Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbon nanohorns allow acceleration of osteoblast differentiation via macrophage activation†
Eri
Hirata
*a,
Eijiro
Miyako
b,
Nobutaka
Hanagata
c,
Natsumi
Ushijima
d,
Norihito
Sakaguchi
e,
Julie
Russier
f,
Masako
Yudasaka
bg,
Sumio
Iijima
g,
Alberto
Bianco
f and
Atsuro
Yokoyama
a
aDepartment of Oral Functional Prosthodontics, Division of Oral Functional Science, Graduate School of Dental Medicine, Hokkaido University, Kita 13, Nishi 7, Kita-ku, Sapporo 060-8586, Japan. E-mail: erieri@den.hokudai.ac.jp; Tel: +81 11706 4270
bNanomaterials Research Institute (NMRI), National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba Central 5, 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan
cNanotechnology Innovation Station, National Institute for Materials Science, 1-2-1 Sengen, Tsukuba, Ibaraki 305-0047, Japan
dSupport Section for Education and Research, Graduate School of Dental Medicine, Hokkaido University, Kita 13, Nishi 7, Kita-ku, Sapporo 060-8586, Japan
eCenter for Advanced Research of Energy and Materials, Faculty of Engineering, Hokkaido University, Kita 13, Nishi 8, Kita-ku, Sapporo 060-8586, Japan
fCNRS, Institut de Biologie Moléculaire et Cellulaire, Laboratoire d'Immunopathologie et Chimie Thérapeutique, 67000 Strasbourg, France
gMeijo University, Graduate School of Science and Technology, 1-501, Shiogamaguchi, Tenpaku, Nagoya, Aichi 468-8502, Japan
First published on 28th June 2016
Abstract
Carbon nanohorns (CNHs), formed by a rolled graphene structure and terminating in a cone, are promising nanomaterials for the development of a variety of biological applications. Here we demonstrate that alkaline phosphatase activity is dramatically increased by coculture of human monocyte derived macrophages (hMDMs) and human mesenchymal stem cells (hMSCs) in the presence of CNHs. CNHs were mainly localized in the lysosome of macrophages more than in hMSCs during coculturing. At the same time, the amount of Oncostatin M (OSM) in the supernatant was also increased during incubation with CNHs. Oncostatin M (OSM) from activated macrophage has been reported to induce osteoblast differentiation and matrix mineralization through STAT3. These results suggest that the macrophages engulfed CNHs and accelerated the differentiation of mesenchymal stem cells into the osteoblast via OSM release. We expect that the proof-of-concept on the osteoblast differentiation capacity by CNHs will allow future studies focused on CNHs as ideal therapeutic materials for bone regeneration.
Introduction
Bone fractures, osteoarthritis, osteoporosis or bone cancers represent common and serious clinical problems. The management and reconstruction of damaged or diseased bone tissues is still an important global healthcare challenge to improve the lives of the patients in order to recover their normal functions and health.1Carbon nanomaterials, such as carbon nanotubes (CNTs), graphene and carbon nanohorns (CNHs), have been studied for biomedical applications because of their unique characteristics.2–11 Carbon nanomaterials are promising candidates for bone tissue engineering applications due to their superior cytocompatible, mechanical and electrical properties.12–18 Some years ago we initiated a program on the applications of carbon nanomaterials for bone tissue regeneration. We have reported that CNT-coated substrates can be effective for the adhesion and differentiation of osteoblasts, while CNT-coated collagen sponges resulted in possessing a favorable biocompatibility profile with bone.19–22 On the other hand, the impurities (e.g. metal catalysts and amorphous carbons) and the high aspect ratio of CNTs might lead to concerns about their safety for clinical uses.23,24
There is currently a great interest in creating biomedical applications using CNHs,25–27 owing to their advantages, such as low toxicity and huge inner nanospaces for drug loading.28,29 We previously found that CNHs promoted bone formation within a period of 2 weeks.25 More interestingly, we observed that a high amount of CNHs was localized inside the macrophages around the newly formed bone.25 However, the mechanism of bone formation by CNHs has not been clarified yet. Therefore, in this study, we focused our attention on the effect of macrophages loaded with CNHs on osteoblast differentiation. Several studies have reported that immune cells including monocytes and macrophages are key players in bone tissue integration with various biomaterials.30 We hypothesized that CNHs will be able to stimulate the macrophages for the production of osteoinductive factors such as cytokines, which are necessary for the differentiation of hMSCs into osteoblasts and the formation of new bone. Nicolaidou et al. reported that monocytes/macrophages cultured on human bone marrow-derived mesenchymal stem cells directly and potently induced hMSC differentiation into osteoblasts.31 On the basis of these findings, in this study, hMDMs were cultured with hMSCs in the presence of CNHs, in order to elucidate the effect of CNHs on macrophages for the differentiation of the stem cells into osteoblasts. First, the influence and localization of CNHs into hMDMs were investigated. The increase in the amount of alkaline phosphatase (ALP) activity from coculturing hMDMs and hMSCs with CNHs was assessed. In addition, we evidenced that the expression of Oncostatin M (OSM), a multifunctional cytokine that induces osteoblast differentiation and matrix mineralization, increased in the presence of CNHs.32 The obtained results show more accurately how CNHs can influence the formation of new bone.
Results
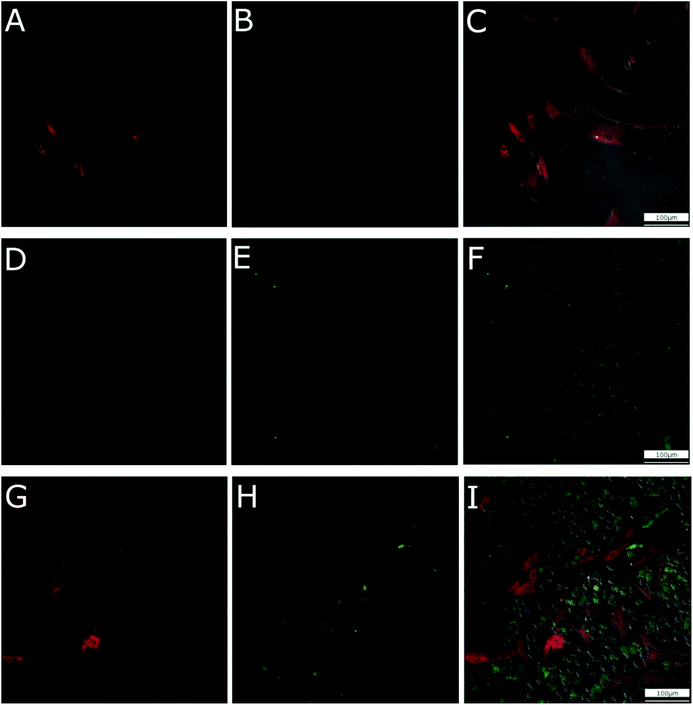
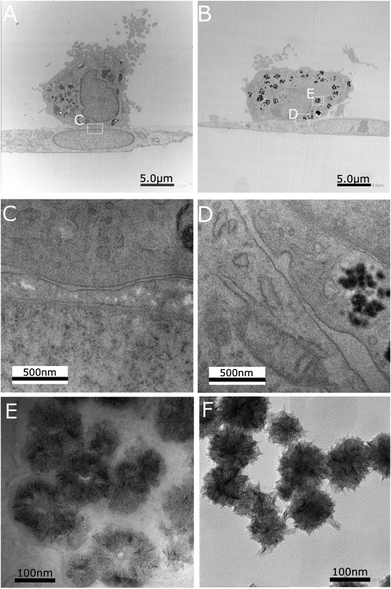
In order to clearly observe the cellular uptake of CNHs during the coculture of hMSCs and hMDMs, stem cells, labelled with CMPTX dye, and the macrophages were cultured with CNHs functionalized with fluorescent Alexa488-BSA (Alexa-BSA-CNHs) for 24 hours (Fig. 1). During cell culturing with increasing concentrations of CNHs, we observed that 50 μg mL−1 (the highest dose used) of CNHs was extensively aggregated in the culture medium. So we decided to use 5 μg mL−1 of CNHs for the subsequent experiments. CNHs at 5 μg mL−1 remained well dispersed for 7 days in cell culture media. The confocal microscopy images showed that very few Alexa-BSA-CNHs were present inside the hMSCs. There were significantly low levels of fluorescence from Alexa-BSA-CNHs in these cells (Fig. 1A–C). On the other hand, most of the hMDMs were able to internalize a large number of fluorescent Alexa-BSA-CNHs (Fig. 1D–F). More interestingly, we could observe that high amounts of Alexa-BSA-CNHs were also present inside the hMDMs in comparison with hMSCs under coculturing conditions (Fig. 1G–I).To further observe the presence of CNHs in these two types of cells, the cellular uptake behavior of CNHs after coculturing for 24 hours was analyzed by TEM (Fig. 2). Many CNHs were clearly observed in the hMDMs that were in close contact with the hMSCs (Fig. 2B). The morphology and structure of the cells were not affected compared to the control cells without CNHs (Fig. 2A). Most of the macrophages were in close contact with stem cells (Fig. 2C and D). We observed many CNHs in the cytoplasmic vesicles. In the lysosomes and the endosomes, CNHs taken up by hMDMs preserved their globular structures (Fig. 2E), similar to control CNHs (Fig. 2F). After 7 days of coculturing, CNHs mainly remained inside the hMDMs (Fig. S1†).
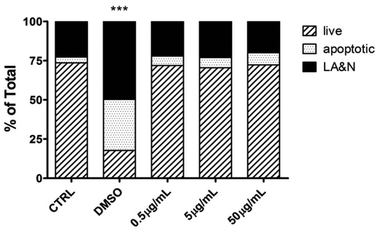
Next, hMDMs were incubated with different concentrations of CNHs (0.5, 5.0, 50 μg mL−1) for 24 hours to explore the effect of CNHs on the cellular viability of human macrophages. At the end of the incubation time, the cells were stained with AnnV and PI to determine the cell viability (Fig. 3). CNHs did not cause any significant necrosis or apoptosis at any concentrations compared with the untreated cells. The quantity of CD86, a co-stimulatory molecule expressed by macrophages upon activation,28 was not affected at the different concentrations of CNHs tested (Fig. S2†).
In order to explore cell response to CNHs by gene expression, microarray analysis was carried out after culturing hMDMs with CNHs for 24 hours. We identified 30 modified genes in hMDMs treated with CNHs. We identified 30 differentially expressed genes whose fold-change represented by the logarithmic ratio (log2 ratio) to the expression level of the control was more than 1 (>1) and less than −1 (<−1). Of these 30 altered genes, 16 were up-regulated and 14 were down-regulated genes (Table 1). By classifying these genes into the Gene Ontology (GO) Biological Process category, we obtained 5 statistically significant (p < 1 × 10−5) GO terms that are related to lymphocyte migration from the CNH up-regulated genes (Table 2). On the other hand, no GO terms were obtained from the CNH down-regulated genes. The up-regulated genes classified into the lymphocyte migration related GO terms included genes that encode chemokines like CCL3, CCL4 and CXCL12 (Table 2). The expression levels of these chemokine-related genes were also analyzed by real time RT-PCR, and this analysis verified the upregulation in hMDMs treated with CNHs (Fig. 4).
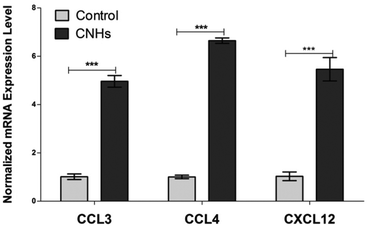 | ||
| Fig. 4 qPCR analysis of relative expression levels of CCL3, CCL4 CXCL12 cytokines for hMDMs cultured with or without CNHs for 24 hours. ***p < 0.001. | ||
| A | |||
|---|---|---|---|
| Gene name | Systematic name | CNHs/CTRL [rep.] | Description |
| CCL4 | NM_002984 | 1.472 | Chemokine (C–C motif) ligand 4 (CCl4), mRNA |
| NFATC2 | NM_173091 | 1.405 | Nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 2 (NFATC2), transcript variant 2, mRNA |
| G0S2 | NM_015714 | 1.372 | G0/G1 switch 2 (G0S2), mRNA |
| ANKRD29 | NM_173505 | 1.365 | Ankyrin repeat domain 29 (ANKRD29), mRNA |
| CCL4L2 | NM_001291470 | 1.343 | Chemokine (C–C motif) ligand 4-like 2 (CCL4L2), transcript variant CCL4L2b2, mRNA |
| FBLN5 | NM_006329 | 1.251 | Fibulin 5 (FBLN5), mRNA |
| CCL3 | NM_002983 | 1.229 | Chemokine (C–C motif) ligand 3 (CCL3), mRNA |
| FBLIM1 | NM_017556 | 1.167 | Filamin binding LIM protein 1 (FBLIM1), transcript variant 1, mRNA |
| CXCL12 | NM_199168 | 1.147 | Chemokine (C–X–C motif) ligand 12 (CXCL12), transcript variant 1, mRNA |
| CTSZ | ENST00000503833 | 1.135 | Cathepsin Z (source: HGNC symbol; Acc: 2547) |
| NAF1 | NM_138386 | 1.119 | Nuclear assembly factor 1 ribonucleoprotein (NAF1), transcript variant 1, mRNA |
| FN1 | NM_054034 | 1.099 | Fibronectin 1 (FN1), transcript variant 7, mRNA |
| P2RY1 | NM_002563 | 1.092 | Purinergic receptor P2Y, G-protein coupled, 1 (P2RY1), mRNA |
| PARP15 | NM_001113523 | 1.085 | Poly(AOP-ribose) polymerase family, member 15 (PARP15), transcript variant 1, mRNA |
| CCL3L3 | NM_001001437 | 1.024 | Chemokine (C–C motif) ligand 3-like 3 (CCL3L3), mRNA |
| NEURL3 | NM_001285486 | 1.000 | Neuralized E3 ubiquitin protein ligase 3 (NEURL3), transcript variant 2, mRNA |
| B | |||
| IGF2BP1 | NM_006548 | −1.942 | Insulin-like growth factor 2 mRNA binding protein 1 (IGF2BP1), transcript variant 1, mRNA |
| TIPARP | NM_001184717 | −1.459 | TCDD-inducible poly(ADP-ribose) polymerase (TIPARP), transcript variant 1, mRNA |
| SULF2 | NM_018837 | −1.291 | Sulfatase 2 (SULF2), transcript variant 1, mRNA |
| CNR2 | NM_001841 | −1.240 | Cannabinoid receptor 2 (macrophage) (CNR2), mRNA |
| CYP1B1 | NM_000104 | −1.151 | Cytochrome P450, family 1, subfamily B, polypeptide 1 (CYP1B1), mRNA |
| XYLT1 | NM_022166 | −1.139 | Xylosyltransferase I (XYLT1), mRNA |
| FRY | NM_023037 | −1.130 | Furry homolog (Drosophila) (FRY), mRNA |
| CTTNBP2 | NM_033427 | −1.106 | Cortactin binding protein 2 (CTTNBP2), mRNA |
| LOC100128288 | NR_024447 | −1.077 | Uncharacterized LOC100128288 (LOC100128288), long non-coding RNA |
| LOC100127886 | AF090938 | −1.070 | Clone HQ0628 PRO0628 mRNA, complete cds. |
| LINC00926 | NR_024433 | −1.067 | Long intergenic non-protein coding RNA 926 (LINC00926), long non-coding RNA |
| CLEC10A | NM_182906 | −1.030 | C-type lectin domain family 10, member A (CLEC10A), transcript variant 1, mRNA |
| S100B | NM_006272 | −1.007 | S100 calcium binding protein B (S100B), mRNA |
| PRRT1 | NM_030651 | −1.002 | Proline-rich transmembrane protein 1 (PRRT1), mRNA |
| GO ID | GO term | P-value | Genes | |||
|---|---|---|---|---|---|---|
| GO:2000403 | Positive regulation of lymphocyte migration | 4.65 × 10−7 | CCL3 | CCL4 | CXCL12 | |
| GO:2000401 | Regulation of lymphocyte migration | 1.37 × 10−6 | CCL3 | CCL4 | CXCL12 | |
| GO:2000503 | Positive regulation of natural killer cell chemotaxis | 5.12 × 10−6 | CCL3 | CCL4 | ||
| GO:0072676 | Lymphocyte migration | 6.26 × 10−6 | CCL3 | CCL4 | CXCL12 | |
| GO:0043270 | Positive regulation of ion transport | 8.20 × 10−6 | CCL3 | CCL4 | CXCL12 | P2RY1 |
ALP is one of the osteoblastic differentiation markers at the early stages. After 7 days, ALP activity was higher in the cocultured hMSCs and hMDMs both with and without CNHs compared with those of MSCs alone. Moreover ALP activity in cocultures is dramatically increased by CNHs at 5 μg mL−1 (Fig. 5A). CNHs further increased the ALP activity of cocultures after 14 days, while the ALP activity of hMSCs cocultured with hMDM did not change in the absence of CNHs (Fig. 5B).
