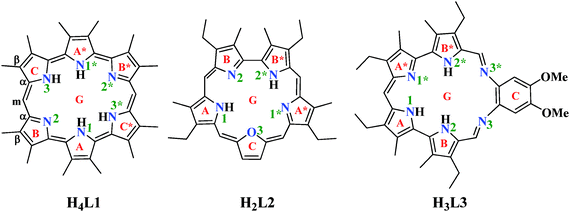
Characterization of the binding of six actinyls AnO22+/+ (An = U/Np/Pu) with three expanded porphyrins by density functional theory†
Meixiu
Yang
ab,
Wanjian
Ding
*a and
Dongqi
Wang
*b
aKey Laboratory of Theoretical and Computational Photochemistry, Ministry of Education, College of Chemistry, Beijing Normal University, Beijing 100875, China. E-mail: dingwanjian@bnu.edu.cn
bCAS Key Laboratory of Nuclear Radiation and Nuclear Energy Techniques, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China. E-mail: dwang@ihep.ac.cn
First published on 4th November 2016
Abstract
We reported a density functional theory study on the complexation of six hydrated actinyl cations, AnO2(H2O)52+/+ (aq) (An = U/Np/Pu), with three expanded porphyrins, amethyrin (H4L1), oxasapphyrin (H2L2), and grandephyrin (H3L3). The geometries have been fully optimized and analyzed, and the electronic structures, the binding free energies, and the NMR properties were calculated. Natural population analysis and Quantum Theory of Atoms in Molecules (QTAIM) topology analysis techniques were applied to understand the interaction modes between two entities of each complex. The calculations show that for the same ligand, PuO22+ and NpO22+ display stronger binding affinity than UO22+, UO2+, NpO2+, and PuO2+, and among the three ligands tested, L22− fits better with the actinyl cations than L33− and H2L12−. The redox process was observed in the complexation of PuO22+ and NpO22+ with specific ligands, which agrees with the experimental results. In the characterization of the nature of the coordination bonding interactions, QTAIM gives a consistent description with the natural population analysis method and shows that the interaction between An and the electron donor atoms in the first coordination shell has a strong ionic feature, while the interaction between An and Oyl atoms of the actinyls in the complexes remains covalent. This work complements the earlier experimental studies by providing a molecular level of understanding on the interaction between actinyls and expanded porphyrins, and is expected to contribute to the communities of the chemistry of actinides and expanded porphyrins.
1. Introduction
As an important ligand, porphyrins (Pors) are known to display strong affinity toward d-transition metal atoms, such as iron and zinc, and play important roles in biomolecular systems and have potential application in dye-sensitized solar cells. While their binding with actinides is rare, and limited examples1–6include ThPor(acac)2, MPor(Cl)2L2 (M = Th, U; L = Py, THF, or Bzn), UPor(Cl)2(THF), and MPor2 (M = Th, U), where the actinides form out-of-plane or sandwich complexes. Similar phenomena were also observed in an earlier work of Cao et al.,7 in which the coordination of three trivalent actinide cations (An(III)), Ac, Cm, and Lr, with texaphyrins and motexafins was studied and An(III) was observed to sit above the polypyrrolic macrocyclic ligand rather than being co-planar with the ligand. The texaphyrins and motexafins have five coordinating N atoms, thus with a cavity larger than that of Pors by about one fifth. This certainly indicates that the host and the guest do not fit each other well, which may be significantly improved if expanded porphyrins (ePors) with appropriate cavity size were developed.Expanded porphyrins are a family of derivatives of normal tetradentate porphyrins, but with much rich chemistry since multiple types of heterocyclic rings may be fused into the macrocycle, leading to diverse conformers with modulated electronic and mechanical properties.8 The enlarged cavity size of ePor also gives them the ability to accommodate bigger cations that Por cannot hold, such as actinides.
The coordination of ePors with actinyls has been observed in early 1990s when Sessler et al.9,10 developed a new class of pyrrole-based ePor which were capable of complexing uranyl (UO22+) effectively. This opened a new avenue of the host–guest chemistry of actinides in addition to calixarenes11–14 and crown ethers,15–18 and a variety of ePors were reported to form stable complexes with actinyls (AnO22+/+), including pentaphyrin,9,19 sapphyrin,10,20 alaskaphyrin,21 grandephyrin,22 isoamethyrin,23 oxasapphyrin,24 and amethyrin.25–27
The ePors provide a better “fit” for larger metal cations, and their affinity to bind with actinyls has been observed, while the nature of the interaction between actinyls and the ePors remains obscure. The specific binding behavior of ePors with actinyls may open a new avenue to the chemistry of actinyls and other cations of large size when coordinating with macrocyclic ligands which Pors cannot offer, and thus are potentially relevant to the communities of chemistry and biology. This motivated us to carry out a computational study to understand the interaction between actinides and ePors, which may facilitate the rational design of new ePor molecular systems for manipulatable binding of actinides.
2. Computational details
Three ePor compounds (Scheme 1) were used as ligands to study their interaction with six actinyls, i.e. uranyl(VI/V), neptunyl(VI/V), and plutonyl(VI/V). The ligands include amethyrin ([24] hexaphyrin(1.0.0.1.0.0) in the nomenclature rule of Franck and Nonn for porphyrinic systems28–30) (H4L1), oxasapphyrin (H2L2), and grandephyrin (H3L3).Density functional theory (DFT)31 has been widely used to study the electronic properties and chemical processes of molecular actinide systems.32–40 In this work, range-separated hybrid functional CAM-B3LYP41 implemented in Gaussian 0942 was employed to optimize and analyze the stationary points. This method has been used to study the nonlinear optical properties of an ePor and was shown to give correct qualitative results.43 It was also tested in a recent comparative study of low valent uranium complexes, and was shown to give comparable geometrical parameters and energies with the recommended methods of B3LYP and B3PW91.44
For the basis set, the locally dense basis set method was adopted, which has been qualified in earlier studies of geometry and electronic properties.45–47 Quasi-relativistic 5f-in-valence small-core ECPs48–50 (SC-RECPs), together with their optimized segmented basis set for the valence shells contracted as (14s13p10d8f6g)/[10s9p5d4f3g], were used to treat the actinide atoms. Such a treatment has been shown to yield reasonable results in many studies of molecular systems containing actinides.51–56 For the N and O atoms of the first coordination sphere, the triple-ζ quality 6-311+G(d)44,57–60 basis set was used, and for the H atoms bonding with N atoms in the native form of the ePors, only polarization functions were included based on the 6-311G basis set. For all the other atoms, including C, O, and H atoms, the double-ζ basis set 6-31G(d)61,62 was used. Spin–orbit coupling is neglected with such treatments of the model systems.
All stationary points reported here were fully optimized and confirmed to be energy minima by frequency calculations. In order to better mimic the experimental condition, taking experimentally used methanol (MeOH) as solvent, the polarizable continuum model (PCM)63,64 was used to re-optimize all of the stationary points followed by vibrational frequency analysis to identify the nature of each stationary point and obtain the zero-point energy (ZPE) and thermal correction. To take the entropy effect into account, the Gibbs free energy is used in the following discussions unless otherwise specified. The free energies in vacuum were calculated from standard determination emerged from the Gaussian 0942 output with ideal-gas translational entropy, and the ones in MeOH solvent were estimated using solution-phase translational entropy.65
The Quantum Theory of Atoms in Molecules (QTAIM)66–70 analysis was performed based on Kohn–Sham orbitals optimized by the Gaussian 09 program. The AIM analysis was done using the Multiwfn 3.2 package.71 The calculations of NMR shielding tensors were carried out by using the Continuous Set of Gauge Transformation (CSGT) method72–75 and the Gauge-Independent Atomic Orbital (GIAO)76,77 method to obtain the anisotropy of the current-induced density (ACID), which was analyzed by the ACID program,78,79 and the Nucleus-independent chemical shifts (NICS) indices,80–82 respectively.
