Perovskites at the nanoscale: from fundamentals to applications
Joshua J.
Choi
*a and
Simon J. L.
Billinge
*bc
aDepartment of Chemical Engineering, University of Virginia, Charlottesville, VA 22904, USA. E-mail: jjc6z@virginia.edu
bDepartment of Applied Physics and Applied Mathematics, Columbia University, New York, NY 10027, USA
cCondensed Matter Physics and Materials Science Department, Brookhaven National Laboratory, Upton, NY 11973, USA. E-mail: sb2896@columbia.edu
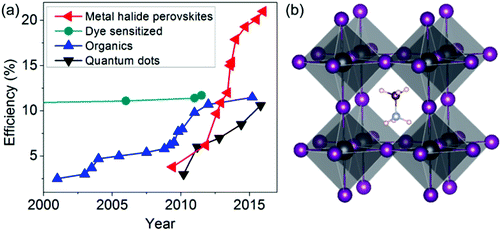
Interestingly, the best solar cell performance has been obtained from hybrid organic–inorganic MHPs such as methylammonium lead iodide (Fig. 1b). The origin of the high performance is far from being understood and a vigorous international research effort is currently directed at this. The presence of organic cations and the structural complexity of these materials both raise interesting research questions. The organic cations have aspherical shape, electric dipole, rapid rotation at room temperature5 and hydrogen bonding to the halides in the inorganic network that cause complex structural dynamics and distortions. Previous studies have shown that the rotation and alignment of electric dipoles in organic cations may play a role in the long carrier lifetime responsible for high solar cell efficiency,6 ferroelectricity-like behavior,7 and low exciton binding energy.8 The precise mechanisms of these phenomena are still actively being studied but establishing the role of the organic cations in the properties of MHPs will be crucially important (Even et al., DOI: 10.1039/c5nr06386h; Leguy et al., DOI: 10.1039/c5nr05435d).
Another characteristic of these materials is the presence of nanoscale structural disorder and a tendency to amorphization, and it will be critical to understand the role this plays in the properties. The presence of local structural distortions in the inorganic framework of these materials was noted early on, well before the materials became ‘plat du jour’.9 Later structural work indicated that solution-processed material had a significant amorphous and nanocrystalline component.10 How can such defective and structurally imperfect materials have such good electronic properties? The charge diffusion length in MHPs has been shown to vary widely depending on the sample preparation method, ranging from hundreds of nanometers in polycrystalline films to 175 microns in single crystals.11–15 These results seem to indicate that higher crystallinity will be beneficial for solar cell performance. However, MHPs turn on its head the conventional notion that high degree of crystallinity is a requirement for achieving high solar cell efficiency (>20%). A majority of MHP solar cells reported to date, including one of the record >20% solar cell results,16 employ solution processing at low temperature that results in polycrystalline thin films with grain sizes typically smaller than 1 micron. Spatially resolved photoluminescence mapping shows wide variation among different MHP grains, which suggests differences in structures at the surfaces as well as crystallinity among the grains.17 Together with the earlier reports of structural disorder,9,10 these results suggest that the origin of high MHP solar cell performance may not be high crystallinity but that the photocarriers are either protected from the disorder, or are somehow using it to their own ends. Answering these questions will be critical to accelerate the progress in MHP solar cell efficiencies as well as rationally identifying other more favorable chemical compositions (more stable and less toxic) that retain the key characteristics for high photovoltaic performance.
To realize the wide-spread commercial deployment of MHP solar cells, arguably the most important challenges to overcome are poor long term stability3 and low reproducibility in the thin film growth and device fabrication process. This is primarily due to insufficient understanding of the MHP nucleation and growth process and material degradation pathways. Accordingly, the MHP research community is devoting significant effort to studying these topics3,18 and this themed issue presents several papers that will help close this knowledge gap (Gangishetty et al., DOI: 10.1039/c5nr04179a; Gouda et al., DOI: 10.1039/c5nr08658b; McLeod et al., DOI: 10.1039/c5nr06217a; Zhou et al., DOI: 10.1039/c5nr06189j). Another important challenge to overcome for commercial deployment of MHP solar cells is the lead toxicity. Identifying non-toxic perovskite compositions with optoelectronic properties suitable for high photovoltaic performance will greatly benefit from rapid materials screening using first-principle calculations. In this issue, Sun et al. (DOI: 10.1039/c5nr04310g) performed first-principle calculations to identify promising lead-free perovskite materials with dual anions such as CH3NH3BiSeI2 and CH3NH3BiSI2.
Improvement in the efficiency and stability of MHP solar cells requires a deeper understanding of the interfacial recombination and charge transfer at the interface with charge transporting layers as well as development of novel materials for better charge collection and light management. Fittingly, the application of nanoscale materials to obtain superior charge transporting layers is an active area of research. These research interests are reflected in a large number of papers that are presented in this themed issue (Cha et al., DOI: 10.1039/c5nr05974g; Jaramillo-Quintero et al., DOI: 10.1039/c5nr06692a; Kim et al., DOI: 10.1039/c5nr04585a; Li et al., DOI: 10.1039/c5nr06177f, Li et al., DOI: 10.1039/c5nr07347b; Liu et al., DOI: 10.1039/c5nr05207f; Long et al., DOI: 10.1039/c5nr05042a; Paek et al., DOI: 10.1039/c5nr05697g; Ponesca et al., DOI: 10.1039/c5nr08622a; Yusoff et al., DOI: 10.1039/c5nr06234a; Zheng et al., DOI: 10.1039/c5nr06715d).
