-free oxygen carrier†
Serena
Singh
,
Ina S.
Dubinsky-Davidchik
and
Ronald
Kluger
*
Davenport Chemical Laboratories, Department of Chemistry, University of Toronto, Toronto, Ontario, Canada M5S 3H6. E-mail: r.kluger@utoronto.ca
First published on 27th September 2016
Abstract
Conventional chemical approaches to protein–protein coupling present challenges due to the intrinsic competition between the desired interactions of reagents with groups of the protein as well as reactions with water. Biorthogonal Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC)-processes provide a basis to direct reactivity without functional group interference. However, the requirement for Cu(I) in CuAAC leads to complications that result from the metal ion's interactions with the protein. In principle, a similar but metal-free alternative approach to coupling could employ the reaction of an alkyne that is strained in combination with an azide (strain-promoted azide–alkyne cycloaddition, SPAAC). The method is exemplified by the combination of a cyclooctyne derivative of hemoglobin with an azide-modified hemoglobin. The bis-hemoglobin tetramer that is produced has properties consistent with those sought for use as a hemoglobin-based oxygen carrier (HBOC).
Introduction
Chemical cross-linking of individual proteins is a highly developed area with many applications and examples.1–4 Extending the approach that creates linkages within proteins to protein–protein coupling can produce novel entities with designed properties.2,5,6 Constructs of two hemoglobin (Hb) proteins coupled together, known as Hb bis-tetramers, have been found useful as circulating oxygen carriers that avoid extravasation from the vasculature.6 Their propensity to evade scavenging of nitric oxide from endothelial nitric oxide synthase7 supports their potential utility and value as protein-based synthetic targets. However, because proteins have many reactive functional groups, directing a specific coupling reaction to unique sites is a chemical challenge. Furthermore, developing reactions between proteins confronts a significant entropic challenge. The concept and implementation of biorthogonal processes led to the successful application of copper-catalyzed azide–alkyne cycloaddition (CuAAC)8 in the formation of useful coupled proteins.9–11 For example, we adapted CuAAC to coupling stabilized Hb tetramers using the solubility-directed combination of cross-linked Hb azides and bis-alkynes in the presence of Cu(I).10,12,13 The process is also applicable to coupling different proteins and can serve as the basis for a more general approach to coupling proteins.9 However, the requirement for Cu(I) can lead to protein denaturation and the ongoing association of protein-bound Cu(I).14,15 Subsequent release of copper in circulation is then concerning due to its capacity to interfere with cellular processes.16 However, the concentrations of copper will be small as we purified the products with this issue in mind.Limitations that result from complications associated with CuAAC17–19 motivated us to develop an alternative biorthogonal coupling that does not require an added metal ion. Bertozzi and co-workers have shown that cycloalkynes are activated for such a process, presumably by strain that is induced by distortion of their triple bond.20,21 We now report that using strain-promoted azide–alkyne cycloaddition (SPAAC) as a metal-free alternative,22–26 we can efficiently produce cross-linked Hb bis-tetramers. The preparation of the necessary modified proteins for the cycloaddition process is convenient and the controlled cycloadditions give readily isolable-coupled proteins as products.
