 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pd-catalyzed cascade allylic alkylation and dearomatization reactions of indoles with vinyloxirane†
Run-Duo
Gao
,
Qing-Long
Xu
,
Li-Xin
Dai
and
Shu-Li
You
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China. E-mail: slyou@sioc.ac.cn
First published on 4th August 2016
Abstract
We have developed Pd-catalyzed intermolecular Friedel–Crafts-type allylic alkylation and allylic dearomatization reactions of substituted indoles bearing a nucleophilic group with vinyloxirane, providing an efficient method to synthesize structurally diverse tetrahydrocarboline and spiroindolenine derivatives under mild conditions.
Substituted indole nucleus and polycyclic skeletons embedded with indoline moieties represent important structural motifs in numerous natural products and pharmaceuticals.1 Meanwhile, transition-metal catalyzed Friedel–Crafts-type allylic alkylation2 and allylic dearomatization3 reactions of indoles have proved to be efficient and successful strategies for the synthesis of structurally diverse indolines in good yields with high diastereoselectivity and enantioselectivity. However, most of the successful examples to date have employed allyl carbonates as electrophiles. Allylic alcohols as electrophiles in allylic substitution reactions in the presence of suitable promoters were reported in the last decade.4 The Tamaru group5 and Trost group6 respectively reported Pd-catalyzed allylic dearomatization of indoles with allylic alcohols in the presence of trialkylborane. After that, Bandini and coworkers7 elegantly described the use of allylic alcohols in the catalytic and enantioselective Friedel–Crafts alkylation reaction in the presence of chiral gold complexes. Moreover, the Carreira8 group disclosed that branch allylic alcohols were suitable allylic precursors under acidic conditions. Recently, we have developed a Ru-catalyzed9 intermolecular dearomatization of indoles with allylic alcohols via a cascade sequence including the allylic dearomatization/cyclization/allylic amination reaction. In addition, we realized an iridium-catalyzed10 intermolecular allylic dearomatization reaction of substituted indoles using the Carreira ligand and Fe(OTf)2 as an additive.
In addition to these reports on employing allylic alcohols as suitable electrophiles in allylic substitution reactions, vinyloxirane under palladium catalysis could in situ generate an allylic alcohol upon the attack by a suitable nucleophile.11 Inspired by these elegant reports, we envisioned the utilization of vinyloxirane might enable a cascade reaction with indole bearing a nucleophile (eqn (1)). Herein, we describe such a Pd-catalyzed cascade allylic alkylation reaction of dimethyl malonate tethered indoles with vinyloxirane, including the intermolecular nucleophilic ring-open reaction of vinyloxirane and the subsequent intramolecular allylic alkylation and dearomatization reactions.
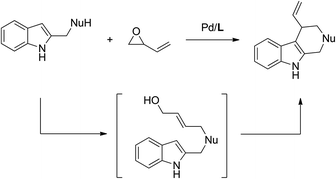 | (1) |
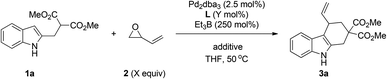
We initiated our investigation with the optimization of the reaction of dimethyl 2-((1H-indol-2-yl)methyl) malonate 1a and vinyloxirane 2. First, various ligands were examined in the presence of Pd2dba3 with Et3B as a promoter in THF at 50 °C. As shown in Table 1, when the monodentate ligand Ph3P or P(OPh)3 was used, no reaction was observed (entries 1 and 2, Table 1). Notably, bidentate ligands such as dppe and dppp could afford the product 3a in the same yield (entries 3 and 4, Table 1, 79% yield as determined by GC-MS). However, the desired product 3a and substrate 1a have a similar polarity and therefore cause the difficulty in separation in the case of incomplete conversion. Dppp was used as a preferred ligand because substrate 1a could not be consumed completely using dppe as the ligand. By reducing the loading of vinyloxirane, better yield could be obtained (entry 7, Table 1). In the presence of 2.5 mol% of Pd2dba3 and 5.5 mol% of dppp, we then tested the effects of various additives such as Na2SO4, CaCl2 and MgSO4 (entries 9–12, Table 1). The reaction with MgSO4 proceeded smoothly, providing the desired product 3a with the best result (82% yield, entry 12, Table 1).