Going beyond the application of MHP thin films for solar cells, there is a growing interest in studying the effect of reduced dimensions and nanostructuring in MHPs. This research pursuit lies at the intersection of MHPs and nanomaterials (quantum dots, two dimensional materials) research fields. Quantum confinement effect and a myriad of properties that arise due to nanostructuring provide a fertile research area as evidenced by papers in this themed issue (Koolyk et al., DOI: 10.1039/c5nr09127f; Sapori et al., DOI: 10.1039/c5nr07175e; Vybornyi et al., DOI: 10.1039/c5nr06890h; Wang et al., DOI: 10.1039/c5nr06262d). The community is making progress in achieving robust methods to synthesize nanostructured MHPs with controlled dimensions with low size dispersion. Theoretical studies on the electronic structure and properties with low dimensions are also being conducted (Sapori et al., DOI: 10.1039/c5nr07175e). Advancing the state of the art for characterizing the atomic and nanoscale structure will be a key to gaining a deeper understanding (Polking, DOI: 10.1039/c5nr06186e). We expect this research area to rapidly advance by drawing upon existing expertise in the nanomaterials research community and open up many exciting new opportunities for fundamental science and technological applications.
In summary, the collection of papers in this themed issue tackles some of the most important and timely questions currently existing in the field of perovskites research. As shown in this issue, there is a great amount of activity and excitement in the rapidly growing perovskite research community. We expect that understanding of perovskites at the nanoscale will be at the heart of advancing the fundamental knowledge and realizing the promised technological applications.
J. J. C. acknowledges support from an Early Career Faculty Award grant from NASA's Space Technology Research Grants Program (NNX15AU43G). S. J. L. B. would like to acknowledge support from NSF through DMR-1534910.
References
- http://www.nrel.gov/ncpv/images/efficiency_chart.jpg .
- T. M. Brenner, D. A. Egger, L. Kronik, G. Hodes and D. Cahen, Nat. Rev. Mater., 2016, 1, 15007 CrossRef.
- T. Leijtens, G. E. Eperon, N. K. Noel, S. N. Habisreutinger, A. Petrozza and H. J. Snaith, Adv. Energy Mater., 2015, 5(20) DOI:10.1002/aenm.201500963.
- S. D. Stranks and H. J. Snaith, Nat. Nanotechnol., 2015, 10, 391–402 CrossRef CAS PubMed.
- T. Chen, B. J. Foley, B. Ipek, M. Tyagi, J. R. D. Copley, C. M. Brown, J. J. Choi and S.-H. Lee, Phys. Chem. Chem. Phys., 2015, 17, 31278–31286 RSC.
- J. Ma and L.-W. Wang, Nano Lett., 2015, 15, 248–253 CrossRef CAS PubMed.
- J. M. Frost, K. T. Butler, F. Brivio, C. H. Hendon, M. van Schilfgaarde and A. Walsh, Nano Lett., 2014, 14, 2584–2590 CrossRef CAS PubMed.
- J. Even, L. Pedesseau and C. Katan, J. Phys. Chem. C, 2014, 118, 11566–11572 CAS.
- R. J. Worhatch, H. Kim, I. P. Swainson, A. L. Yonkeu and S. J. L. Billinge, Chem. Mater., 2008, 20, 1272–1277 CrossRef CAS.
- J. J. Choi, X. Yang, Z. M. Norman, S. J. L. Billinge and J. S. Owen, Nano Lett., 2014, 14, 127–133 CrossRef CAS PubMed.
- A. Buin, P. Pietsch, J. Xu, O. Voznyy, A. H. Ip, R. Comin and E. H. Sargent, Nano Lett., 2014, 14, 6281–6286 CrossRef CAS PubMed.
- Q. Dong, Y. Fang, Y. Shao, P. Mulligan, J. Qiu, L. Cao and J. Huang, Science, 2015, 347, 967–970 CrossRef CAS PubMed.
- D. Shi, V. Adinolfi, R. Comin, M. Yuan, E. Alarousu, A. Buin, Y. Chen, S. Hoogland, A. Rothenberger, K. Katsiev, Y. Losovyj, X. Zhang, P. A. Dowben, O. F. Mohammed, E. H. Sargent and O. M. Bakr, Science, 2015, 347, 519–522 CrossRef CAS PubMed.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–344 CrossRef CAS PubMed.
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Grätzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 342, 344–347 CrossRef CAS PubMed.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Science, 2015, 348, 1234–1237 CrossRef CAS PubMed.
- D. W. D. Quilettes, S. M. Vorpahl, S. D. Stranks, H. Nagaoka, G. E. Eperon, M. E. Ziffer, H. J. Snaith and D. S. Ginger, Science, 2015, 348, 683–686 CrossRef PubMed.
- Y. Zhou, O. S. Game, S. Pang and N. P. Padture, J. Phys. Chem. Lett., 2015, 6, 4827–4839 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |