Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of novel pyrophosphorothiolate-linked dinucleoside cap analogues in a ball mill†
Olga
Eguaogie
a,
Leonie A.
Cooke
a,
Patricia M. L.
Martin
a,
Francesco
Ravalico
a,
Louis P.
Conway
b,
David R. W.
Hodgson
b,
Christopher J.
Law
c and
Joseph S.
Vyle
*a
aSchool of Chemistry and Chemical Engineering, Queen's University Belfast, David Keir Building, Stranmillis Road, Belfast BT9 5AG, UK. E-mail: j.vyle@qub.ac.uk; Fax: +44 (0)28 9097 6524
bDepartment of Chemistry, University of Durham, Science Labs, South Road, Durham DH1 3LE, UK
cSchool of Biological Sciences, Queen's University Belfast, Medical Biology Centre, 97 Lisburn Road, Belfast BT9 7BL, UK
First published on 13th November 2015
Abstract
Michaelis–Arbuzov reactions of S-aryl disulfide derivatives of 3′-thiothymidine or 5′-thioadenosine with tris(trimethylsilyl) phosphite proceeded in high yields to the corresponding phosphorothiolate monoesters. Subsequent hydrolytic desilylation and phosphate coupling were effected in one-pot using liquid-assisted grinding in a vibration ball mill. Novel 3′,5′- and 5′,5′-pyrophosphorothiolate-linked dinucleoside cap analogues were thereby prepared.
Enzymes catalysing the transfer of a ribonucleotidyl moiety (typically AMP) to DNA or RNA strands bearing terminal phosphate monoesters are ubiquitous across all three domains of life.1 The capped products of these transfer reactions contain 3′,5′- and 5′,5′- internucleoside pyrophosphate linkages and have been identified at the termini of DNA, RNA or DNA–RNA chimera.2 These motifs are substrates of both ligases3 and cyclases4 and the different cofactor requirements for prokaryote ligases have led to interest in the potential of these enzymes as novel targets for antimicrobial agents.5
Reflecting the structural diversity of naturally-occurring pyrophosphate-appended nucleosides, a wide variety of methodologies have been developed for their synthesis and that of their analogues.6 One of the most commonly applied strategies involves acidic activation of nucleoside phosphoromorpholidates in the presence of (poly)anionic phosphate substrates.7,8 Anhydrous conditions are required and rendering charged species soluble in organic solvents typically involves cation exchange and extensive pre-drying. This pre-treatment may be incompatible with unstable phosphate monoester substrates and in this context, S-alkyl phosphorothiolate monoesters are susceptible to P–S bond scission especially under acidic conditions.9 In the limited reports of the use of such monoesters as intermediates in, e.g., the preparation of 5′-thioadenosine 5′-triphosphate10 or the corresponding cAMP analogue,11 or as substrates for enzymatic ligation,12 highly attenuated yields were recorded. Displacement of halides or sulfonate esters from primary or allylic positions by inorganic pyrophosphorothioate has been described.13 However, this is not applicable to the installation of the PSC-3′ linkage as considerably more forcing conditions are required which are not compatible with the integrity of the pyrophosphorothiolate moiety.14 Hata and coworkers described the synthesis of an S-aryl phosphoryl imidazolide from which the corresponding pyrophosphorothiolate guanosine and 9-methyl guanosine derivatives were prepared.15 However, to the authors’ knowledge, there have been no reports of the synthesis of an internucleoside pyrophosphorothiolate linkage.
Using mechanochemical activation in a ball mill in the presence of stoichiometric quantities of solvent, efficient phosphate coupling between adenosine-5′-monophosphoromorpholidate (AMP-morpholidate) and a variety of phosphoryl donors as their sodium or barium salts was recently pioneered in the Vyle laboratory.16 In the absence of a requirement to render nucleoside phosphorothiolate monoesters soluble in organic solvents and with expeditious coupling using liquid assisted grinding (LAG), we describe here the preparation of unprecedented dinucleoside pyrophosphorothiolate cap analogues.
Initial attempts to prepare a phosphorothiolate monoester by grinding 5′-chloro-5′-deoxyadenosine and excess inorganic thiophosphate17 using a steel ball and vessel were unsuccessful. Although minimal consumption of the halonucleoside was found, pitting of the steel components and compete degradation of the thiophosphate (by 31P NMR) were observed. We assume that this arises due to leeching of metal ions18 known to catalyse desulfurisation of phosphorothioates.19
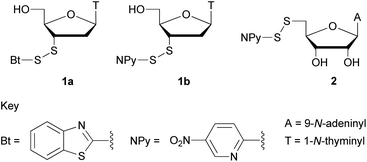
Alternatively, phosphorothiolate monoesters have been prepared following oxidative coupling of P(III) intermediates with thiols20 or under Michaelis–Arbuzov (M–A) conditions.21 In the latter case, tris(trimethylsilyl) phosphite – (TMSO)3P – was reacted with diphenyl disulfide and the monoester liberated following cleavage of the silyl ester functions. However, it was reported that the stability of the product of this reaction was dependent upon the solvent.22 In the current study, we examined the directed M–A reaction between (TMSO)3P and activated S-aryl disulfide derivatives of thionucleosides; 3′-deoxy-3′-(benzothiazoyl-2-disulfanyl)thymidine (1a), 3′-deoxy-3′-(5-nitropyridyl-2-disulfanyl)thymidine (1b) or 5′-deoxy-5′-(5-nitropyridyl-2-disulfanyl)adenosine (2) – Fig. 1.
The 3′-thiothymidine derivatives 1a and 1b were prepared from the corresponding monomethoxytrityl- and acetyl-protected thionucleoside23 adapting literature protocols.24 The previously unreported activated disulfide 1a was accessed using the vulcanization accelerator 2,2′-dithiobis(benzothiazole)25 which is approximately 2% of the price of 2,2′-dithiobis(5-nitropyridine) used in the preparation of 1b and 2.26 The procedure of Schroll et al.27 was adapted to the preparation of 2: trifluoroacetic acid-mediated deprotection of 5′-S-(p-methoxybenzyl)-5′-thioadenosine and in situ disulfide exchange with the nitropyridyl disulfide in the presence of thioanisole proceeded in good yield.
Following reaction of 1a with (TMSO)3P, attempted isolation of pure 3′-thiothymidine 3′-S-phosphorothiolate monoester (dTSMP) was not successful: reversed-phase HPLC purification of the reaction mixture and analysis of the fractions derived from the major peak by 31P NMR showed two resonances at δ ∼ 15 (dTSMP) and δ ∼ 0 (inorganic phosphate). This degradation was further monitored at 25 °C (pH 6.5) by RP-HPLC which confirmed >95% hydrolytic dephosphorylation of dTSMP to 3′-thiothymidine and the corresponding symmetrical disulfide after 27.5 hours.
The M–A reactions of the activated disulfide derivatives of 3′- or 5′-thionucleosides in the presence of the neutral silylating agent N,O-bis(trimethylsilyl)acetamide – BTMSA28 (e.g., Scheme 1) were therefore investigated in more detail by 31P NMR. The thionucleosides were dissolved in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform
1 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTMSA over 30 minutes and a solution of 1.1 equivalents (TMSO)3P (δ 113.5) in the same solvent was added (a total of 13.6 equivalents of BTMSA were thus present).
BTMSA over 30 minutes and a solution of 1.1 equivalents (TMSO)3P (δ 113.5) in the same solvent was added (a total of 13.6 equivalents of BTMSA were thus present).
Rapid consumption of this phosphite was observed and a single major product resonance at δ 2.2 was evident in the reactions of either 1a (Fig. 2) or 1b. This is assumed to originate from the corresponding persilylated 3′-phosphorothiolate (3). Although the benzothiazole-2-thiyl moiety has been used to activate thiols towards cross-coupling, only a single report in the patent literature appears of its application in Michaelis–Arbuzov reactions.29 Under such conditions, these data suggest that the benzothiazole-2-thiolate is an effective leaving group for the directed M–A reaction.