| Entry | Ligand | X | Y | Additive | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 0.2 mmol of 1a, 2.5 mol% Pd2dba3, 0.5 mL Et3B (1 M in THF) in THF (2.0 mL) at 50 °C. b Isolated yield. c Determined by GC-MS (normal dodecane as the internal standard). d 5.0 equiv. additive was used. e 1.0 equiv. additive was used. | |||||
| 1 | PPh3 | 1.3 | 11 | — | Trace |
| 2 | P(OPh)3 | 1.3 | 11 | — | Trace |
| 3 | dppe | 1.3 | 5.5 | — | 79c |
| 4 | dppp | 1.3 | 5.5 | — | 79c (64) |
| 5 | dppb | 1.3 | 5.5 | — | 71c |
| 6 | dppf | 1.3 | 5.5 | — | 19c |
| 7 | dppp | 1.1 | 5.5 | — | 69 |
| 8 | dppp | 2.0 | 5.5 | — | 32 |
| 9d | dppp | 1.1 | 5.5 | Na2SO4 | 79 |
| 10d | dppp | 1.1 | 5.5 | CaCl2 | 20 |
| 11d | dppp | 1.1 | 5.5 | MgSO4 | 83 |
| 12e | dppp | 1.1 | 5.5 | MgSO4 | 82 |
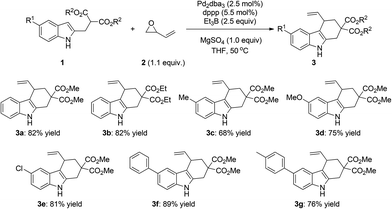
Under the optimized reaction conditions (entry 12, Table 1), we next turned our attention to explore the substrate scope of the reaction. As summarized in Scheme 1, substrates bearing different ester groups could be tolerated, giving the corresponding products in 82% yield in both cases (3a, 3b, Scheme 1). The reactions of 5-substituted indole substrates with either an electron-donating group (Me, OMe, Ph) or an electron-withdrawing group (Cl) proceeded smoothly under the optimal conditions, affording the desired products in good yields (68–89%).
By taking advantage of the nucleophilicity of the C-3 position of indoles, we have developed an allylic dearomatization reaction of indoles with an intramolecular design. The Pd-catalyzed allylic cascade reaction of C-3 substituted indoles with vinyloxirane has also been successful in a dearomatization fashion. The results are summarized in Scheme 2. With dppb as the ligand, the reactions of substrates 4a and 4b, bearing an electron-donating group on the indole core, could give the desired products with satisfactory yields (84–88% yields) and moderate diastereoselectivity (5a: 80/20, 5b: 78/22, Scheme 2). For the 6-F substituted indole 4c, moderate yield and diastereoselectivity could also be achieved (76% yield, 74/26 dr). Notably, substrate 4d and its dearomatized product have a similar polarity, which led to the difficulty in product separation. To address this problem, a one-pot reaction including an in situ reduction was carried out to convert the indolenine to the corresponding indoline (54% yield with 61/39 dr). Interestingly, for the more bulky substrate 4e, allylic alcohol 5e was obtained in 53% yield under the same reaction conditions. These results indicated that the current Pd-catalyzed cascade reaction firstly generates the corresponding allylic alcohol.
In conclusion, we have developed Pd-catalyzed intermolecular Friedel–Crafts-type allylic alkylation and allylic dearomatization of indoles with vinyloxirane through a cascade sequence. These protocols provide a rapid construction of structurally diverse tetrahydrocarboline and spiroindolenine derivatives under mild conditions.