Experimental section
Materials
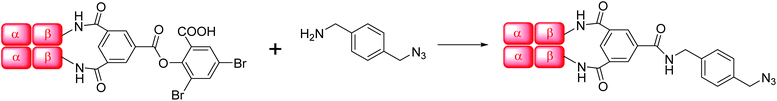
Human hemoglobin A (HbA) was obtained from Oxygenix Ltd. N-[(1R,8S,9S)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane (amine-cyclooctyne), 3,6,9,12,15-pentaoxaheptadecane-1,17-diyl bis-azide and amino-PEG4-alkyne (amine-alkyne) were obtained from commercial sources. Trimesoyl tris(3,5-dibromosalicylate) (TTDS) was synthesized according to Kluger et al.27 4-Azidomethyl-benzylamine (amine-azide) and the reference bis-tetramer were prepared as described by Yang et al.12 4,4′-Diazidediphenylsulfone (bis-azide) was synthesized according to Li et al.17Native gel electrophoresis
Two-dimensional Tris-HCl polyacrylamide gels contained 12% separating gel (pH 8.8) and 4% stacking gel (pH 8.8). Sample buffer was adjusted to pH 6.8 and running buffer was adjusted to pH 8.3. The finished gels were stained with Coomassie Brilliant Blue. Polyacrylamide gel electrophoresis (PAGE) followed standard operations.28Hb bis-tetramers by SPAAC
Results and discussion
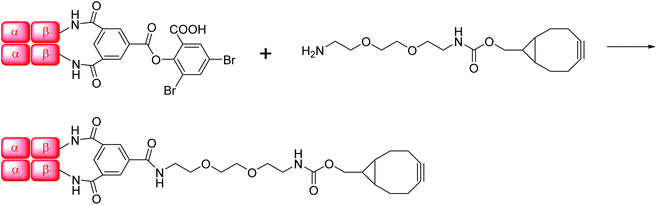
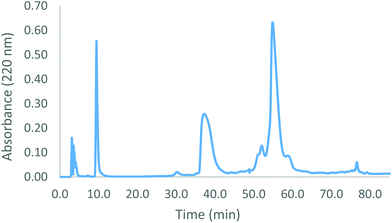
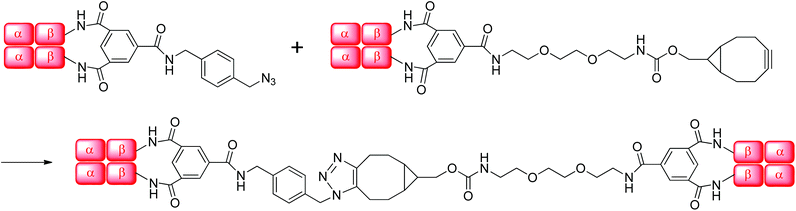
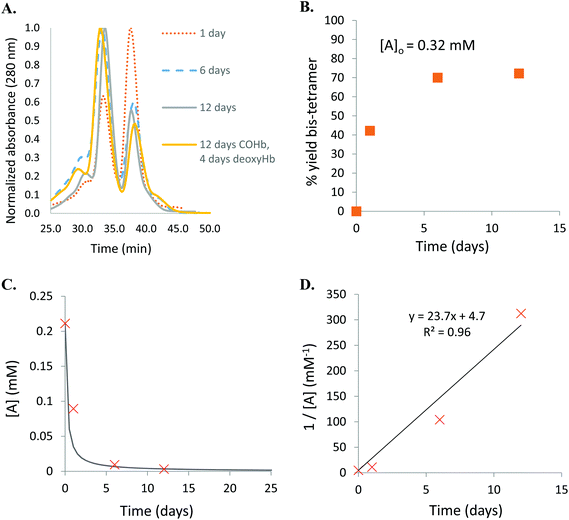
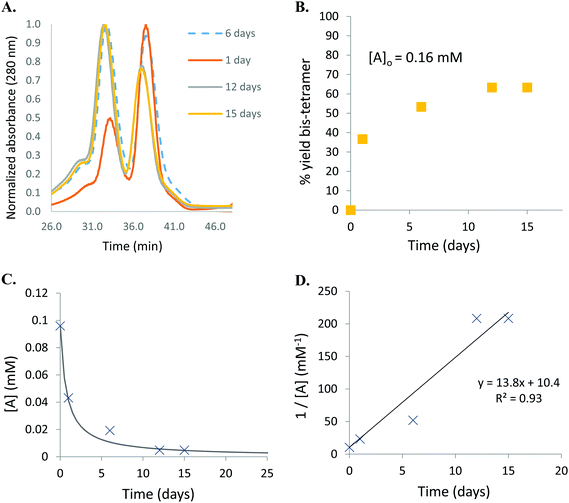
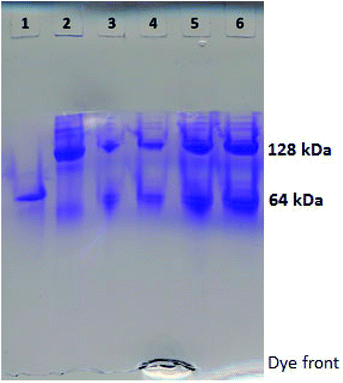
Human Hb was cross-linked with trimesoyl tris(3,5-dibromosalicylate) (Scheme 1), a trifunctional reagent that reacts with the ε-amino groups of each β-lys-82, leaving the third ester available for further reaction.27,29 The polyanionic electrophile reacts site-specifically with residues residing within Hb's cationic funnel that normally associate with 2,3-diphosphoglycerate. Addition of an amine-functionalized hydrocarbon derivative of cyclooctyne30 to the cross-linked protein ester produces the desired conjugate (also in Scheme 1). Addition of an amino azide to the cross-linked protein ester produces an azido conjugate (Scheme 2). These derivatives were characterized by reverse-phase HPLC of the product solutions. Fig. 1 shows the chromatogram for the reaction products from the Hb–cyclooctyne conjugation. Fig. 2 shows the chromatogram for formation of the Hb–azide conjugate. Both samples contained small amounts of non-conjugated products that result from a side reaction that is typically due to competitive hydrolysis of the initial ester.The cycloaddition of Hb–azide and the Hb–cyclooctyne was initiated by combining solutions of each reactant (Scheme 3). Progress of the reaction was followed by HPLC until an optimal conversion was achieved. After 12 days the reactants were converted in 70% yield to the cycloaddition product, which is a cross-linked bis-tetramer connected by the triazole from the SPAAC process (Fig. 3). Gel electrophoretic analysis confirmed that the peak that elutes earlier than the modified Hb tetramers in the size-exclusion HPLC is the bis-tetrameric species (Fig. 6). Allowing the reaction to proceed for up to 18 more days did not increase the yield. Other modes of addition and combination of the reactants did not improve the final outcome. Notably, addition of amine-cyclooctyne/azide reagents to CO-Hbs to secure uniformity of the product conformation did not enhance the subsequent SPAAC reaction. The yield increases to 76% by keeping the mixed proteins in their deoxygenated states for four more days. We expect that access to the central channel of the protein is improved in the conformation favored by the deoxy heme.13 However, this is impractical for a long-term reaction because of the competing formation of non-functional methemoglobin from small amounts of residual oxygen. There was no added benefit neither from heating the solution at 70 °C for 30 min nor by stirring for 12 h at 40 °C.