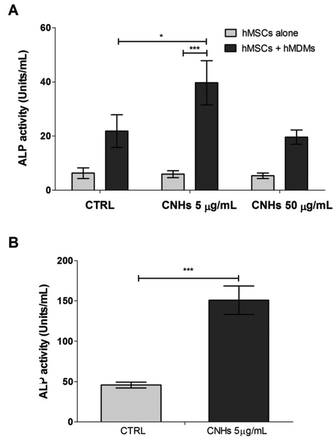 | ||
| Fig. 5 ALP activity of hMSC alone or cocultured hMDMs and hMSCs with or without CNHs after 7 days (A) and ALP activity cocultured after 14 days (B). ***p < 0.001. | ||
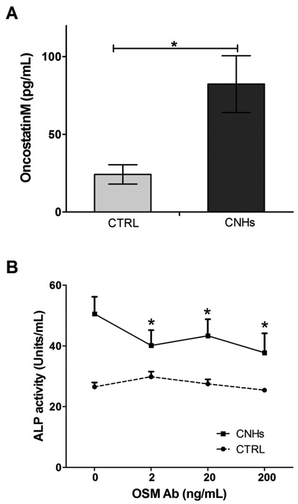
Several studies have reported that monocytes and macrophages directly regulate the osteogenic differentiation of MSCs through a mechanism that involves cell contact, leading to the production of OSM by the monocytes.31,33 In this study, OSM levels in supernatants from hMSCs cocultured with hMDM treated with and without CNHs were measured in order to investigate whether OSM is one of the soluble factors increased by CNHs during coculturing. The amount of OSM in the supernatant with CNHs was 3 times higher than that of the control experiment without CNHs (Fig. 6A). To measure how much the OSM in the coculturing medium with CNHs affects the induction of ALP, an OSM-neutralized antibody was added to hMSC and hMDM cocultures at increasing concentrations (2, 20 and 200 ng ml−1) with and without CNHs. ALP activity was quantified after 7 days. The addition of the OSM-neutralizing antibody in the cocultured hMSCs and hMDMs with CNHs prevented the ALP induction (Fig. 6B).
Discussion
In our previous studies, we found that CNHs accelerated bone regeneration. In order to elucidate the mechanism of bone formation, the behavior of macrophages in the presence of CNHs and the effect on mesenchymal stem cells were investigated in cocultured cells.According to the results of confocal microscopy, a large number of CNHs were located in the hMDMs rather than hMSCs. TEM observations confirmed that CNHs were present in the subcellular compartments of the macrophages (i.e. lysosomes and endosomes). It was already reported that phagocytic cells commonly internalize carbon nanohorns via endocytosis,34 and accumulate them in the lysosomes.35 These results definitely show that CNHs are taken up by macrophages with high selectivity, although the elucidation of the precise process beyond the selective cellular internalization of the CNHs, is an issue of future research.
CNHs did not increase cell apoptosis and necrosis at least up to 50 μg mL−1 as shown by flow cytometry analyses, although CNHs were highly accumulated into the lysosomes. Indeed, many researchers have reported that the cytotoxicity of CNHs was very low.6,29,35,36 However, a high uptake level of CNHs in RAW 264.7, a well-known murine macrophage cell line, seemed to generate reactive oxygen species (ROS), lysosomal membrane destabilization, cell apoptosis and necrosis.35 Russier et al. reported that human macrophages appeared less responsive to carbon nanomaterials in comparison with murine macrophage. This work suggests that hMDMs likely respond to CNHs less than murine macrophage. Our results are similar to those obtained with other types of nanomaterials and nanoparticles, designed for different applications (i.e. as contrast agents for imaging or for drug delivery), which resulted immune compatible or could exert an immune specific action depending on their composition and surface coating.37,38
Microarray analysis indicates that chemokine-related genes, including CCL3, CCL4, and CXCL12, were expressed significantly higher in hMDMs treated with CNHs than without CNHs. GO analysis suggests that these up-regulated genes regulate lymphocyte migration. Furthermore, it has been reported that these chemokines are involved not only in immunoregulatory and inflammatory processes but also in tissue repair.39 For example, CXCL12 was reported to play a role in the maintenance, survival, and osteogenic capacity of immature bone marrow stromal stem cell populations.40 Our results clearly indicate that CNHs might be promising regulators for a variety of immune system reactions without triggering any cytotoxicity.
In order to elucidate the relation between macrophages with internalized CNHs and bone formation, human macrophage and mesenchymal stem cells were cocultured in the presence of CNHs (Fig. 5). CNHs dramatically increased the ALP activity of the cocultures. According to the TEM observations (Fig. 2C and D), hMDMs have the possibility to communicate with hMSCs via molecular signaling because of the tight contact observed between these two types of cells. Several studies have reported that macrophages directly regulate osteogenic differentiation of MSCs through a mechanism that involves cell contact leading to the production of Oncostatin M by monocytes and STAT3 signaling in MSCs.31,33 OSM, which is produced by activated monocytes, is a multifunctional cytokine that influences the growth and differentiation of several cell types.32,41In vitro studies on osteoblastic models have demonstrated that OSM stimulates osteogenic differentiation in MSCs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 and inhibits adipogenic differentiation of hMSCs.43 In support of these studies, we found that OSM was increased in the medium of cocultured hMSCs and hMDMs. Moreover, OSM was significantly increased in the presence of CNHs (Fig. 6A). An OSM-neutralizing antibody prevented ALP induction in the presence of CNHs but had no effect on ALP activity without CNHs (Fig. 6B). These data suggested that ALP activity is enhanced by OSM produced during coculturing hMDMs and hMSCs in the presence of CNHs.