3. Results and discussion
3.1. The ligands and the binding modes offered to actinyls
The three ePor ligands studied here differ from each other in their backbone and their coordinating atoms, as shown in Scheme 1. In their neutral forms, all of the three ligands may adopt multiple protonation schemes. In H4L1, there are six pyrrole rings, with rings A and A*, B and B*, and C and C* being equivalent, respectively, leading to six potential neutral isomers according to different protonation sites, i.e. H4L1131*3*, H4L1131*2*, H4L11*233*, H4L11*2*23, H4L1232*3*, and H4L11231*, where the four digits of the subscript stand for the protonated N atoms of the pyrrole rings defined in Scheme 1, respectively. Isomer H4L1131*3* was found to be the most stable, and the Gibbs free energies (Table S1, ESI†) of the other five isomers with respect to H4L1131*3*, ΔGvacuum/ΔGMeOH, were calculated to be 0.2/0.2, 13.6/10.5, 17.8/13.1, 22.9/18.8, and 33.2/19.7 kcal mol−1 for H4L1131*2*, H4L11*233*, H4L11*2*23, H4L1232*3*, and H4L11231*, respectively. This suggests that the two isomers, H4L1131*3* and H4L1131*2*, are essentially degenerated in view of free energy.H2L2 differs from H4L1 in the number of pyrrole rings, and the presence of one furan ring, leading to two types of N atoms of H2L2, numbered as 1 and 1*, and 2 and 2*, respectively, as seen in Scheme 1, and one O atom in the first coordination sphere when interacting with actinyls. There are four possible protonation isomers, with H2L212* more stable by 1.7, 5.8, and 17.8 kcal mol−1 in vacuum than H2L211*, H2L222*, and H2L212, respectively.
H3L3 is a Schiff-base type macrocyclic compound. It has four pyrrole rings and two imines which are bridged by a phenyl ring. The extension of the ring backbone makes H3L3 more flexible. Similarly, there are ten different isomers (Table S1, ESI†) with the most energetically favorable structure of H3L3122* staying 3.4–44.6 kcal mol−1 lower in vacuum than other isomers. The relative stability changes to 2.8–34.2 kcal mol−1 when taking into account the solvent effect of MeOH. Such a protonation scheme is consistent with the structure of the grandephyrin methanolic adduct reported in a crystallographic study22 (see Fig. 7 and Table 2 in ref. 22).
These compounds in principle can appear as hexadentate (L14− and L33−) or pentadentate ligands (L22−). In view of the structures, it is reasonable to consider the pentadentate and hexadentate binding modes offered by H2L2 and H3L3 in their fully deprotonated states, respectively, while for H4L1, it is more complex due to the enlarged cavity size and the potential competence of the six pyrrole N sites to binding with the electron-deficient actinide cations. To figure out the most favorable binding mode, we have investigated the thermodynamics of the binding processes featured by a sequential deprotonation of H4L1 assisted by Et3N. These reaction pathways are discussed in the ESI.†
According to our calculations, starting from the thermodynamically more stable conformers H4L1131*3* and H4L1131*2*, the first step, which involves the complexation of the deprotonated ligand and the dehydrated actinyl and the protonation of Et3N, is strongly exothermic in the cases of hexavalent actinyls while endothermic for pentavalent actinyls. Taking into account the solvent effect of MeOH, the exothermicity decreases in both cases, while the preference of the ligand to bind to An(VI) over An(V) does not change. The further deprotonation in MeOH solvent brings more exothermicity for the An(VI) complexes due to the removal of repulsive interaction between the proton and the actinyl, while makes the An(V) complexes even less stable.
The exothermicity for the deprotonation of An(VI) complexes is reversed starting from the third step in MeOH solvent. Concerning the thermodynamics of the various channels, the product of the optimal doubly deprotonation processes, i.e. H2L12− with only N1 and N3 remaining protonated, was considered as the most favorable precursor for the binding of actinyls to H4L1, and the following discussion is based on the complexes of AnO2(H2L1)0/−.
The optimized geometries of the three ligands in their deprotonated forms, i.e. H2L12−, L22−, and L33−, and their complexes with actinyls bound are shown in Fig. 1 and Fig. S1 (ESI†), and the key geometrical parameters, including bond distances dAn–N and dAn–O, and bond angle O–An–O, are given in Table 1. In their unbound deprotonated forms, the hetero atoms of the ligands are nearly coplanar as a result of the backbone strain and the conjugation.
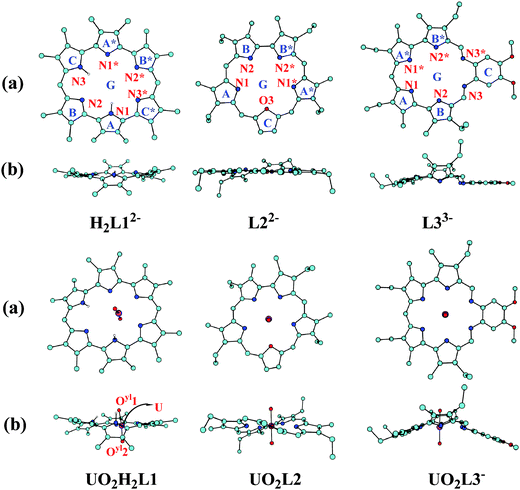 | ||
| Fig. 1 The optimized structures of H2L12−, L22−, and L33− ligands and their complexes with UO22+ in vacuum (hydrogens binding with carbon atoms are omitted for clarity): (a) top view; (b) side view. The schematic structures of complexes with other antinyls are similar and given in Fig S1 (ESI†). | ||
| UVI | NpVI | PuVI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H2L12− | L22− | L33− | H2L12− | L22− | L33− | H2L12− | L22− | L33− | |
| a The distances between An and N1* and N2* are the same as their counterparts for AnO2L20/− and AnO2L3−/2−, thus are not shown; for AnO2H2L10/−, they are given as An–N1*/An–N1 and An–N2*/An–N2. b An–N3*/An–N3 for AnO2H2L10/− and AnO2L3−/2−, and An–O3 for AnO2L20/−. c Arithmetic mean of the bond lengths in the equatorial plane of actinyls. d The distances in the penta-aquo complexes of actinyls AnO2(H2O)52+/+. The data in parenthesis are obtained with solvent effect of MeOH taken into account. | |||||||||
| An–N1a | 2.49/2.81 | 2.58 | 2.67 | 2.58/2.94 | 2.65 | 2.75 | 2.57/2.94 | 2.65 | 2.68 |
| An–N2a | 2.41/3.64 | 2.48 | 2.54 | 2.52/3.51 | 2.56 | 2.59 | 2.51/3.50 | 2.56 | 2.51 |
| An–N3(O3)b | 2.48/3.68 | 2.70 | 2.83 | 2.60/3.50 | 2.73 | 2.88 | 2.60/3.49 | 2.73 | 2.83 |
![[d with combining overline]](https://www.rsc.org/images/entities/i_char_0064_0305.gif)
|
2.92 | 2.56 | 2.68 | 2.94 | 2.63 | 2.74 | 2.93 | 2.63 | 2.67 |
| An–Oyl | 1.74/1.75 | 1.75 | 1.75 | 1.77/1.79 | 1.79 | 1.79 | 1.75/1.77 | 1.76 | 1.72 |
| ∠O–An–O | 171.8 | 175.5 | 178.5 | 174.6 | 177.1 | 179.1 | 176.3 | 178.3 | 179.2 |
| ν s(An–Oyl) | 923 | 926 | 913 | 833 | 837 | 820 | 833 | 836 | 895 |
| ν as(An–Oyl) | 999 | 999 | 986 | 908 | 898 | 877 | 916 | 912 | 1000 |
| An–Oyl (hyd.)d | 1.73(1.74) | 1.71(1.71) | 1.69(1.69) | ||||||
| An–Ow (hyd.)d | 2.47(2.44) | 2.46(2.43) | 2.44(2.41) | ||||||
| bareAn–Oyl | 1.68 | 1.66 | 1.64 | ||||||
| UV | NpV | PuV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H2L12− | L22− | L33− | H2L12− | L22− | L33− | H2L12− | L22− | L33− | |
| An–N1a | 2.56/3.02 | 2.57 | 2.59 | 2.57/3.02 | 2.69 | 2.74 | 2.56/3.01 | 2.69 | 2.75 |
| An–N2a | 2.50/3.61 | 2.48 | 2.56 | 2.49/3.64 | 2.57 | 2.61 | 2.48/3.62 | 2.57 | 2.60 |
| An–N3(O3)b | 2.59/3.55 | 2.73 | 2.98 | 2.57/3.58 | 2.77 | 2.95 | 2.57/3.57 | 2.77 | 2.94 |
![[d with combining overline]](https://www.rsc.org/images/entities/i_char_0064_0305.gif)
|
2.97 | 2.57 | 2.71 | 2.98 | 2.66 | 2.77 | 2.97 | 2.65 | 2.76 |
| An–Oyl | 1.80/1.82 | 1.75 | 1.76 | 1.78/1.80 | 1.79 | 1.80 | 1.76/1.78 | 1.77 | 1.78 |
| ∠O–An–O | 171.2 | 175.3 | 176.8 | 173.4 | 176.7 | 178.7 | 175.5 | 178.0 | 179.0 |
| ν s(An–Oyl) | 812 | 912 | 900 | 818 | 827 | 807 | 820 | 826 | 806 |
| ν as(An–Oyl) | 874 | 983 | 973 | 891 | 884 | 866 | 902 | 899 | 881 |
| An–Oyl (hyd.)d | 1.79(1.81) | 1.76(1.78) | 1.75(1.77) | ||||||
| An–Ow (hyd.)d | 2.55(2.53) | 2.54(2.52) | 2.55(2.53) | ||||||
| bareAn–Oyl | 1.73 | 1.71 | 1.70 | ||||||
Upon the binding of actinyl cations, significant deformation was observed in the conformations of the ligands. The deformation energy (ΔEdeform) was calculated as a measure of the energy required to deform the ligand in order to adapt to its coordination with actinyl cations, and was calculated as the energy difference between the conformations before (EePor0) and after (EePor1) the coordination of the ligand with actinyl, i.e. ΔEdeform = EePor1 − EePor0. The results are plotted in Fig. 2.
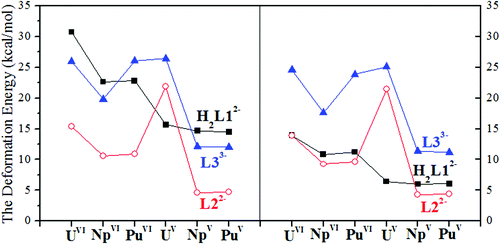 | ||
| Fig. 2 The deformation energy of the three ligands when coordinated with the antinyls in vacuum (left) and in MeOH (right). | ||
We note that the deformation energies of the ligands decrease going from An(VI) to An(V), with an exception of U(V) for L22− and L33− and Pu(VI) for L33−. Population analysis shows that there is electron transfer from U(V) to the macrocyclic ligands L22− and L33− while Pu(VI) remains in its hexavalent state when binding with L33−. This indicates that more energy is needed for the ligands in these cases to adopt the conformation with their spin density increased or unchanged to interact with the dicationic actinyls, and is in contrast to the case of NpO2L3− where L33− donates one electron to Np(VI) and less energy is needed to deform its conformation. These show the delicate balance between the electron affinity of L33− and its ability to resist the deformation of its geometry when interacting with oxidizing dications (NpO22+ and PuO22+) and reducing monocation (UO2+). The calculations also suggest that upon complexation, less energy is needed for L22− to adapt to the coordination to the actinyl moiety than that for H2L12− and L33−, as shown in Fig. 2, except for the case of U(V) where U(V) is oxidized when coordinating with the L22− and L33− ligands.
The larger deformation of H2L12− and L33− than L22− may be explained in terms of aromaticity. In their neutral states, there are 24 and 22 π electrons in the shortest conjugation paths of H4L1 and H2L2, respectively, suggesting that H2L2 is aromatic globally, while the aromaticity of H4L1 displays a localized feature, according to Hückel's definition of aromaticity. In H3L3, though there are 22 π electrons in its shortest conjugation path, the phenyl ring does not favor the delocalization of its electrons through the Schiff-base N atoms, leading to a broken ring current, which is to be shown later in the discussion of ACID.
In AnO2(H2L1)0/− (An = U/Np/Pu) (Fig. 1 and Fig. S1, ESI,†Table 1), for all of the six actinyl cations, the actinide atoms bind stronger with N1*, N2*, and N3* than with the other three N atoms, as seen from the shorter An–N1*/N2*/N3* bond lengths of 2.4–2.6 Å compared to the values of 2.8–3.7 Å for the other three An–N bonds. The protons on N1 and N3 deviate from the planar significantly due to the repulsive interaction rising from the binding of actinyls. As a result of interaction between AnO22+/+ and the ligand H2L12−, the An–Oyl bonds were perturbed with a bond length increment of 0.01–0.08 Å, and larger changes were observed for Np(VI) and Pu(VI), which is in agreement with the larger charge transfer character in these two complexes.
The fully deprotonated L33− is a hexadentate ligand with higher backbone flexibility. In our calculations, this ligand, both in its intact form and binding state, was found to be strongly deformed, which is different from the conformation solved from the crystallographic experiment22 where a planar conformation was reported. The difference may be due to the lack of the crystal packing in our calculations. In view of key geometric parameters of UO2L3−, including the U–Oyl and U–N distances, there is good agreement between the calculated values and the experimental data with 1.75 vs. 1.76 Å for U–Oyl, (2.54, 2.67, 2.83 Å) vs. (2.54, 2.72, 2.86 Å) for the three pairs of equivalent U–N bonds. The ligand deformation was also observed by Shamov and Schreckenbach in their study83 of the interaction between uranyl and alaskaphyrin, which is a hexadentate macrocyclic Schiff-base ligand.
In the actinyl oxasapphyrin complexes AnO2L20/−, for all of the six actinyl moieties, the An–O bond length is longer than that of An–N bonds by 0.08–0.25 Å. Such a difference is relevant to the distinct nucleophilicity of the two types of heteroatoms in view of their atomic charges and the unsaturated valence of N atoms that bring more orbital overlap between actinyl and N atoms.
3.2. Free energy of the binding process
In experimental studies, triethyl amine (Et3N) appeared as an additive when actinyl compounds were mixed with ePors in MeOH. By assuming that the ePor ligands exist in their neutral forms in MeOH, the binding of actinyls to the ePor ligands studied here accompanies with the deprotonation of the ligands assisted by Et3N, which may be described as below:| HkL + AnO2(H2O)5n+ + (k − m)Et3N → AnO2LH(k − n − m)−m + (k − m)Et3NH+ + (H2O)5 |
| ΔG = G(AnO2LH(k − n − m)−m) + (k − m)G(Et3NH+) + G((H2O)5) − G(HkL) − G(AnO2(H2O)5n+) − (k − m)G(Et3N) |
As shown in Table 2, in vacuum, the complexation of plutonyl(VI) with H2L12− or L22− is in general much more exothermic than that of uranyl(VI) and neptunyl(VI), while for L33−, the reaction energies of the three hexavalent actinyls are comparable within a difference of 5.3 kcal mol−1.
| UVI | NpVI | PuVI | UV | NpV | PuV | |
|---|---|---|---|---|---|---|
| Vacuum | ||||||
| H2L12− | −82.0 | −104.9 | −123.0 | 99.7 | 92.5 | 99.9 |
| L22− | −113.7 | −113.3 | −134.4 | 88.5 | 79.2 | 83.4 |
| L33− | −21.3 | −17.9 | −23.2 | 255.5 | 240.2 | 247.5 |
| MeOH | ||||||
| H2L12− | −19.7 | −45.3 | −60.6 | 4.4 | 2.3 | 8.1 |
| L22− | −42.4 | −52.5 | −72.4 | 5.9 | −17.7 | −12.5 |
| L33− | −35.1 | −46.7 | −35.3 | 32.5 | 1.9 | 10.6 |
The actinyls with U, Np, and Pu in their pentavalent states display strong propensity to resist their binding with the deprotonated L33− ligand, and their interaction with H2L12− and L22− ligands is also much weaker than that between the ligands and the three actinyls(VI). This is consistent with their low ability to form corrdination complexes. The much stronger binding strength between actinyls(VI) and the ligands than that between actinyls(V) and the ligands is relevant to the more positive charge the actinyls(VI) carry, and in the cases of Np(VI) and Pu(VI), the redox reaction occurred upon their binding to the ligands, which is to be discussed later.