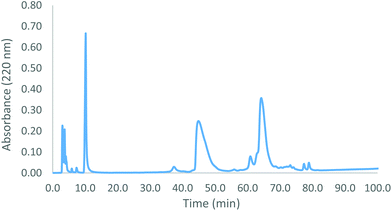
We assessed the outcome of the SPAAC-based protein-coupling process in comparison to that from CuAAC by coupling a strain-free Hb–alkyne with the Hb–azide in the presence of Cu(I) (Scheme 4). The alkyne-functionalized tether is comparable in length to amine-cyclooctyne so we chose this to lead to effective coupling. A bathophenanthroline ligand (4 eq.), CuSO4 (2 eq.) and ascorbic acid (40 eq.) were added to the protein mixture. This ratio of reagents has previously been shown to be optimal for the coupling of Hb–azides to bis-alkynes.10 However, with the alkyne covalently tethered to Hb, little product formation occurred. After one hour, only a small fraction of the protein present was coupled (Fig. 4). Leaving the reaction mixture longer (greater than one hour) resulted in a significant amount of oxidation of the heme and denaturation of the protein. Methemoglobins were apparent from the deepening colour of the reaction mixture and successive denaturation was confirmed from the observed precipitate. We observed a similar outcome under CuAAC conditions using a Hb–alkyne and a bis–azide.
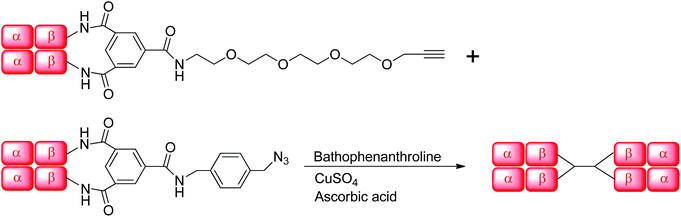
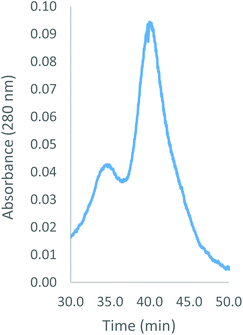
Despite negative results with other combinations (e.g. Hb–alkyne with Hb–azide or bis-azide), Hb–cyclooctyne reacts effectively with a bis-azide (Scheme 5). Approximately 63% bis-tetramer results after incubation at 4 °C for 15 days (Fig. 5). The long incubation does not affect the protein in the absence of copper ion and exclusion of oxygen. Native gel electrophoresis analysis revealed that the species eluting first in the size-exclusion HPLC is the bis-tetramer (Fig. 6). The mass spectrum of the product of the reaction of Hb–cyclooctyne with an excess of bis-azide (see the ESI†) confirms that the bis-azide is capable of reacting with the entire pool of Hb–cyclooctyne. Since every Hb cyclooctyne appendage is accessible to the small molecule bis-azide, then half an equivalent of bis-azide modifies half of the total Hb–cyclooctyne in solution to produce a mixture of approximately 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 azido Hb to Hb–cyclooctyne. The yield outcome of the bis-tetramer-forming reaction is then analogous to the combination of the singly modified Hb–azide with the Hb–cyclooctyne noted above. Replacing the rigid bis-azide with an extended linkage derived from condensed ethylene glycols (3,6,9,12,15-pentaoxaheptadecane-1,17-diyl bis-azide) did not improve the outcome. The addition of 2.0 or 10.0 eq. of bis-azide resulted in addition of each azide to no more than one Hb-cyclooctyne in contrast to the reaction with a hydrocarbon bis-alkyne.10 The alternative strategy in this case would be inefficient due to the complexity of the reagent.