42 and inhibits adipogenic differentiation of hMSCs.43 In support of these studies, we found that OSM was increased in the medium of cocultured hMSCs and hMDMs. Moreover, OSM was significantly increased in the presence of CNHs (Fig. 6A). An OSM-neutralizing antibody prevented ALP induction in the presence of CNHs but had no effect on ALP activity without CNHs (Fig. 6B). These data suggested that ALP activity is enhanced by OSM produced during coculturing hMDMs and hMSCs in the presence of CNHs.
Even with the addition of an OSM-neutralizing antibody, ALP activity was still higher than control. Therefore there might be other factors involved in the increase of ALP activity. For instance, several studies have reported that CXCL12 promotes the growth, survival, and development of hMSCs,44 and bone formation.12,45 Further studies must be performed to find the other factors increasing bone formation by CNHs. However, these data suggested that OSM is one of the possible factors to induce hMSC differentiation into osteoblasts in cocultures with hMDM loaded with CNHs.
The immune cell responses to biomaterial interactions and subsequent effects of factors released by immune cells on osteoblastic cells are important.46 Few studies with different biomaterials have described bone formation via macrophage activation. For example, a recent systematic review of dental implants reported that over 90% of research in this area focused primarily on the in vitro behavior of osteoblasts on implant surfaces while only a small percentage (roughly 10%) was dedicated to immune cells.47 Almost all of the studies about carbon nanomaterials and bone also focused mainly on osteoblasts. For instance, Shimizu et al. showed that multi-walled carbon nanotubes (MWCNTs) can promote bone formation by interacting with osteoblasts by accumulating calcium which adhered to MWCNTs.48 In an in vitro study Saito et al. found that CNTs are suitable to stimulate osteoblast functions.49 Misra et al. reported that CNHs and graphene oxide inside polymeric materials are able to enhance osteoblast functions and cellular interactions.13,50 As far as we know, there have been no studies investigating the mechanisms of bone formation using carbon nanomaterials with the focus on the relationship between macrophages, mesenchymal stem cells and these nanomaterials.
This study demonstrates one of the possible mechanisms for bone formation with CNHs. Our findings may be an important milestone and inspire a new design of therapeutic materials for bone regeneration using CNHs such as dental implant and osteoblast cell culture scaffolds.
Experimental section
Preparation of CNH dispersion
CNHs were produced by CO2 laser ablation of graphite without the metal catalysts.28 CNHs were oxidized with air by increasing the temperature at 1 °C min−1 from room temperature to 500 °C, followed by cooling.51 Functionalization of CNHs with Alexa488-BSA (Alexa-BSA-CNHs) was performed as previously described.36 The CNHs or Alexa-BSA-CNHs were dispersed in bovine serum albumin (BSA) at a concentration of 1 mg mL−1. The general medium consisted of DMEM Glutamax media (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS: MP Biomedicals, OH) and streptomycin/penicillin (Gibco). The CNH dispersions by BSA were diluted with the general medium by serial dilution (0.5, 5.0, 50 μg mL−1) and used for human hMDM culture and cocultures of hMDMs and hMSCs.Cell culture
Ethical approval for the use of peripheral blood from healthy donors was obtained from the Hokkaido University Graduate School of Dental Medicine Ethics Committee (No. 2014-6). Whole blood used in this study was obtained from donors with written informed consent. Peripheral blood mononuclear cells (PBMCs) from healthy adult donors were collected by centrifugation over a Ficoll-Histpaque-1077 (Sigma, MO). CD14+ cells were magnetically labeled with CD14 microbeads and positively selected by MACS Technology (Miltenyi Biotec, Germany). The medium for PBMCs consisted of RPMI 1640 (Sigma) including 10% heat inactivated FBS, 10 mM HEPES (Lonza) and streptomycin/penicillin. PBMCs were cultured at 37 °C, 5% CO2, in 12-well plates at a density of 3 × 106 cells per well in the medium for PBMC for one day.52 hMDMs were obtained by culturing PBMCs in the medium supplemented with 12.5 ng ml−1 of a macrophage colony stimulating factor (M-CSF; ImmunoTools) for an additional 6 days. hMSCs were commercially purchased (Product No. PT-2501, Lonza, Switzerland). hMSCs were maintained in a general medium and used between passages 3 and 8. Four hours after seeding, each medium was replaced with the general medium with and without CNHs.Confocal laser scanning electron microscopy observation
hMSCs were subcultured and stained by the Cell Tracker Red CMPTX dye (Molecular Probes) one day before seeding. Each cell was seeded on an 8-well cell culture slide (Falcon) in 200 μL medium at a density of 5 × 104 cells per mL in the general medium for hMSCs or 5 × 105 cells per mL for hMDMs in the medium for PBMCs. For the cocultures, hMSCs were seeded at first and then hMDMs were seeded on them. Four hours after seeding, each medium was replaced with the medium with and without Alexa-BSA-CNHs (5 μg mL−1). After 24 hours, the cellular uptake of CNHs was observed using an inverted microscope (Nikon Ti-E, Japan) with a confocal laser scanning system (Nikon A1, Japan).Transmission electron microscopy observation
hMSCs were seeded at first on glass coverslips in 24-well plates at a density of 5000 cells per well and hMDMs were seeded on them at a density of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well and cultured with and without CNHs (5 μg mL−1) for 24 hours. For TEM samples, polymerized blocks with embedded cells were prepared as previously described.52 Afterwards, the glass coverslips were removed from the polymerized block surface. A cube-shaped sample was taken from the capsule and a resin was applied to the surface. Then, the cube was laid on its side and cut into ultrathin sections. The ultrathin sections were obtained using an ultramicrotome (Leica) with a diamond knife (DiATOME). The ultrathin sections were examined by TEM (JEM1400 80 V and Titan cubed G2 60-300 operated at 60 kV).