In MeOH, the solvation effect significantly mitigates the binding strength of An(VI) with H2L12− and L22−, while brings enhancement to the coordination of An(VI) with L33− moderately and that of An(V) with the three ligands strongly. The difference rises from their distinct charge features. The coordination of actinyls(VI) with H2L12− and L22− gives neutral species, while their binding with L33− and the coordination of actinyls(V) with the three ligands produce charged complexes. This is similar to that observed in the study84 of [AnO2(18-crown-6)]2+/+ (An = U/Np/Pu), in which Schreckenbach et al. reported that polar solvent brought more negative solvation free energy change (ΔΔGsolv) for the ligand exchange reaction of hydrated monocationic actinyls(V) with the crown ether ligand than that of hydrated dicationic actinyls(VI), and proposed that this resulted from the dependence of the stabilization of a charged species due to solvation on its charge and its distance with the polarizable solvent medium. This suggests that the smaller a charged solute and the more charge it carries, the stronger stabilization it feels from the polarizable medium. This rationale still holds in the current case.
3.3. Electronic structure
| An | Oyl | AnO22+/+ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aquo | H2L12− | L22− | L33− | Aquo | H2L12− | L22− | L33− | Aquo | H2L12− | L22− | L33− | |
| UVI | 1.88 | 1.86 | 1.74 | 1.57 | −0.47 | −0.54 | −0.53 | −0.54 | 0.93 | 0.78 | 0.69 | 0.49 |
| NpVI | 1.63 | 1.53 | 1.39 | 1.28 | −0.35 | −0.64 | −0.62 | −0.64 | 0.92 | 0.25 | 0.16 | 0.00 |
| PuVI | 1.45 | 1.44 | 1.30 | 1.27 | −0.26 | −0.58 | −0.56 | −0.36 | 0.93 | 0.28 | 0.18 | 0.56 |
| UV | 1.61 | 1.71 | 1.73 | 1.62 | −0.70 | −0.75 | −0.54 | −0.56 | 0.21 | 0.21 | 0.65 | 0.50 |
| NpV | 1.42 | 1.56 | 1.44 | 1.32 | −0.60 | −0.66 | −0.64 | −0.66 | 0.22 | 0.25 | 0.15 | 0.01 |
| PuV | 1.40 | 1.49 | 1.34 | 1.23 | −0.54 | −0.60 | −0.58 | −0.60 | 0.32 | 0.30 | 0.18 | 0.04 |
Upon binding actinyls with the ligands, there is significant ligand-to-metal charge transfer in the actinyl(VI) complexes, as seen from the decrease of group charges of the actinyl moiety with respect to those of hydrated actinyls shown in Table 3. Compared to the charge distribution of the hydrated actinyl ions, the charge transfer does not always neutralize the positive charge on the actinide atoms, but increases the negative charge accumulation of the Oyl atoms. This may be the consequence, in other words, an indication of the strong interaction between the actinide atoms and the ePor ligands, which repels the electron clouds of Oyl atoms that were in the An–Oyl bonding region, leading to the weakening of the An–Oyl covalent bond feature, as observed from the increase of the bond lengths and the decrease of the Wiberg bond index (WBI) of the An–Oyl bonds.
In the neptunyl(VI) (NpVIO2H2L10/L20/L3−) and plutonyl(VI) (PuVIO2H2L10/L20) complexes, except for the charge redistribution, we also observed that the spin density of Np(VI) in all three complexes and Pu(VI) in PuO2H2L1 and PuO2L2 becomes larger than in their formal oxidation states, which should be 1e and 2e, respectively. As seen in Fig. 3, the spin densities of Np(VI) and Pu(VI) were calculated to be about 2.2e and 3.4e, respectively, which is comparable to that of Np(V) and Pu(V) upon their coordination with the corresponding ligands. This suggests that redox reaction may occur upon the binding of neptunyl(VI) and plutonyl(VI) with these ligands, i.e. reduction of the actinide(VI) and oxidation of the macrocyclic ligands. This is consistent with the experimental studies22 where the reduction of Np(VI) and Pu(VI) has been observed in view of the presence of the characteristic Np(V) UV-Vis signals or the disappearance of the Pu(VI) UV-Vis signals except for Pu(VI) in its grandephyrin (PuO2L3−) complex.
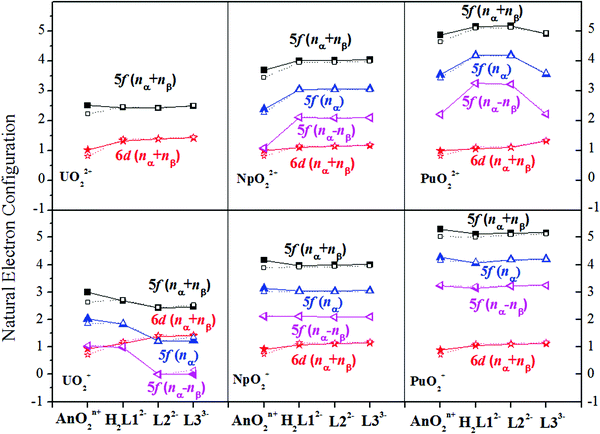
The population analysis shows that the transferred electron mainly locates in the 5f orbitals of Np(VI) and Pu(VI), and the occupation in the 5f orbitals increases moderately compared to the values in the bare cations, as seen in Fig. 3. Analysis of the frontier orbitals of these complexes shows that in the frontier orbitals of these complexes, there is significant 5f contribution from Np(VI) and Pu(VI), which displays much little overlap with the p-component of the ligand atoms in the same orbital, leading to an assignment of α-spin density in the 5f-component and the β-spin density in the p-components of the ligand atoms. Such a phenomenon has been observed and discussed by Kaltsoyannis85 and Prodan et al.86 in their studies of AnCp3 and AnCp4 (An = Th-Cm; Cp = η5-C5H5) and AnO2 (An = Th-Es), respectively.
We note that the reduction of Np(VI) and Pu(VI) was also observed in the fragments of NpVIO2(TMOGA)22+ and PuVIO2(TMOGA)22+, where TMOGA represents tetramethyl-3-oxa-glutaramide, via collision induced dissociation (CID) by electrospray ionization,103 which otherwise remains in its +6 oxidation state. In these complexes, actinyl is coordinated to the O atom of the carbonyl or the ether group of the ligand, which has stronger electronic affinity, or “harder”, than N atoms in the ligands studied in this work. Thus, in the native complexes, Np(VI) and Pu(VI) cannot abstract one electron from TMOGA, while they are reduced when complexing with the macrocyclic ligands L1 and L2 or the fragmented TMOGA, showing the delicate balance of the electron distribution in the complexes.