50 azido Hb to Hb–cyclooctyne. The yield outcome of the bis-tetramer-forming reaction is then analogous to the combination of the singly modified Hb–azide with the Hb–cyclooctyne noted above. Replacing the rigid bis-azide with an extended linkage derived from condensed ethylene glycols (3,6,9,12,15-pentaoxaheptadecane-1,17-diyl bis-azide) did not improve the outcome. The addition of 2.0 or 10.0 eq. of bis-azide resulted in addition of each azide to no more than one Hb-cyclooctyne in contrast to the reaction with a hydrocarbon bis-alkyne.10 The alternative strategy in this case would be inefficient due to the complexity of the reagent.
The bis-tetramer from SPAAC of Hb–cyclooctyne with bis-azide was separated from the reactants prior to the acquisition of its oxygen-binding curve (Fig. 7). The oxygen affinity of the purified bis-tetramer (P50 = 8.1 ± 0.3 torr) is similar to that of native Hb (P50 = 5 torr) and the cooperativity remains significant in the bis-tetramer (n50 = 2.0 ± 0.1). These oxygen binding properties are comparable to those previously reported for bis-tetramers with structurally analogous features.6,12 Hb properties may be further customized by modifying the internal covalent cross-links31 and/or by adjusting the hybridization of the spacer bridging the two Hb proteins.32
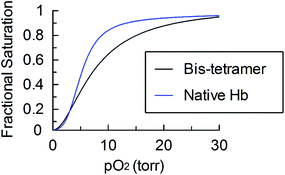 | ||
| Fig. 7 Oxygen desaturation curve of the purified bis-tetramer from SPAAC of Hb–cyclooctyne with bis-azide compared to that of native Hb. | ||
Conclusions
Metal-free strain-promoted azide–alkyne cycloaddition (SPAAC) has been successfully applied for the first time to the coupling cross-linked Hbs. The presence of residual copper from CuAAC is potentially problematic for processes involving heme proteins and the SPAAC process overcomes that limitation. The SPAAC method results in the formation of homogeneous Hb-based bis-tetramers in the highest yields of any process that has been utilized to form members of this class of double proteins (see Table 1 for comparison).Combining heme proteins under a carbon monoxide atmosphere in a stable non-denaturing environment prevents degradation of the product. This architecture of two coupled Hb tetramers cross-linked between the β-subunits has the desirable physical characteristics of a potential candidate for safe and effective circulatory delivery of oxygen as a supplement to red cells.
Acknowledgements
We thank the Canadian Blood Services for support through an operating grant. S. S. is the recipient of an Ontario Graduate Scholarship.References
- S. Deroo, F. Stengel, A. Mohammadi, N. Henry, E. Hubin, E. M. Krammer, R. Aebersold and V. Raussens, ACS Chem. Biol., 2015, 10, 1010–1016 CrossRef CAS PubMed.
- R. Kluger and A. Alagic, Bioorg. Chem., 2004, 32, 451–472 CrossRef CAS PubMed.
- T. Schiffner, N. de Val, R. A. Russell, S. W. De Taeye, A. T. De la Pena, G. Ozorowski, H. J. Kim, T. Nieusma, F. Brod, A. Cupo, R. W. Sanders, J. P. Moore, A. B. Ward and Q. J. Sattentau, J. Virol., 2016, 90, 813–828 CrossRef CAS PubMed.
- A. Sinz, C. Arlt, D. Chorev and M. Sharon, Protein Sci., 2015, 24, 1193–1209 CrossRef CAS PubMed.
- A. Alagic, A. Koprianiuk and R. Kluger, J. Am. Chem. Soc., 2005, 127, 8036–8043 CrossRef CAS PubMed.
- F. E. Lui, B. Yu, D. M. Baron, C. Lei, W. M. Zapol and R. Kluger, Transfusion, 2012, 52, 974–982 CrossRef CAS PubMed.
- D. B. Kim-Shapiro, A. N. Schechter and M. T. Gladwin, Arterioscler., Thromb., Vasc. Biol., 2006, 26, 697–705 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- E. M. J. Siren, S. Singh and R. Kluger, Org. Biomol. Chem., 2015, 13, 10244–10249 CAS.
- J. S. Foot, F. E. Lui and R. Kluger, Chem. Commun., 2009, 7315–7317 RSC.
- R. Kluger, J. S. Foot and A. A. Vandersteen, Chem. Commun., 2010, 46, 1194–1202 RSC.
- Y. Yang and R. Kluger, Chem. Commun., 2010, 46, 7557–7559 RSC.
- S. Singh, I. S. Dubinsky-Davidchik, Y. Yang and R. Kluger, Org. Biomol. Chem., 2015, 13, 11118 CAS.
- J. M. Rifkind, L. D. Lauer, S. C. Chiang and N. C. Li, Biochemistry, 1976, 15, 5337–5343 CrossRef CAS PubMed.
- V. Hong, S. I. Presolski, C. Ma and M. G. Finn, Angew. Chem., Int. Ed., 2009, 48, 9879–9883 CrossRef CAS PubMed.
- L. M. Gaetke and C. K. Chow, Toxicology, 2003, 189, 147–163 CrossRef CAS PubMed.
- D. Zeng, B. M. Zeglis, J. S. Lewis and C. J. Anderson, J. Nucl. Med., 2013, 54, 829–832 CrossRef CAS PubMed.
- G. R. Abel, Z. A. Calabrese, J. Ayco, J. E. Hein and T. Ye, Bioconjugate Chem., 2016, 27, 698–704 CrossRef CAS PubMed.
- C. Gruttner, K. Muller and J. Teller, IEEE Trans. Magn., 2013, 49, 172–176 CrossRef.
- N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046–15047 CrossRef CAS PubMed.
- P. V. Chang, J. A. Prescher, E. M. Sletten, J. M. Baskin, I. A. Miller, N. J. Agard, A. Lo and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 1821–1826 CrossRef CAS PubMed.
- C. Wendeln, I. Singh, S. Rinnen, C. Schulz, H. F. Arlinghaus, G. A. Burley and B. J. Ravoo, Chem. Sci., 2012, 3, 2479–2484 RSC.
- H. Yang, P. Srivastava, C. Zhang and J. C. Lewis, ChemBioChem, 2014, 15, 223–227 CrossRef CAS PubMed.
- C. Ornelas, J. Broichhagen and M. Weck, J. Am. Chem. Soc., 2010, 132, 3923–3931 CrossRef CAS PubMed.
- S. Schoffelen, J. Beekwilder, M. F. Debets, D. Bosch and J. C. M. V. Hest, Bioconjugate Chem., 2013, 24, 987–996 CrossRef CAS PubMed.
- K. D. Webster, D. Dahhan, C. Frosti, W. Dean, J. B. Chaires and K. W. Olsen, FASEB J., 2016, 30, 825.3 CrossRef PubMed.
- R. Kluger, Y. Song, J. Wodzinska, C. Head, T. S. Fujita and R. T. Jones, J. Am. Chem. Soc., 1992, 114, 9275–9279 CrossRef CAS.
- C. Arndt, S. Koristka, H. Bartsch and M. Bachmann, in Protein Electrophoresis, ed. B. T. Kurien and R. H. Scofield, Humana Press, 2012, vol. 869, ch. 5, pp. 49–53 Search PubMed.
- R. Kluger and Y. Song, J. Org. Chem., 1994, 59, 733–736 CrossRef CAS.
- J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793–16797 CrossRef CAS PubMed.
- M. A. Schumacher, M. M. Dixon, R. Kluger, R. T. Jones and R. G. Brennan, Nature, 1995, 375, 84–87 CrossRef CAS PubMed.
- D. Hu and R. Kluger, Biochemistry, 2008, 47, 12551–12561 CrossRef CAS PubMed.
- F. E. Lui and R. Kluger, Biochemistry, 2009, 48, 11912–11919 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Mass spectra of modified protein subunits and HPLC traces. See DOI: 10.1039/c6ob01817c |
| This journal is © The Royal Society of Chemistry 2016 |