000 cells per well and cultured with and without CNHs (5 μg mL−1) for 24 hours. For TEM samples, polymerized blocks with embedded cells were prepared as previously described.52 Afterwards, the glass coverslips were removed from the polymerized block surface. A cube-shaped sample was taken from the capsule and a resin was applied to the surface. Then, the cube was laid on its side and cut into ultrathin sections. The ultrathin sections were obtained using an ultramicrotome (Leica) with a diamond knife (DiATOME). The ultrathin sections were examined by TEM (JEM1400 80 V and Titan cubed G2 60-300 operated at 60 kV).
Detection of apoptotic cells
hMDMs were seeded in 98-well plates (1.5 × 105 cells per well) and cultured with each CNH medium (0, 0.5, 5.0, 50 μg mL−1) while DMSO 10% was used as positive control for cell death. Flow cytometry analysis was carried out as previously reported,52 using APC-Annexin V (AnnV; BD Pharmingen 550475) and propidium iodide (PI, 0.2 μg mL−1; Sigma-Aldrich) in a calcium containing buffer. The percentage of live (AnnV−/PI−), early apoptotic (AnnV+/PI−) and late apoptotic/necrotic (AnnV+/PI+) and AnnV−/PI+) cells was determined by acquiring at least 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events using a FACS Flow Cytometer (Gallios, Beckman Coulter) and by analyzing the data on CD14+ hMDM (FITC-Mouse anti-Human CD14, Clone M5E2, BD Pharmingen 555397) gated populations with FlowJo software.
000 events using a FACS Flow Cytometer (Gallios, Beckman Coulter) and by analyzing the data on CD14+ hMDM (FITC-Mouse anti-Human CD14, Clone M5E2, BD Pharmingen 555397) gated populations with FlowJo software.
Microarray analysis
The procedure of DNA microarray analysis has been described in detail previously.53 Briefly, the total RNA of hMDMs cultured for one day with CNHs (5 μg mL−1) was extracted with ISOGEN (Nippon Gene, Tokyo, Japan) according to the manufacturer's instructions. mRNA was amplified with the Animo Allyl MessageAmp II aRNA amplification kit (Thermo Fisher Scientific, Waltham, MA, USA) and labeled with Cy3 or Cy5. The global gene expression analysis was performed with the Whole Human Genome Microarray Kit 4 × 44 K (Agilent, Santa Clara, CA, USA). The fluorescent intensity of Cy3 and Cy5 in each spot was scanned with a GenePix 4000B and detected with a GenePix Pro (Molecular Devices, Sunnyvale, CA, USA). Gene expression data obtained from fluorescent intensity were globally normalized, and locally weighted scatter plot smoothing adjustment was applied. The DNA microarray experiment was conducted twice, and genes whose expression level ratios from two experiments were less than double were identified as valid data. The extracted up-regulated and down-regulated genes were placed in Gene Ontology bioprocess categories using the PANTHER gene expression analysis/compare gene lists.Real-time polymerase chain reaction
Total RNA was extracted from one day hMDM cultures using ISOGEN (Nippon gene). First-strand cDNA was synthesized from 500 ng total RNA using Primescript (Takara). The real-time polymerase chain reaction (PCR) contained 10 ng reverse transcribed total RNA, 400 nM primers (Table S1†), and SYBR Premix Ex Taq (Takara). Quantitative PCRs (qPCRs) were carried out on a StepOnePlus RealTime PCR System (Applied Biosystems). Relative quantification was made against serial dilution of GAPDH cDNA which was used as a house-keeping gene.Measurement of ALP activity
hMSCs were seeded in 24-well plates at a density of 5000 cells per well and hMDMs were seeded on them at a density of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well for coculture. After 7 and 14 days of cell culture with and without CNHs (0.5, 5, 50 μg mL−1), the amount of the ALP activity in the cells was measured as previously reported.22
000 cells per well for coculture. After 7 and 14 days of cell culture with and without CNHs (0.5, 5, 50 μg mL−1), the amount of the ALP activity in the cells was measured as previously reported.22
Measurement of OSM levels and ALP activity with OSM neutralizing antibody
After a 7 day coculture, the OSM levels in supernatants were measured using the Human OSM DuoSet (R&D systems). The OSM neutralizing antibody was added to the cocultures at increasing concentrations (2, 20, 200 ng ml−1) in the general medium and ALP activity was quantified after additional 7 days.Statistical analysis
All data are presented ± standard error of the mean (SEM). Statistical analyses were performed using GraphPad software and two-way ANOVA followed by Bonferroni's post-test or Student's t test. All p values <0.05 were considered significant.Acknowledgements
E. H. wishes thank the KAKENHI Grant-in-Aid for Young Scientists B (ID No. 26861616). A. Y. wishes to thank the Ministry of Education, Science, Culture and Sport of Japan for Grant-in Aid for Scientific Research B (ID No. 25293389). A. B. wishes to thank JSPS (Japanese Society for the Promotion of Science) for the Invitation Fellowship in Japan (ID No. L15526). E. M. wishes to thank for KAKENHI Grant-in-Aid for Scientific Research (B) (16H03834) and KAKENHI Grant-in-Aid for Challenging Exploratory Research (16K13632). A part of this work was conducted at Hokkaido University, supported by the “Nanotechnology Platform” Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This work was also partly supported by CNRS. Confocal images were acquired at the Nikon Imaging Center at Hokkaido University.Notes and references
- J. J. Li, D. L. Kaplan and H. Zreiqat, J. Mater. Chem. B, 2014, 2, 7272–7306 RSC.
- I. Marangon, C. Ménard-Moyon, A. K. A. Silva, A. Bianco, N. Luciani and F. Gazeau, Carbon, 2016, 97, 110–123 CrossRef CAS.
- E. Miyako, T. Deguchi, Y. Nakajima, M. Yudasaka, Y. Hagihara, M. Horie, M. Shichiri, Y. Higuchi, F. Yamashita, M. Hashida, Y. Shigeri, Y. Yoshida and S. Iijima, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 7523–7528 CrossRef CAS PubMed.
- E. Miyako, K. Kono, E. Yuba, C. Hosokawa, H. Nagai and Y. Hagihara, Nat. Commun., 2012, 3, 1226 CrossRef PubMed.
- T. Murakami, K. Ajima, J. Miyawaki, M. Yudasaka, S. Iijima and K. Shiba, Mol. Pharm., 2004, 1, 399–405 CrossRef CAS.
- J. Wang, Z. Hu, J. Xu and Y. Zhao, NPG Asia Mater., 2014, 6, e84 CrossRef CAS.
- K. Yang, L. Feng, X. Shi and Z. Liu, Chem. Soc. Rev., 2013, 42, 530–547 RSC.
- A. Battigelli, C. Ménard-Moyon and A. Bianco, J. Mater. Chem. B, 2014, 2, 6144–6156 RSC.
- A. Bianco, H. M. Cheng, T. Enoki, Y. Gogotsi, R. H. Hurt, N. Koratkar, T. Kyotani, M. Monthioux, C. R. Park, J. M. D. Tascon and J. Zhang, Carbon, 2013, 65, 1–6 CrossRef CAS.
- M. Orecchioni, R. Cabizza, A. Bianco and L. G. Delogu, Theranostics, 2015, 5, 710–723 CrossRef CAS PubMed.
- M. Orecchioni, D. A. Jasim, M. Pescatori, R. Manetti, C. Fozza, F. Sgarrella, D. Bedognetti, A. Bianco, K. Kostarelos and L. G. Delogu, Adv. Healthcare Mater., 2016, 5, 276–287 CrossRef CAS PubMed.
- P. A. Tran, L. Zhang and T. J. Webster, Adv. Drug Delivery Rev., 2009, 61, 1097–1114 CrossRef CAS PubMed.
- R. D. K. Misra and P. M. Chaudhari, J. Biomed. Mater. Res., Part A, 2013, 101 A, 528–536 CrossRef PubMed.
- D. Depan and R. D. K. Misra, Nanoscale, 2012, 4, 6325–6335 RSC.
- R. D. K. Misra, D. Depan and J. S. Shah, Acta Biomater., 2012, 8, 1908–1917 CrossRef CAS PubMed.
- R. D. K. Misra and Q. Yuan, Mater. Sci. Eng., C, 2012, 32, 902–908 CrossRef CAS.
- B. Girase, J. S. Shah and R. D. K. Misra, Adv. Eng. Mater., 2012, 14, 101–111 CrossRef.
- R. D. K. Misra, B. Girase, D. Depan and J. S. Shah, Adv. Eng. Mater., 2012, 14, 93–100 CrossRef.
- E. Hirata, T. Akasaka, M. Uo, H. Takita, F. Watari and A. Yokoyama, Appl. Surf. Sci., 2012, 262, 24–27 CrossRef CAS.
- E. Hirata, M. Uo, Y. Nodasaka, H. Takita, N. Ushijima, T. Akasaka, F. Watari and A. Yokoyama, J. Biomed. Mater. Res., Part B, 2010, 93, 544–550 CrossRef PubMed.
- E. Hirata, M. Uo, H. Takita, T. Akasaka, F. Watari and A. Yokoyama, J. Biomed. Mater. Res., Part B, 2009, 90, 629–634 CrossRef PubMed.
- E. Hirata, M. Uo, H. Takita, T. Akasaka, F. Watari and A. Yokoyama, Carbon, 2011, 49, 3284–3291 CrossRef CAS.
- C. A. Poland, R. Duffin, I. Kinloch, A. Maynard, W. A. H. Wallace, A. Seaton, V. Stone, S. Brown, W. Macnee and K. Donaldson, Nat. Nanotechnol., 2008, 3, 423–428 CrossRef CAS PubMed.
- K. Kostarelos, Nat. Biotechnol., 2008, 26, 774–776 CrossRef CAS PubMed.
- T. Kasai, S. Matsumura, T. Iizuka, K. Shiba, T. Kanamori, M. Yudasaka, S. Iijima and A. Yokoyama, Nanotechnology, 2011, 22, 065102 CrossRef PubMed.
- D. Depan and R. D. K. Misra, Acta Biomater., 2013, 9, 6084–6094 CrossRef CAS PubMed.
- T. Murakami, H. Sawada, G. Tamura, M. Yudasaka, S. Iijima and K. Tsuchida, Nanomedicine, 2008, 3, 453–463 CrossRef CAS PubMed.
- S. Iijima, M. Yudasaka, R. Yamada, S. Bandow, K. Suenaga, F. Kokai and K. Takahashi, Chem. Phys. Lett., 1999, 309, 165–170 CrossRef CAS.
- J. Miyawaki, M. Yudasaka, T. Azami, Y. Kubo and S. Iijima, ACS Nano, 2008, 2, 213–226 CrossRef CAS PubMed.
- R. J. Miron and D. D. Bosshardt, Biomaterials, 2016, 82, 1–19 CrossRef CAS PubMed.
- V. Nicolaidou, M. M. Wong, A. N. Redpath, A. Ersek, D. F. Baban, L. M. Williams, A. P. Cope and N. J. Horwood, PLoS One, 2012, 7, e39871 CAS.
- M. J. Gómez-Lechón, Life Sci., 1999, 65, 2019–2030 CrossRef.
- P. Guihard, Y. Danger, B. Brounais, E. David, R. Brion, J. Delecrin, C. D. Richards, S. Chevalier, F. Rédini, D. Heymann, H. Gascan and F. Blanchard, Stem Cells, 2012, 30, 762–772 CrossRef CAS PubMed.
- S. Lacotte, A. García, M. Décossas, W. T. Al-Jamal, S. Li, K. Kostarelos, S. Muller, M. Prato, H. Dumortier and A. Bianco, Adv. Mater., 2008, 20, 2421–2426 CrossRef CAS.
- Y. Tahara, M. Nakamura, M. Yang, M. Zhang, S. Iijima and M. Yudasaka, Biomaterials, 2012, 33, 2762–2769 CrossRef CAS PubMed.
- M. Horie, L. K. Komaba, H. Fukui, H. Kato, S. Endoh, A. Nakamura, A. Miyauchi, J. Maru, E. Miyako, K. Fujita, Y. Hagihara, Y. Yoshida and H. Iwahashi, Carbon, 2013, 54, 155–167 CrossRef CAS.
- S. Dolci, V. Domenici, G. Vidili, M. Orecchioni, P. Bandiera, R. Madeddu, C. Farace, M. Peana, M. R. Tiné, R. Manetti, F. Sgarrella and L. G. Delogu, RSC Adv., 2016, 6, 2712–2723 RSC.
- C. Farace, P. Sánchez-Moreno, M. Orecchioni, R. Manetti, F. Sgarrella, Y. Asara, J. M. Peula-García, J. A. Marchal, R. Madeddu and L. G. Delogu, Sci. Rep., 2016, 6, 18423–18437 CrossRef CAS PubMed.
- R. Gillitzer and M. Goebeler, J. Leukocyte Biol., 2001, 69, 513–521 CAS.
- A. Kortesidis, A. Zannettino, S. Isenmann, S. Shi, T. Lapidot and S. Gronthos, Blood, 2005, 105, 3793–3801 CrossRef CAS PubMed.
- M. Tanaka and A. Miyajima, Rev. Physiol., Biochem. Pharmacol., 2003, 149, 39–52 CAS.
- C. Chipoy, M. Berreur, S. Couillaud, G. Pradal, F. Vallette, C. Colombeix, F. Rédini, D. Heymann and F. Blanchard, J. Bone Miner. Res., 2004, 19, 1850–1861 CrossRef CAS PubMed.
- H. Y. Song, E. S. Jeon, J. Il Kim, J. S. Jung and J. H. Kim, J. Cell. Biochem., 2007, 101, 1238–1251 CrossRef CAS PubMed.
- T. Kitaori, H. Ito, E. M. Schwarz, R. Tsutsumi, H. Yoshitomi, S. Oishi, M. Nakano, N. Fujii, T. Nagasawa and T. Nakamura, Arthritis Rheum., 2009, 60, 813–823 CrossRef CAS PubMed.
- S. Otsuru, K. Tamai, T. Yamazaki, H. Yoshikawa and Y. Kaneda, Stem Cells, 2008, 26, 223–234 CrossRef CAS PubMed.
- Q.-L. Ma, L.-Z. Zhao, R.-R. Liu, B.-Q. Jin, W. Song, Y. Wang, Y.-S. Zhang, L.-H. Chen and Y.-M. Zhang, Biomaterials, 2014, 35, 9853–9867 CrossRef CAS PubMed.
- G. Thalji and L. F. Cooper, Int. J. Oral Maxillofac. Implants, 2014, 29, e171–e199 CrossRef PubMed.
- M. Shimizu, Y. Kobayashi, T. Mizoguchi, H. Nakamura, I. Kawahara, N. Narita, Y. Usui, K. Aoki, K. Hara, H. Haniu, N. Ogihara, N. Ishigaki, K. Nakamura, H. Kato, M. Kawakubo, Y. Dohi, S. Taruta, Y. A. Kim, M. Endo, H. Ozawa, N. Udagawa, N. Takahashi and N. Saito, Adv. Mater., 2012, 24, 2176–2185 CrossRef CAS PubMed.
- N. Saito, Y. Usui, K. Aoki, N. Narita, M. Shimizu, N. Ogiwara, K. Nakamura, N. Ishigaki, H. Kato, S. Taruta and M. Endo, Curr. Med. Chem., 2008, 15, 523–527 CrossRef CAS PubMed.
- R. D. K. Misra and P. M. Chaudhari, J. Biomed. Mater. Res., Part A, 2013, 101 A, 1059–1068 CrossRef PubMed.
- J. Fan, M. Yudasaka, J. Miyawaki, K. Ajima, K. Murata and S. Iijima, J. Phys. Chem. B, 2006, 110, 1587–1591 CrossRef CAS PubMed.
- J. Russier, E. Treossi, A. Scarsi, F. Perrozzi, H. Dumortier, L. Ottaviano, M. Meneghetti, V. Palermo and A. Bianco, Nanoscale, 2013, 5, 11234–11247 RSC.
- N. Hanagata, F. Zhuang, S. Connolly, J. Li, N. Ogawa and M. Xu, ACS Nano, 2011, 5, 9326–9338 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nr02756c |
| This journal is © The Royal Society of Chemistry 2016 |