In the framework of AIM, a bond critical point (BCP) is defined as the point along the bond path of an atom pair where the gradient of the electron density is zero, i.e. depending on the property of a bond, the electron density may accumulate (a shared bond) or be depleted (a closed-shell bond) at a BCP. Four descriptors68 are used to identify the nature of chemical bonding and non-bonding interactions, i.e. the electron density ρb and its Laplacian ∇2ρb, the total electron energy density Hb(r) at a BCP, and the delocalization index between atom pair A and B δ(A, B).
According to our calculations (Table S14, ESI†), the electron density ρb at the BCP of the U–Oyl bond is 0.32–0.33 e− bohr−3 in the vacuum, which is similar to those of the U![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O triple bonds,66,87,88 suggesting a covalent bond between U and Oyl in the complexes of UO22+ with H2L12−, L22− and L33−, according to Bader70 that a ρb value greater than 0.2 e− bohr−3 may be used as the lower bound to judge a covalent bond and a value less than 0.1 e− bohr−3 as an upper bound for closed-shell interactions including ionic interactions. The values of ρb of An–Oyl for the other actinyls are also over 0.2 e− bohr−3, i.e. 0.29–0.30, 0.30–0.34, 0.26–0.32, 0.28–0.29, and 0.29–0.31 e− bohr−3 for NpVI, PuVI, UV, NpV, and PuV, showing similar bonding character. This is also supported by the value of Hb(r) (Table S14, ESI†), the positive and negative values of which is used, respectively, as an alternative way of defining ionic and covalent bonds. In the calculations, the values of Hb(r) fall between −0.22 and −0.34 for all the actinyls studied here. Since the An–Oyl bond is of multiple bond feature, the delocalization index between An and O was also calculated (Table S16, ESI†), and all are in the range of 2.7–3.0, suggesting a triple bond character of An–Oyl.
O triple bonds,66,87,88 suggesting a covalent bond between U and Oyl in the complexes of UO22+ with H2L12−, L22− and L33−, according to Bader70 that a ρb value greater than 0.2 e− bohr−3 may be used as the lower bound to judge a covalent bond and a value less than 0.1 e− bohr−3 as an upper bound for closed-shell interactions including ionic interactions. The values of ρb of An–Oyl for the other actinyls are also over 0.2 e− bohr−3, i.e. 0.29–0.30, 0.30–0.34, 0.26–0.32, 0.28–0.29, and 0.29–0.31 e− bohr−3 for NpVI, PuVI, UV, NpV, and PuV, showing similar bonding character. This is also supported by the value of Hb(r) (Table S14, ESI†), the positive and negative values of which is used, respectively, as an alternative way of defining ionic and covalent bonds. In the calculations, the values of Hb(r) fall between −0.22 and −0.34 for all the actinyls studied here. Since the An–Oyl bond is of multiple bond feature, the delocalization index between An and O was also calculated (Table S16, ESI†), and all are in the range of 2.7–3.0, suggesting a triple bond character of An–Oyl.
In contrast to the An–Oyl bond, there is essentially little build-up of electron density between An and the coordinating atoms of the ligands in the first coordination shell, suggesting a dominant contribution of ionic interactions. It is recommended that the positive value of ∇2ρb is for ionic bonds while negative for a single covalent bond. In these complexes, the Laplacian is positive with a ρb-value smaller than the value of 0.08 e− bohr−3 for the typical ionic LiF molecule,66,67 suggesting only minor electron accumulation between actinide and the ligands, and the interaction between An and the electron donor atoms in the first coordination shell is best described as ionic interaction.
Taking the solvent effect of MeOH into account (Tables S15 and S16, ESI†), the values of the four descriptors fluctuate marginally, but does not change the bonding character.
In the framework of QTAIM, δ(A, B) is defined as the integration of the electron density ρb in the bonding region between two atoms A and B, thus it is strongly correlated with ρb. Matta and Hernandez-Trujillo89 proposed that δ(A, B) may be used to calibrate the correlation between ρb and bond order via the relation δ(A, B) = exp[A(ρb − B)], where A and B are constants depending on the nature of the two atoms evaluated. Here we will not fit the relation due to insufficient samples, while it is interesting to see how δ(A, B) is correlated with ρb in the model systems under study, which is plotted in Fig. 4.
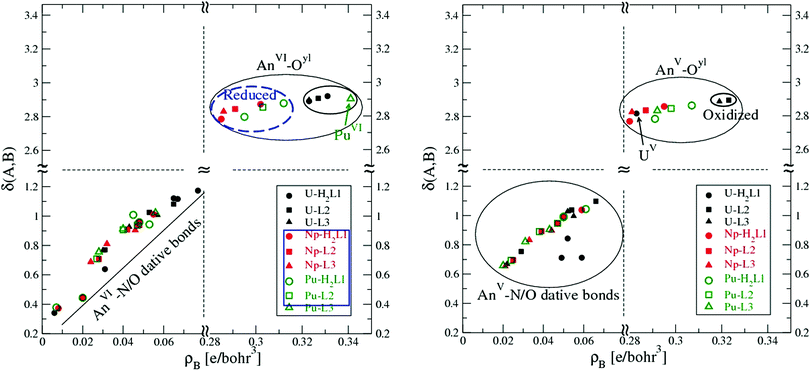 | ||
| Fig. 4 The delocalization index δ of key bonds as a function of the electron density ρb in the complexes of actinyls with H2L12−, L22−, and L33−. Left: An(VI), right: An(V). | ||
It is clear that δ(An, Oyl) is not sensitive to the variation of ρb for U, Np, and Pu both in their hexa- and penta-oxidation states. In contrast, for the An⋯N/O dative bonds, δ(An, N/O) increases nearly linearly as ρb becomes higher. The inert response of the An–Oyl bond in its δ(An, Oyl) to the variation of ρb may reflect the saturation of electron density in the bond region. The variation of ρb is a consequence of the distinct polarization mode of the An–Oyl bonds in the models studied here.
As mentioned above, upon the binding of actinyls to the ligands, Np(VI) was reduced to Np(V) in all of the three complexes, and so does Pu(VI) in its complexes with H2L12− and L22−. While for actinyl(V), all remain in their +5 oxidation state except for U(V) when coordinated to L22− and L33−, where U(V) was oxidized to U(VI). Keeping this in mind, we observe in Fig. 4 that the electron density at the BCP, ρb, of AnVI–Oyl bonds, including the oxidized ones in the UVO2L2− and UVO2L32−, is always larger than that in the AnV–Oyl bonds, including the reduced ones.
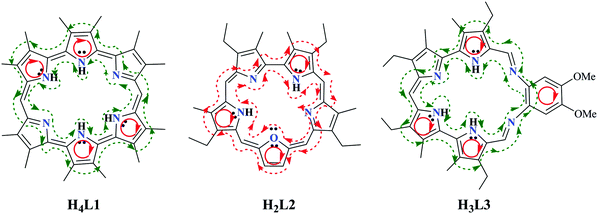
In general, the induced current density mainly circulates around nuclei where the electron density is high. For ring-shaped molecules, the current density may flow around the molecular ring. In these molecules, aromaticity is associated with diatropic ring current, which results in induced magnetic field with opposite direction to the external field, while paratropic current enhances the external field and indicates antiaromaticity.
According to our calculations, macrocyclic paratropic current was observed in H4L1, H3L3 and their deprotonated states, while macrocyclic diatropic current was observed in H2L2/L22−, as shown in Fig. 5. Note that, in H2L12− and L33−, there is diatropic current in local pyrrolic and phenyl rings, which is similar to that observed by Wu, Fernandez and Schleyer in their study90 of aromaticity in porphyrinoids. Such a phenomenon may be illustrated as below in Scheme 2.