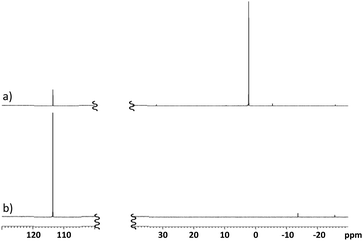 | ||
Fig. 2
31P NMR spectra of: (a) 63 mM (TMSO)3P (1.1 eq.) and disulfide 1a in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CHCl3 1 CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTMSA after 30 min at room temperature; or (b) 63 mM (TMSO)3P in 4 BTMSA after 30 min at room temperature; or (b) 63 mM (TMSO)3P in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CHCl3 1 CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTMSA. BTMSA. | ||
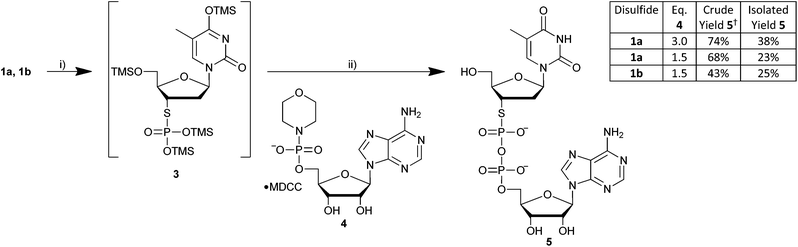
Liquid-assisted grinding in the presence of water has been found to facilitate selective reaction in phosphate coupling reactions performed in a vibration ball mill. Therefore, hydrolytic desilylation of putative 3 in the presence of sub-stoichiometric water and coupling of this hydrolysate with excess AMP-morpholidate (4) in one pot was attempted. M–A reaction mixtures were transferred to zirconia-lined vessels, the volatiles removed in vacuo and argon used to equilibrate to atmospheric pressure. Successive addition of Brønsted (tetrazole) and Lewis (Mg(II)) acid promoters, AMP-morpholidate (1.5 or 3 eq.) and finally water (12 eq.) was then performed prior to commencement of LAG.
After 90 minutes milling at 30 Hz, the reaction mixture derived from the nitropyridyl disulfide (1b) was readily extracted from the vessel using only water. Reaction mixtures derived from the benzothiazole analogue (1a) were less tractable and required both aqueous and organic washes to fully remove all material from the vessels. The crude extracts were subsequently analysed by 31P NMR. In the presence of three equivalents of the phosphoromorpholidate (4) 12% of this reactant (δ ∼ 7) remained unconsumed and very low levels (<2%) of residual dTSMP (δ ∼ 15) were evident (Fig. 3).
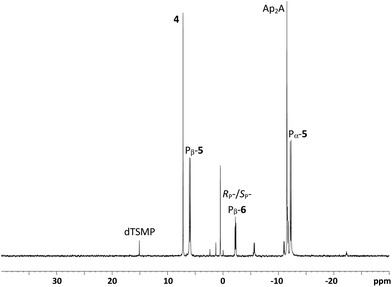 | ||
| Fig. 3 31P NMR spectrum of crude ball milling reaction mixture following hydrolysis and phosphate coupling of the putative persilylated M–A product 3 in the presence of 3 eq. AMP-morpholidate (4). | ||
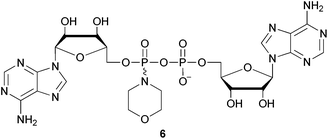
In contrast, ca. 5% unreacted dTSMP was observed in the presence of 1.5 equivalents of 4 which underwent essentially complete consumption. In all extracts, two doublets were observed at δ ∼ 6 and δ ∼ −12 corresponding to the β- and α- phosphoryl groups of dTSppA (5) respectively. These values are consistent with the reported NMR of a pyrophosphorothiolate analogue of UDP-N-acetylglucosamine (δ 5.7 and δ −11.3).30 In addition, 31P NMR signals associated with side-products previously observed31 using such coupling conditions (such as AMP and inorganic phosphate (δ ∼ 0), Ap2A (δ −11.5) and also varying levels of the two diastereoisomers of homocoupled AMP-morpholidate7 (6: Fig. 4) were present. Although the M–A reactions of 1a or 1b gave different heteroaryl thiolate side-products, these did not appear to influence the efficiency of the subsequent phosphate coupling reactions.
Most of these side-products were removed during purification of the crude material by C18 RP-HPLC using triethylammonium acetate (TEAA) buffers. However, dTSppA was found to co-elute with the less hydrophobic isomer of 6 indicated by the presence of additional resonances (ca. 6%–14%) at δ ∼ 2 and δ ∼ −11 in the 31P NMR spectra of all three of the purified reaction mixtures. Separation of this monoanionic pyrophosphoramidate from the desired, dianionic product (5) required application of an ion-pair buffer system which included tetrabutylammonium (TBA) hydrogen sulfate.32 The impurity levels found using 31P NMR were confirmed by the relative peak sizes determined at 260 nm.‡ Pure dTSppA was isolated as the triethylammonium salt (with some residual TBA) following desalting.16
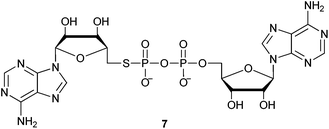
31P NMR analysis of the reaction between 2 and (TMSO)3P under the same M–A reaction conditions used previously, gave one major resonance at δ 4.8 (the putative persilylated adenosine 5′-phosphorothiolate). In contrast to the instability of dTSMP found under aqueous conditions, no significant loss of the signal at δ 4.8 was observed after six days at ambient temperature in the absence of moisture and air. The solvent was then removed and desilylation/phosphate coupling with AMP-morpholidate (1.5 eq.) was performed as above. Following aqueous work-up, analysis of the crude reaction mixture by 31P NMR showed a similar profile to those derived from the 3′,5′ coupling reactions with major product resonances observed at δ 8.0 and δ −12.2 corresponding to the β- and α-phosphoryl groups of dASppA (7): Fig. 5.
Efficient consumption of the putative 5′-thioadenosine 5′-S-phosphorothiolate monoester (dASMP) was observed such that less than 2% of the peak at δ 16.8 remained. In contrast with the protracted procedures required to isolate pure dTSppA, RP-HPLC purification of the less hydrophobic dASppA using only triethylammonium acetate (TEAA) buffers removed all of the side-products (including the isomers of pyrophosphoromorpholidate 6). Subsequent desalting enabled the product to be isolated in 25% yield.
Conclusions
In this communication we have demonstrated the application of novel phosphate coupling chemistry in a ball mill thereby facilitating the preparation of previously inaccessible 3′,5′- and 5′,5′-pyrophosphorothiolate-linked dinucleosides. We are currently investigating the application of this methodology to the synthesis of alkylated guanosine triphosphate caps. As isosteric and isoelectronic analogues of ubiquitous DNA and RNA cap structures, such dinucleotide motifs may provide useful insight into enzymes which process these structures. In addition, we note that the hydrolytic lability of phosphorothiolate monoesters compared with pyrophosphorothiolates may facilitate analysis of decapping activities which, by their pyrophosphatase activities, unmask 3′- or 5′-thiol functions.Acknowledgements
Funding was provided by: the School of Chemistry and Chemical Engineering, QUB (FR, PMLM); the EU (LAC); EPSRC (LPC) and by the authors (JSV, OE). We acknowledge Conor McGrann for performing mass spectra and Richard Murphy for help with NMR.Notes and references
- J. M. Pascal, Curr. Opin. Struct. Biol., 2008, 18, 96 CrossRef CAS PubMed.
- I. R. Lehman, Science, 1974, 186, 790 Search PubMed; H. Cahova, M. L. Winz, K. Hofer, G. Nubel and A. Jaschke, Nature, 2015, 519, 374 CrossRef CAS PubMed; W. Filipowicz, K. Strugala, M. Konarska and A. J. Shatkin, Proc. Natl. Acad. Sci. U. S. A, 1985, 82, 1316 CrossRef; P. Tumbale, J. S. Williams, M. J. Schellenberg, T. A. Kunkel and R. S. Williams, Nature, 2014, 506, 111 CrossRef PubMed.
- A. M. Zhelkovsky and L. A. McReynolds, J. Biol. Chem., 2014, 289, 33608 CrossRef CAS PubMed.
- A. K. Chakravarty, R. Subbotin, B. T. Chait and S. Shuman, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 6072 CrossRef CAS PubMed.
- E.g., G. Pergolizzi, M. M. D. Cominetti, J. N. Butt, R. A. Field, R. P. Bowater and G. K. Wagner, Org. Biomol. Chem., 2015, 13, 6380 CAS.
- Q. Dai, M. Saikia, N. S. Li, T. Pan and J. A. Piccirilli, Org. Lett., 2009, 11, 1067 CrossRef CAS PubMed; Y. Ahmadibeni and K. Parang, Angew. Chem., Int. Ed., 2007, 46, 4739 CrossRef PubMed; H. Tanaka, Y. Yoshimura, M. R. Jorgensen, J. A. Cuesta-Seijo and O. Hindsgaul, Angew. Chem., Int. Ed., 2012, 51, 11531 CrossRef PubMed; H. J. Jessen, N. Ahmed and A. Hofer, Org. Biomol. Chem., 2014, 12, 3526 Search PubMed; P. Wanat, S. Walczak, B. A. Wojtczak, M. Nowakowska, J. Jemielity and J. Kowalska, Org. Lett., 2015, 17, 3062 CrossRef PubMed.
- J. G. Moffatt and H. G. Khorana, J. Am. Chem. Soc., 1961, 83, 649 CrossRef CAS.
- N. Russo and R. Shapiro, J. Biol. Chem., 1999, 274, 14902 CrossRef CAS PubMed.
- S. Akerfeldt, Acta Chem. Scand., 1961, 15, 575 CrossRef CAS; P. Brear, G. R. Freeman, M. C. Shankey, M. Trmčić and D. R. W. Hodgson, Chem. Commun., 2009, 4980 RSC.
