Total synthesis based on the originally claimed structure of mucosin†
Abstract
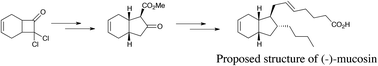
The first total synthesis aimed at the naturally occurring eicosanoid bicycle mucosin is reported. A practical route has been devised allowing the issues relating to the previous assignment of stereochemistry to be examined. X-ray crystallography was performed on a late stage intermediate to pinpoint the topological relationship displayed by the featured bicyclo[4.3.0]non-3-ene scaffold.

 Please wait while we load your content...
Please wait while we load your content...