NICSs have been proposed to be used as a measure of the aromaticity of a molecular system.91–93 Negative NICS values in interior positions of rings or cages, i.e. magnetically shielded, indicate the presence of induced diatropic ring currents or “aromaticity”, whereas positive values, i.e., deshielded, at each point denote paratropic ring currents and “antiaromaticity”. Thus, NICS is typically computed at ring centers, which is referred to as NICS(0), or at points 1 Å above the ring center for planar or nearly planar molecules, where the π orbitals have their maximum density, and NICS(1) was recommended as a better measure of the π electron delocalization than NICS(0).93,94
Note that NICS has been shown95–98 to be able to identify the aromaticity of organic molecules, while suffers from its limitations to work always as reliable descriptor of aromaticity,90,99–101 and its use for some model systems has been warned, including the organometallic and metallic cluster systems.102 Here, as a complement of the ACID calculations, we also obtained the NICSs of the macrocyclic and the pyrrolic and furanic subunits and tried to analyze the data with caution. The values of NICS(0) and NICS(1) are −13.62 and −10.09 for pyrrole, −11.88 and −9.38 for furan, and −8.03 and −10.20 for benzene at the B3LYP/6-311+G(d,p) level, according to Chen and Schleyer and coworkers.82
In this work, the NICS(0) and NICS(1) values of H2L12−, L22−, and L33− and their corresponding complexes AnO2Ln− (An = U, Np, Pu) have been calculated at the same level as optimization based on the fully optimized structures to investigate how the presence of actinyls and the deformation of the ligands upon their complexation with actinyls may modulate the aromaticity of the polycyclic systems. In the calculations, the presence of actinyls was mimicked by putting the Mulliken atomic charges of An and the two Oyl atoms at the corresponding sites, and the NICS(1) results are listed in Table 4. Due to the limitation of the program we used in the calculation of NICS, we have only investigated the model systems that did not experience electron transfer between the actinyl and the macrocyclic ligands.
| Actinide | A Ring | B Ring | C Ring | A* Ring | B* Ring | C* Ring | G Ring | |
|---|---|---|---|---|---|---|---|---|
| H2L12− | H2L12− | −9.11 | −6.01 | −3.83 | −11.66 | −6.76 | −7.70 | 4.26 |
| UVI | −17.12 | −5.15 | −13.89 | −5.89 | −1.41 | −6.15 | −0.93 | |
| UV | −12.62 | −4.64 | −7.95 | −9.90 | −4.88 | −6.80 | 4.01 | |
| NpV | −12.62 | −4.66 | −7.87 | −9.96 | −4.77 | −6.84 | 4.12 | |
| PuV | −12.88 | −4.67 | −7.79 | −9.92 | −4.81 | −6.77 | 4.11 | |
| L22− | L22− | −5.43 | −7.62 | −8.05 | −5.59 | −7.23 | — | −4.63 |
| UVI | −14.74 | −10.76 | −14.64 | −13.46 | −10.50 | — | −7.91 | |
| NpV | −7.34 | −8.43 | −12.25 | −7.33 | −8.25 | — | −9.23 | |
| PuV | −7.28 | −8.28 | −12.14 | −7.30 | −8.10 | — | −8.98 | |
| L33− | L33− | −7.17 | −18.37 | −9.18 | −6.88 | −20.40 | — | 1.39 |
| UVI | −8.56 | −11.77 | −11.51 | −8.56 | −13.09 | — | −1.51 | |
| PuVI | −8.54 | −11.53 | −11.34 | −8.55 | −12.89 | — | −0.67 | |
| NpV | −7.29 | −11.51 | −10.64 | −7.22 | −12.83 | — | 0.15 | |
| PuV | −7.37 | −11.32 | −10.61 | −7.25 | −12.70 | — | 0.30 | |
According to our calculations, the aromaticity of the deprotonated H2L12− and L33− ligands displays a highly localized feature in view of their positive NICS(0) (Table S17, ESI†) and NICS(1) values for the macrocycles, while negative for their ring subunits. For H2L12−, both the NICS(0) and NICS(1) indices of A and A* rings are about double of the values of the other rings, suggesting stronger π character of A and A* rings than the others. As seen in Scheme 1, the difference between the two equivalent A and A* rings and the others is that these two rings are directly neighboured to two aromatic pyrrole rings, while the others are bridged by a meso-C atom, leading to more significant delocalization of π electrons to be redistributed in a broader region. Similar phenomena exist in L33−, where B and B* displays stronger π character than A and A* rings. For ligand L22−, both the NICS(0) and NICS(1) indices of the whole ligand and its ring subunits are negative, among which ring C, i.e. the furan ring, has the larger indices. These results are consistent with the ACID results discussed above.
Upon the complexation with actinyl cations, both the NICS(1) indices of the sub-rings of H2L12− and L33− become less negative except for A and C rings of H2L12− and A, A* and C rings of L33−. In contrast, the NICS(1) indices of L22− become much more negative in the complexes, indicating that the deformation due to the complexation with actinyls enhances the aromaticity of L22−. These suggest that ligand L22− benefits more than the other two ligands upon complexation with actinyls, and explains the larger exothermicity of L22− than H2L12− and L33− when coordinating with the same actinyl.
4. Conclusion
The complexation of six penta-aquo actinyl complexes, AnO2(H2O)52+/+ (An = U/Np/Pu), with three expanded porphyrins (ePors), amethyrin (H4L1), oxasapphyrin (H2L2), and grandephyrin (H3L3), have been studied by range-separated hybrid functional CAM-B3LYP. The geometries, the electronic structures, the binding free energies, and the NMR properties have been analyzed, and quantum theory of atom-in-molecules (QTAIM) topology analysis was also done to understand the interaction modes between to two entities of each complex.In view of binding free energy, in MeOH, PuO22+ and NpO22+ display stronger propensity to coordination with the ePor ligands than UO22+, UO2+, NpO2+, and PuO2+ for the same ligand, and the binding affinity of the L22− ligand to the actinyls is larger than the other two ligands, suggesting that the introduction of the furan ring in L22− favors the binding of actinyls. In the complexes of Np(VI) and Pu(VI), we observed higher spin density accumulation on the actinide atoms. The stronger electron affinity of Np(VI) and Pu(VI) than that of U(VI), U(V), Np(V), and Pu(V) enables the former to more significantly influence the electronic structures of the ligands than the latter. This leads to electronic configurations of f0, f2, f3, f1, f2 and f3 for U(VI), Np(VI), Pu(VI), U(V), Np(V) and Pu(V) in their H2L12− complexes, f0, f2, f3, f0, f2, and f3 in their L22− complexes, and f0, f2, f2, f0, f2, and f3 in their L33− complexes, respectively. The redox reactions upon the binding of Np(VI) and Pu(VI) to the specific ligands are consistent with the experimental observations.
The QTAIM was used here to analyze the bonding mode of AnO22+/+ (An = U/Np/Pu) with H2L12−/L22−/L33− and agrees well with the messages delivered by the analysis of geometric parameters and free energies along the ligand exchange reactions. Our calculations suggest that the interaction between An and the electron donor atoms in the first coordination shell is best described as ionic interaction, and covalency remains between An and Oyl in the actinyl upon their complexation with the ligands studied here.
Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China (21573021, 21073013, and 91226105) and the CAS Hundred Talents Program (Y2291810S3) for financial support.References
- A. Dormond, B. Belkalem and R. Guilard, Polyhedron, 1984, 3, 107–112 CrossRef CAS.
- G. S. Girolami, S. N. Milam and K. S. Suslick, Inorg. Chem., 1987, 26, 343–344 CrossRef CAS.
- G. S. Girolami, S. N. Milam and K. S. Suslick, J. Am. Chem. Soc., 1988, 110, 2011–2012 CrossRef CAS.
- O. Bilsel, S. N. Milam, G. S. Girolami, K. S. Suslick and D. Holten, J. Phys. Chem., 1993, 97, 7216–7221 CrossRef CAS.