- B. K. Patel and F. Eckstein, Tetrahedron Lett., 1997, 38, 1021 CrossRef CAS; A. Stutz and K. H. Scheit, Eur. J. Biochem., 1975, 50, 343 CrossRef PubMed.
- D. A. Shuman, J. P. Miller, M. B. Scholten, L. N. Simon and R. K. Robins, Biochemistry, 1973, 12, 2781 CrossRef CAS PubMed.
- J. M. Thomas and D. M. Perrin, J. Am. Chem. Soc., 2009, 131, 1135 CrossRef CAS PubMed.
- R. M. Phan and C. D. Poulter, J. Org. Chem., 2001, 66, 670 CrossRef.
- W. Dabkowski, M. Michalska and I. Tworowska, Chem. Commun., 1998, 427 RSC; R. Cosstick and J. S. Vyle, J. Chem. Soc., Chem. Commun., 1988, 992 RSC.
- T. Kamimura, Y. Osaki, M. Sekine and T. Hata, Tetrahedron Lett., 1984, 25, 2683 CrossRef CAS.
- F. Ravalico, I. Messina, M. V. Berberian, S. L. James, M. E. Migaud and J. S. Vyle, Org. Biomol. Chem., 2011, 9, 6496 CAS.
- S. K. Yasuda and J. L. Lambert, J. Am. Chem. Soc., 1954, 76, 5356 CrossRef CAS.
- G. Štefanić, S. Krehula and I. Štefanić, Chem. Commun., 2013, 49, 9245 RSC; J. G. Hernández and T. Friščić, Tetrahedron Lett., 2015, 56, 4253 CrossRef.
- J. T. Kodra, J. Kehler and O. Dahl, Nucleic Acids Res., 1995, 23, 3349 CrossRef CAS PubMed.
- W. L. Santos, B. H. Heasley, R. Jarosz, K. M. Carter, K. R. Lynch and T. L. Macdonald, Bioorg. Med. Chem. Lett., 2004, 14, 3473 CrossRef CAS PubMed.
- T. Hata and M. Sekine, J. Am. Chem. Soc., 1974, 96, 7363 CrossRef CAS.
- M. Sekine and T. Hata, Curr. Org. Chem., 1999, 3, 25 CAS.
- B. Gonzalez-Perez, M. Lucas, L. A. Cooke, J. S. Vyle, F. de la Cruz and G. Moncalian, EMBO J., 2007, 26, 3847 CrossRef CAS PubMed.
- D. J. Hansen, I. Manuguerra, M. B. Kjelstrup and K. V. Gothelf, Angew. Chem., Int. Ed., 2014, 53, 14415 CrossRef CAS PubMed; E. Brzezinska and A. L. Ternay, J. Org. Chem., 1994, 59, 8239 CrossRef.
- A. Y. Coran, J. Appl. Polym. Sci., 2003, 87, 24 CrossRef CAS.
- N.-S. Li, J. K. Frederiksen, S. C. Koo, J. Lu, T. J. Wilson, D. M. J. Lilley and J. A. Piccirilli, Nucleic Acids Res., 2011, 39, e31 CrossRef CAS PubMed.
- A. L. Schroll, R. J. Hondal and S. Flemer, J. Pept. Sci., 2012, 18, 1 CrossRef CAS PubMed.
- S. Battaggia and J. S. Vyle, Tetrahedron Lett., 2003, 44, 861 CrossRef CAS.
- S. Torii, K. Uneyama, H. Tanaka, J. Nokami, M. Sasaoka, N. Saito and T. Shiroi, Ger. Offen, DE 3213264, 1982 Search PubMed; Chem. Abstr. 1983 98 125751 Search PubMed.
- I. Jancan and M. A. MacNaughtan, Anal. Chim. Acta, 2012, 749, 63 CrossRef CAS PubMed.
- F. Ravalico, PhD thesis, Queen's University Belfast, 2012.
- J. S. Vyle, N. H. Williams and J. A. Grasby, Tetrahedron Lett., 1998, 39, 7975 CrossRef CAS.
- E. Holler, B. Holmquist, B. L. Vallee, K. Taneja and P. Zamecnik, Biochemistry, 1983, 22, 4924 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ob02061a |
| ‡ The ε260 nm values of the pyrophosphorothiolate-linked dinucleosides are assumed to be the same as the corresponding 3′,5′-8 or 5′,5′-33 linked pyrophosphates. |
| This journal is © The Royal Society of Chemistry 2016 |