Acknowledgements
We acknowledge the National Basic Research Program of China from MOST (2015CB856600, 2016YFA0202900) and the National Natural Science Foundation of China (21332009, 21421091) for generous financial support.Notes and references
- (a) Medicinal Natural Products: A Biosynthetic Approach, ed. P. M. Dewick, Wiley, New York, 2002 Search PubMed; (b) Modern Alkaloids, ed. E. Fattorusso and O. T. Scafati, Wiley-VCH, Weinheim, 2008 Search PubMed.
- For selected recent reviews on Friedel–Crafts-type reactions of indoles, see: (a) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903 CrossRef CAS PubMed; (b) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC; (c) M. Zeng and S.-L. You, Synlett, 2010, 1289 CAS. For selected recent examples on Friedel–Crafts-type reactions of indoles, see: (d) Q.-L. Xu, C.-X. Zhuo, L.-X. Dai and S.-L. You, Org. Lett., 2013, 15, 5909 CrossRef CAS PubMed; (e) C.-X. Zhuo, Q.-F. Wu, Q. Zhao, Q.-L. Xu and S.-L. You, J. Am. Chem. Soc., 2013, 135, 8169 CrossRef CAS PubMed; (f) Z.-L. Zhao, Q. Gu, X.-Y. Wu and S.-L. You, Chin. J. Catal., 2015, 36, 15 CrossRef CAS.
- For selected catalytic intermolecular allylic dearomatization reactions of indoles, see: (a) N. Kagawa, J. P. Malerich and V. H. Rawal, Org. Lett., 2008, 10, 2381 CrossRef CAS PubMed; (b) Y. Liu and H. Du, Org. Lett., 2013, 15, 740 CrossRef CAS PubMed; (c) T. D. Montgomery, A. E. Nibbs, Y. Zhu and V. H. Rawal, Org. Lett., 2014, 16, 3480 CrossRef CAS PubMed; (d) R.-D. Gao, C. Liu, L.-X. Dai, W. Zhang and S.-L. You, Org. Lett., 2014, 16, 3919 CrossRef CAS PubMed; (e) A. E. Nibbs, T. D. Montgomery, Y. Zhu and V. H. Rawal, J. Org. Chem., 2015, 80, 4928 CrossRef CAS PubMed. For selected catalytic intramolecular allylic dearomatization reactions of indoles, see: (f) Q.-F. Wu, H. He, W.-B. Liu and S.-L. You, J. Am. Chem. Soc., 2010, 132, 11418 CrossRef CAS PubMed; (g) Q.-F. Wu, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 1680 CrossRef CAS PubMed; (h) Q.-L. Xu, L.-X. Dai and S.-L. You, Chem. Sci., 2013, 4, 97 RSC; (i) J. Chen and M. J. Cook, Org. Lett., 2013, 15, 1088 CrossRef PubMed; (j) T. D. Montgomery, Y. Zhu, N. Kagawa and V. H. Rawal, Org. Lett., 2013, 15, 1140 CrossRef CAS PubMed; (k) X. Zhang, W.-B. Liu, Q.-F. Wu and S.-L. You, Org. Lett., 2013, 15, 3746 CrossRef CAS PubMed; (l) T. M. Kaiser and J. Yang, Eur. J. Org. Chem., 2013, 3983 CrossRef CAS; (m) S.-Z. Jiang, X.-Y. Zeng, X. Liang, T. Lei, K. Wei and Y.-R. Yang, Angew. Chem., Int. Ed., 2016, 55, 4044 CrossRef CAS PubMed; (n) Z.-X. Zhang, S.-C. Chen and L. Jiao, Angew. Chem., Int. Ed., 2016, 55, 8090 CrossRef CAS PubMed.
- For selected recent reviews on allylic alcohols as electrophiles, see: (a) B. Sundararaju, M. Achard and C. Bruneau, Chem. Soc. Rev., 2012, 41, 4467 RSC; (b) M. Bandini, G. Cera and M. Chiarucci, Synthesis, 2012, 44, 504 CrossRef CAS; (c) A. Baeza and C. Nájera, Synthesis, 2014, 46, 25 CrossRef. For selected recent examples on allylic alcohols as electrophiles in natural product synthesis, see: (d) J. Deng, S. Zhou, W. Zhang, J. Li, R. Li and A. Li, J. Am. Chem. Soc., 2014, 136, 8185 CrossRef CAS PubMed; (e) S. Zhou, H. Chen, Y. Luo, W. Zhang and A. Li, Angew. Chem., Int. Ed., 2015, 54, 6878 CrossRef CAS PubMed.
- M. Kimura, M. Futamata, R. Mukai and Y. Tamaru, J. Am. Chem. Soc., 2005, 127, 4592 CrossRef CAS PubMed.
- B. M. Trost and J. Quancard, J. Am. Chem. Soc., 2006, 128, 6314 CrossRef CAS PubMed.
- (a) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9533 CrossRef CAS PubMed; (b) G. Cera, M. Chiarucci, A. Mazzanti, M. Mancinelli and M. Bandini, Org. Lett., 2012, 14, 1350 CrossRef CAS PubMed; (c) M. Bandini, A. Bottoni, M. Chiarucci, G. Cera and G. P. Miscione, J. Am. Chem. Soc., 2012, 134, 20690 CrossRef CAS PubMed.
- (a) M. A. Schafroth, D. Sarlah, S. Krautwald and E. M. Carreira, J. Am. Chem. Soc., 2012, 134, 20276 CrossRef CAS PubMed; (b) O. F. Jeker, A. G. Kravina and E. M. Carreira, Angew. Chem., Int. Ed., 2013, 52, 12166 CrossRef CAS PubMed; (c) J. Y. Hamilton, N. Hauser, D. Sarlah and E. M. Carreira, Angew. Chem., Int. Ed., 2014, 53, 10759 CrossRef CAS PubMed; (d) J. Y. Hamilton, D. Sarlah and E. M. Carreira, J. Am. Chem. Soc., 2014, 136, 3006 CrossRef CAS PubMed; (e) S. Krautwald, M. A. Schafroth, D. Sarlah and E. M. Carreira, J. Am. Chem. Soc., 2014, 136, 3020 CrossRef CAS PubMed; (f) T. Sandmeier, S. Krautwald, H. F. Zipfel and E. M. Carreira, Angew. Chem., Int. Ed., 2015, 54, 14363 CrossRef CAS PubMed.
- X. Zhang, Z.-P. Yang, C. Liu and S.-L. You, Chem. Sci., 2013, 4, 3239 RSC.
- X. Zhang, L. Han and S.-L. You, Chem. Sci., 2014, 5, 1059 RSC.
- For recent review on vinyl epoxides, see: J. He, J. Ling and P. Chiu, Chem. Rev., 2014, 114, 8037 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data, and copies of 1H and 13C NMR spectra. See DOI: 10.1039/c6ob01523a |
| This journal is © The Royal Society of Chemistry 2016 |

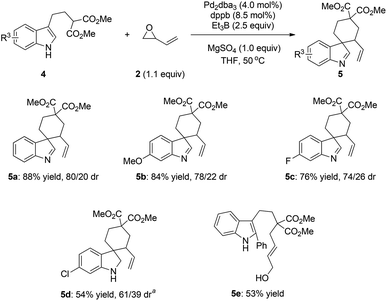
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions: 7.5 mol% Pd2dba3, 16 mol% dppb, 0.5 mL Et3B (1 M in THF) and 0.2 mmol of MgSO4 in THF (2.0 mL) at 50 °C, and then reduced by NaBH3CN.
Reaction conditions: 7.5 mol% Pd2dba3, 16 mol% dppb, 0.5 mL Et3B (1 M in THF) and 0.2 mmol of MgSO4 in THF (2.0 mL) at 50 °C, and then reduced by NaBH3CN.