- K. Kadish, G. Moninot, Y. Hu, D. Dubois, A. Ibnlfassi, J. Barbe and R. Guilard, J. Am. Chem. Soc., 1993, 115, 8153–8166 CrossRef CAS.
- G. S. Girolami, P. A. Gorlin, S. N. Milam, K. S. Suslick and S. R. Wilson, J. Coord. Chem., 1994, 32, 173–212 CrossRef CAS.
- X. Cao, Q. Li, A. Moritz, Z. Xie, M. Dolg, X. Chen and W. Fang, Inorg. Chem., 2006, 45, 3444–3451 CrossRef CAS PubMed.
- V. V. Roznyatovskiy, C.-H. Lee and J. L. Sessler, Chem. Soc. Rev., 2013, 42, 1921–1933 RSC.
- A. K. Burrell, G. Hemmi, V. Lynch and J. L. Sessler, J. Am. Chem. Soc., 1991, 113, 4690–4692 CrossRef CAS.
- A. K. Burrell, M. J. Cyr, V. Lynch and J. L. Sessler, J. Chem. Soc., Chem. Commun., 1991, 1710–1713 RSC.
- P. Thuéry and M. Nierlich, J. Inclusion Phenom. Macrocyclic Chem., 1997, 27, 13–20 CrossRef.
- P. Thuéry, M. Nierlich, B. Masci, Z. Asfari and J. Vicens, J. Chem. Soc., Dalton Trans., 1999, 3151–3152 RSC.
- P. Thuéry, M. Nierlich, J. Vicens and B. Masci, J. Chem. Soc., Dalton Trans., 2001, 867–874 RSC.
- P. Thuéry, M. Nierlich, J. Vicens, B. Masci and H. Takemura, Eur. J. Inorg. Chem., 2001, 637–643 CrossRef.
- A. Navaza, F. Villain and P. Charpin, Polyhedron, 1984, 3, 143–149 CrossRef CAS.
- L. Deshayes, N. Keller, M. Lance, A. Navaza, M. Nierlich and J. Vigner, Polyhedron, 1994, 13, 1725–1733 CrossRef CAS.
- D. L. Clark, D. W. Keogh, P. D. Palmer, B. L. Scott and C. D. Tait, Angew. Chem., Int. Ed., 1998, 37, 164–166 CrossRef CAS.
- R. D. Rogers, A. H. Bond, W. G. Hipple, A. N. Rollins and R. F. Henry, Inorg. Chem., 1991, 30, 2671–2679 CrossRef CAS.
- J. L. Sessler, D. Seidel, C. Bucher and V. Lynch, Tetrahedron, 2001, 57, 3743–3752 CrossRef CAS.
- J. L. Sessler, N. A. Tvermoes, J. Davis, P. Anzenbacher, K. Jursikova, W. Sato, D. Seidel, V. Lynch, C. B. Black and A. Try, Pure Appl. Chem., 1999, 71, 2009–2018 CrossRef CAS.
- J. L. Sessler, T. D. Mody and V. Lynch, Inorg. Chem., 1992, 31, 529–531 CrossRef CAS.
- J. L. Sessler, A. E. Gorden, D. Seidel, S. Hannah, V. Lynch, P. L. Gordon, R. J. Donohoe, C. D. Tait and D. W. Keogh, Inorg. Chim. Acta, 2002, 341, 54–70 CrossRef CAS.
- J. L. Sessler, D. Seidel, A. E. Vivian, V. Lynch, B. L. Scott and D. W. Keogh, Angew. Chem., Int. Ed., 2001, 40, 591–594 CrossRef CAS.
- J. Sessler, Chem. Commun., 1998, 1835–1836 RSC.
- J. L. Sessler, S. J. Weghorn, Y. Hiseada and V. Lynch, Chem. – Eur. J., 1995, 1, 56–67 CrossRef CAS.
- J. Sessler, A. Vivian, D. Seidel, A. Burrell, M. Hoehner, T. Mody, A. Gebauer, S. Weghorn and V. Lynch, Coord. Chem. Rev., 2001, 216, 411–434 CrossRef.
- S. Hannah, D. Seidel, J. L. Sessler and V. Lynch, Inorg. Chim. Acta, 2001, 317, 211–217 CrossRef CAS.
- D. C. M. Gosmann and B. Franck, Angew. Chem., Int. Ed. Engl., 1986, 25, 1100–1101 CrossRef.
- T. Wessel, B. Franck, M. Möller, U. Rodewald and M. Läge, Angew. Chem., Int. Ed. Engl., 1993, 32, 1148–1151 CrossRef.
- B. Franck and A. Nonn, Angew. Chem., Int. Ed. Engl., 1995, 34, 1795–1811 CrossRef CAS.
- M. Holthausen and W. Koch, A Chemist's Guide to Density Functional Theory, Wiley Verlag Chemie, New York, 2000 Search PubMed.
- G. Schreckenbach, P. J. Hay and R. L. Martin, J. Comput. Chem., 1999, 20, 70–90 CrossRef CAS.
- N. Kaltsoyannis, Chem. Soc. Rev., 2003, 32, 9–16 RSC.
- E. R. Batista, R. L. Martin, P. J. Hay, J. E. Peralta and G. E. Scuseria, J. Chem. Phys., 2004, 121, 2144–2150 CrossRef CAS PubMed.
- N. Kaltsoyannis, P. Hay, J. Li, J. Blaudeau and B. Bursten, The Chemistry of The Actinide and Transactinide Elements, Dordrecht, The Netherlands, 2006 Search PubMed.
- V. Vallet, P. Macak, U. Wahlgren and I. Grenthe, Theor. Chem. Acc., 2006, 115, 145–160 CrossRef CAS.
- G. A. Shamov, G. Schreckenbach and T. N. Vo, Chem. – Eur. J., 2007, 13, 4932–4947 CrossRef CAS PubMed.
- F. Réal, V. Vallet, U. Wahlgren and I. Grenthe, J. Am. Chem. Soc., 2008, 130, 11742–11751 CrossRef PubMed.
- J.-H. Lan, W.-Q. Shi, L.-Y. Yuan, J. Li, Y.-L. Zhao and Z.-F. Chai, Coord. Chem. Rev., 2012, 256, 1406–1417 CrossRef CAS.
- D. Wang, W. F. van Gunsteren and Z. Chai, Chem. Soc. Rev., 2012, 41, 5836–5865 RSC.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- M. Torrent-Sucarrat, J. M. Anglada and J. M. Luis, J. Chem. Phys., 2012, 137, 184306 CrossRef PubMed.
- W. Ding, W. Fang, Z. Chai and D.-Q. Wang, Acta Phys.-Chim. Sin., 2015, 31, 1283–1301 CAS.
- D. Chesnut, B. Rusiloski, K. Moore and D. Egolf, J. Comput. Chem., 1993, 14, 1364–1375 CrossRef CAS.
- G. DiLabio, J. Phys. Chem. A, 1999, 103, 11414–11424 CrossRef CAS.
- A. Wu and X. Xu, J. Comput. Chem., 2012, 33, 1421–1432 CAS.
- W. Küchle, M. Dolg, H. Stoll and H. Preuss, J. Chem. Phys., 1994, 100, 7535–7542 CrossRef.
- X. Cao, M. Dolg and H. Stoll, J. Chem. Phys., 2003, 118, 487–496 CrossRef CAS.
- X. Cao and M. Dolg, THEOCHEM, 2004, 673, 203–209 CrossRef CAS.
- Y.-K. Han and K. Hirao, J. Chem. Phys., 2000, 113, 7345–7350 CrossRef CAS.
- Y. K. Han, J. Comput. Chem., 2001, 22, 2010–2017 CrossRef CAS.
- G. A. Shamov and G. Schreckenbach, J. Phys. Chem. A, 2005, 109, 10961–10974 CrossRef CAS PubMed , correction note: G. A. Shamov and G. Schreckenbach, J. Phys. Chem. A, 2006, 110, 12072 CrossRef.
- G. Schreckenbach, Int. J. Quantum Chem., 2005, 101, 372–380 CrossRef CAS.
- W. Ding, W. Fang, Z. Chai and D. Wang, J. Chem. Theory Comput., 2012, 8, 3605–3617 CrossRef CAS PubMed.
- W. Ding and D. Wang, Organometallics, 2014, 33, 7007–7010 CrossRef CAS.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650–654 CrossRef CAS.
- A. McLean and G. Chandler, J. Chem. Phys., 1980, 72, 5639–5648 CrossRef CAS.
- T. Clark, J. Chandrasekhar, G. W. Spitznagel and P. V. R. Schleyer, J. Comput. Chem., 1983, 4, 294–301 CrossRef CAS.
- M. J. Frisch, J. A. Pople and J. S. Binkley, J. Chem. Phys., 1984, 80, 3265–3269 CrossRef CAS.
- W. J. Hehre, R. Ditchfield and J. A. Pople, J. Chem. Phys., 1972, 56, 2257–2261 CrossRef CAS.
- M. M. Francl, W. J. Pietro, W. J. Hehre, J. S. Binkley, M. S. Gordon, D. J. DeFrees and J. A. Pople, J. Chem. Phys., 1982, 77, 3654–3665 CrossRef CAS.
- M. Cossi, V. Barone, R. Cammi and J. Tomasi, Chem. Phys. Lett., 1996, 255, 327–335 CrossRef CAS.
- G. Scalmani and M. J. Frisch, J. Chem. Phys., 2010, 132, 114110 CrossRef PubMed.
- D. C. Fang, Thermo, Beijing Normal University, Beijing, 100875, People's Republic of China.
- V. R. Vallet, U. Wahlgren and I. Grenthe, J. Phys. Chem. A, 2012, 116, 12373–12380 CrossRef CAS PubMed.
- R. F. Bader, J. Phys. Chem. A, 1998, 102, 7314–7323 CrossRef CAS.
- A. Becke, C. F. Matta and R. J. Boyd, The quantum theory of atoms in molecules: from solid state to DNA and drug design, Wiley-VCH, Weinheim, 2007 Search PubMed.
- M. J. Tassell and N. Kaltsoyannis, Dalton Trans., 2010, 39, 6719–6725 RSC.
- R. F. Bader, Atoms in Molecules: A Quantum Theory, Oxford University Press, Oxford, 1990 Search PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- T. Keith and R. Bader, Chem. Phys. Lett., 1992, 194, 1–8 CrossRef CAS.
- T. A. Keith and R. F. Bader, Chem. Phys. Lett., 1993, 210, 223–231 CrossRef CAS.
- J. R. Cheeseman, G. W. Trucks, T. A. Keith and M. J. Frisch, J. Chem. Phys., 1996, 104, 5497–5509 CrossRef CAS.
- T. A. Keith and R. F. Bader, J. Chem. Phys., 1993, 99, 3669–3682 CrossRef CAS.
- R. Ditchfield, Mol. Phys., 1974, 27, 789–807 CrossRef CAS.
- K. Wolinski, J. F. Hinton and P. Pulay, J. Am. Chem. Soc., 1990, 112, 8251–8260 CrossRef CAS.
- R. Herges and D. Geuenich, J. Phys. Chem. A, 2001, 105, 3214–3220 CrossRef CAS.
- D. Geuenich, K. Hess, F. Köhler and R. Herges, Chem. Rev., 2005, 105, 3758–3772 CrossRef CAS PubMed.
- C. Corminboeuf, T. Heine, G. Seifert, P. von Ragué Schleyer and J. Weber, Phys. Chem. Chem. Phys., 2004, 6, 273–276 RSC.
- H. Fallah-Bagher-Shaidaei, C. S. Wannere, C. Corminboeuf, R. Puchta and P. V. R. Schleyer, Org. Lett., 2006, 8, 863–866 CrossRef CAS PubMed.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. V. R. Schleyer, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS PubMed.
- G. A. Shamov and G. Schreckenbach, J. Phys. Chem. A, 2006, 110, 9486–9499 CrossRef CAS PubMed.
- G. A. Shamov, G. Schreckenbach, R. L. Martin and P. J. Hay, Inorg. Chem., 2008, 47, 1465–1475 CrossRef CAS PubMed.
- N. Kaltsoyannis, Inorg. Chem., 2013, 52, 3407–3413 CrossRef CAS PubMed.
- I. D. Prodan, G. E. Scuseria and R. L. Martin, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 033101 CrossRef.
- C.-C. Wang, T.-H. Tang and Y. Wang, J. Phys. Chem. A, 2000, 104, 9566–9572 CrossRef CAS.
- J. Zeng, X. Yang, J. Liao, N. Liu, Y. Yang, Z. Chai and D. Wang, Phys. Chem. Chem. Phys., 2014, 16, 16536–16546 RSC.
- C. F. Matta and J. Hernández-Trujillo, J. Phys. Chem. A, 2003, 107, 7496–7504 CrossRef CAS , Correction: J. Phys. Chem. A, 2005, 109, 10798.
- J. I. Wu, I. Fernández and P. v. R. Schleyer, J. Am. Chem. Soc., 2013, 135, 315–321 CrossRef CAS PubMed.
- M. Bühl and C. van Wüllen, Chem. Phys. Lett., 1995, 247, 63–68 CrossRef.
- P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. v. E. Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS.
- P. V. R. Schleyer, H. Jiao, N. J. V. E. Hommes, V. G. Malkin and O. L. Malkina, J. Am. Chem. Soc., 1997, 119, 12669–12670 CrossRef CAS.
- P. Schleyer, M. Manoharan, Z.-X. Wang, B. Kiran, H. Jiao, R. Puchta and N. Hommes, Org. Lett., 2001, 3, 2465–2468 CrossRef CAS.
- M. K. Cyrañski, T. M. Krygowski, M. Wisiorowski, N. J. van Eikema Hommes and P. V. R. Schleyer, Angew. Chem., Int. Ed., 1998, 37, 177–180 CrossRef.
- T. M. Krygowski and M. K. Cyranski, Chem. Rev., 2001, 101, 1385–1420 CrossRef CAS PubMed.
- J. Jusélius and D. Sundholm, J. Org. Chem., 2000, 65, 5233–5237 CrossRef.
- J. Jusélius and D. Sundholm, Phys. Chem. Chem. Phys., 2000, 2, 2145–2151 RSC.
- P. Lazzeretti, Phys. Chem. Chem. Phys., 2004, 6, 217–223 RSC.
- F. Feixas, E. Matito, J. Poater and M. Solà, J. Comput. Chem., 2008, 29, 1543–1554 CrossRef CAS PubMed.
- P. Bultinck, Faraday Discuss., 2007, 135, 347–365 RSC.
- C. Foroutan-Nejad, Theor. Chem. Acc., 2015, 134, 8 CrossRef.
- Y. Gong, H.-S. Hu, L. Rao, J. Li and J. K. Gibson, J. Phys. Chem. A, 2013, 117, 10544–10550 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The optimized structures of the complexes with H2L12−, L22−, and L33− ligands and their complexes with AnO2(H2O)52+/+ (An = U, Np, Pu) in vacuum and in methanol; the calculated energies of different isomers of the three ligands in vacuum and in methanol; the influence of protonation on the complexation; key geometrical parameters of the actinyls and the corresponding complexes; the atomic charges and the atomic spin densities of key atoms and groups, and the bond orders of the complexes in methanol; the QTAIM analysis of the complexes in methanol; the NICS values of the ligands and their complexation states with AnO22+/+ in methanol; the integration accuracy and the convergence criteria in our calculations; and the coordinates and energies of stationary points. See DOI: 10.1039/c6nj01615d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |