Downsizing the K-CHA zeolite by a postmilling-recrystallization method for enhanced base-catalytic performance†
Chokkalingam
Anand
a,
Takeshi
Kaneda
b,
Satoshi
Inagaki
c,
Sae
Okamura
c,
Hiroki
Sakurai
c,
Kyosuke
Sodeyama
a,
Taiji
Matsumoto
b,
Yoshihiro
Kubota
c,
Tatsuya
Okubo
a and
Toru
Wakihara
*a
aDepartment of Chemical System Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: wakihara@chemsys.t.u-tokyo.ac.jp
bDepartment of Materials Technology, Industrial Technology Centre of Tochigi Prefecture, 1-5-20, Yuinomori, Utsunomiya, 321-3226, Japan
cDivision of Materials Science and Chemical Engineering, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama 240-8501, Japan
First published on 6th November 2015
Abstract
Herein, we report the preparation of potassium chabazite (K-CHA) zeolite nanocrystals with enhanced base-catalytic performance using a bead-milling and postmilling-recrystallization technique. Particles as small as 60 nm were produced by this approach. The surface area of the downsized K-CHA samples showed a significant improvement, increasing from 11 m2 g−1 to 65 m2 g−1. The catalytic activity of the K-CHA nanocrystals in the Knoevenagel condensation of benzaldehyde with ethyl cyanoacetate was found to be twofold higher than that of the raw and the milled samples, achieving 80% product yield within a reaction time of 60 min. In addition, the enhanced catalytic behavior of the K-CHA nanocrystals, despite possessing low surface area relative to the intermediate milled sample, is attributable to the postmilling-recrystallization step, which played a pivotal role in restoring the crystallinity to around 80%.
Introduction
Catalysis is an indispensable phenomenon in chemical sciences and is currently inseparable from our daily lives. Industrial estimates have confirmed that 90% of products, ranging from bulk chemicals to consumer goods, involve a catalyst at some stage of their manufacturing processes. Due to stringent environmental legislation, heterogeneous catalysts are considered clean and are highly sought after because of their activity, selectivity, and reusability.1–3 The discovery of zeolites revolutionized the field of heterogeneous catalysis. The dimensionality, crystallinity, and porosity of zeolites are considered essential to overcome the tremendous industrial challenges associated with heterogeneous catalysis.Zeolites are inorganic crystalline porous solids built up of corner-sharing ‘Si’ and ‘Al’ tetrahedral framework atoms (TO4) linked by four oxygen atoms. Zeolites are associated with enormous structural and textural diversity, and their features can be modified to meet specific needs. Zeolites have been pivotal in solving increasingly complex environmental and economic problems faced by the energy and chemical industries.4–13 The active centers of zeolites that are responsible for catalytic activities can be classified into acidic, basic, and redox, which are in turn generated by proton, alkali, and metal (e.g. alkali earth metals) modifications, respectively. Among these, the acid-catalytic properties of zeolites have been the subject of various research efforts both academically and industrially for a long time. By comparison, there have been far fewer reports concerning the base-catalytic properties of zeolites, despite the superior potential of these properties to be used to produce fine and specialty chemicals and intermediates with minimal amounts of waste and at low costs.14,15
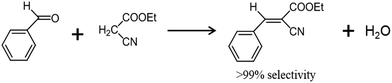
In this report, we discuss the base-catalytic properties of potassium chabazite (K-CHA) nanocrystals in the Knoevenagel condensation reaction of benzaldehyde with ethyl cyanoacetate. A unique top-down method that combines bead-milling and postmilling-recrystallization using dilute silicate or alkali solutions, which was introduced and used in our previous studies on downsizing micrometer-sized zeolites to their respective nanocrystals, was also employed in this study. Previously, this technique was applied to prepare zeolites such as A and X of particle sizes <100 nm with high reproducibility and uniformity.16,17 The heterogeneous base-catalytic properties of zeolites have since been found to be advantageous over those of homogeneous catalysts in terms of separation and regeneration. In particular, zeolites downsized to the nanometer scale are believed to possess large external surface areas and superior catalytic activities since their diffusion paths are shorter than those in their micrometer-sized counterparts.18 Therefore, we decided to investigate the influence of milling-induced size reduction on the base-catalytic potential of K-CHA in the Knoevenagel condensation reaction of benzaldehyde (a carbonyl compound) with ethyl cyanoacetate (a carbanion compound).19,20 The Knoevenagel condensation is a significant step in the commercial production of bulk pharmaceuticals, whereby a carbanion is added to an electrophilic carbonyl group through nucleophilic attraction with subsequent dehydration, for which a weakly basic medium is required.20
In this study, a K-CHA zeolite with an average particle size of 60 nm was prepared by a bead-milling and the subsequent postmilling-recrystallization technique. Compared to the performances of raw K-CHA zeolites, the size reduction of the K-CHA particles resulted in a dramatic enhancement of their catalytic performances in the Knoevenagel condensation reaction. Recrystallization from the KOH solution proved to be necessary to obtain K-CHA nanoparticles with high crystallinity, which in turn was essential to enhance their catalytic performance. The influences of the amount of KOH, recrystallization time, and temperature on the crystallinity of the K-CHA nanocrystals were also investigated to optimize the conditions to prepare smaller K-CHA nanocrystals.
Experimental
K-CHA zeolite was prepared by introducing slight modification to the previous recipe21 with the following gel composition: 1 SiO2: 0.0328 Na2O: 0.386 K2O: 0.193 Al2O3: 43.2 H2O: 21. In a typical synthesis, the requisite quantity of KOH pellets (KOH, Wako, Japan) was dissolved in water and mixed. Zeolite Y (Tosoh Corporation, Japan) was added to this solution, and the mixture was stirred for 30 min to attain the maximum dispersion. The resulting gel was heated at 368 K for several hours. Finally, the K-CHA samples were separated from the mother gel by centrifugation at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The pH of each K-CHA sample was adjusted to near neutral by washing three times with deionized water prior to drying overnight at 333 K. The dried K-CHA sample was then subjected to size reduction by bead-milling.
000 rpm. The pH of each K-CHA sample was adjusted to near neutral by washing three times with deionized water prior to drying overnight at 333 K. The dried K-CHA sample was then subjected to size reduction by bead-milling.
The K-CHA samples thus obtained were bead-milled using an LMZ015 milling apparatus (Ashizawa Finetech Ltd., Japan). Prior to the milling, the K-CHA crystals (40 g) were made into a well-dispersed slurry with H2O (250 g) by means of an ultrasonic vibrator. The resulting slurry was fed into the milling apparatus packed with zirconia beads of diameter 300 μm and pulverized for 2 h at a rotation rate of 9 ms−1. The milled K-CHA particles were separated by centrifugation and dried at 373 K overnight in a hot-air oven. They were then recrystallized in batches of 2 g inside a stainless steel autoclave under autogenous pressure using 2 M KOH solution as a recrystallization agent. The conditions of the postmilling-recrystallization were optimized by varying the amount of 2 M KOH (0.4–1.2 g), the time (2–5 h), and temperature (393–413 K), the details of which are given in Table 1. The products thus obtained were recovered by centrifugation at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 293 K, washed twice with water to bring them to pH 8, and dried at 333 K overnight.
000 rpm at 293 K, washed twice with water to bring them to pH 8, and dried at 333 K overnight.
| Sample | 2 M KOH added (g) | RC temperature (K) | RC duration (hour) | Crystallinity (%) | Yield (%) |
|---|---|---|---|---|---|
| Raw | 0 | 0 | 0 | 100 | 100 |
| Milled | 0 | 0 | 0 | 11 | 100 |
| RC1 | 0.4 | 413 | 5 | 46 | 94 |
| RC2 | 0.8 | 393 | 2 | 20 | 92 |
| RC3 | 0.8 | 393 | 5 | 80 | 96 |
| RC4 | 1.2 | 413 | 2 | 79 | 92 |
| RC5 | 1.2 | 413 | 5 | 80 | 91 |
X-ray diffraction analysis (XRD, Ultima IV, Rigaku) was used to identify the phase as well as to calculate the crystallinity. The crystallinities of the milled and recrystallized samples were estimated through the peak-fitting technique by comparing the area of the peaks observed between 2θ values of 19.5° and 38° with those of a raw K-CHA sample. The surface areas of the samples were measured at 77 K by using a Quantachrome Autosorb-iQ sorption analyzer. All samples were outgassed at 673 K for 6 h prior to nitrogen adsorption measurements. The compositions of the samples were measured by XRF (XRF, JSX-3400R II, JEOL) and ICP-AES (ICP-E9000, Shimadzu). The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method. The framework coordination of the aluminum and silicon species was analyzed on a 600 MHz Bruker AVANCE III spectrometer. These measurements were conducted in accordance with our previous work reported elsewhere.20 The particle sizes and morphologies of the samples were observed using a field-emission scanning electron microscope (FESEM, JEOL JSM-7400F) with an electron-beam accelerating voltage of 5 keV.
The catalytic activity of the K-CHA nanocrystals was tested in a Knoevenagel condensation reaction of benzaldehyde (1.2 mmol; 130 mg) with ethyl cyanoacetate (1.4 mmol; 160 mg). The reactions were performed in a liquid-phase reactor setup at 353 K with a zeolite catalyst (30 mg) and ethanol (3 mL) as a solvent. The influences of the reduced particle size and the degree of crystallinity on the catalytic performances of the K-CHA nanocrystals were studied in comparison with those of the raw and milled K-CHA zeolites. The products were analyzed using a gas-chromatograph (GC-2014, Shimadzu) equipped with a flame ionization detector (FID) and a capillary column (DB-5MS; length, 30 m; inner diameter, 0.25 mm; film thickness, 0.25 μm).
Results and discussion
The effects of the 2 M KOH content, recrystallization temperature, and time on the crystallinity and yield of the K-CHA nanocrystals were tabulated (Table 1). The K-CHA nanocrystals thus prepared are designated as “RC-x”, where “RC” denotes recrystallization and “x” denotes the batch number (1–5). It is evident that the amount of KOH as well as the temperature and the duration for the recrystallization are crucial to obtain K-CHA nanocrystals with higher crystallinity. Somewhere between RC3 and RC5 conditions, the crystallinity seems to be saturated. Of the listed conditions, RC5, which was prepared using 1.2 g of 2 M KOH solution at 413 K for 5 h, was chosen to perform further characterization as it achieved high crystallinity as well as highly uniform nanocrystals with an average size of 60 nm.We explored other physico-chemical properties and the catalytic activity of RC5 and compared the results with those of the raw and milled K-CHA samples. Furthermore, the yield of recrystallized K-CHA nanocrystals was found to be essentially consistent, lying in the range from 91% to 96%, despite significant variations in the amount of KOH used, temperature, and the duration for the individual recrystallizations.
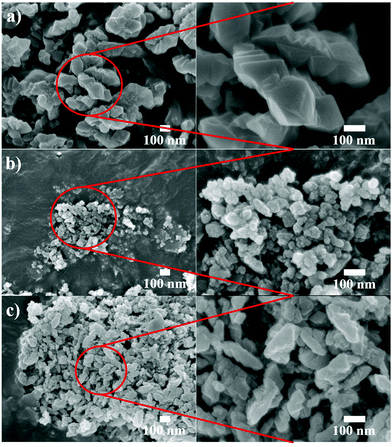
Fig. 1 shows the XRD patterns of the raw, milled, and recrystallized K-CHA nanocrystals (RC5). A decrease in intensity of the XRD peaks is observed for both the milled and recrystallized (RC5) samples as compared to the raw K-CHA, which is evidently due to crystallinity differences. Furthermore, the observed peak-broadening in the milled and recrystallized samples could be attributable to the formation of K-CHA nanocrystals. Although milling is favorable since it effectively downsizes crystallites, it also affects the degree of crystallinity because of the resulting structural deformation, yielding less intense XRD peaks (Fig. 1). The crystallinity of the milled sample with respect to that of raw K-CHA (100%) was found to be 11%. However, the degree of crystallinity of the milled sample significantly recovered to 80% after recrystallization, as estimated from the XRD pattern of RC5. Fig. 2a and b show the influences of bead-milling and the following postmilling-recrystallization on the morphology and crystallite size of the K-CHA zeolites, as observed by FESEM. Bead-milling led to extensive morphological changes from raw to milled K-CHA and further to RC-5 as we attempted to reduce the particle size. In relation to the raw K-CHA, a drastic particle size reduction is clearly evident for both the milled and RC5 samples. However, the particle size of RC5 as observed from FESEM images (Fig. 2b and c) is relatively larger than the milled K-CHA. This growth in particle size might be due to Ostwald's ripening.
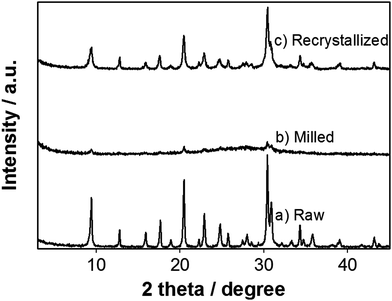 | ||
| Fig. 1 XRD patterns of products: (a) raw K-CHA, (b) milled K-CHA, and (c) recrystallized K-CHA (RC5). | ||
The catalytic activities of the raw, milled, and RC5 samples were tested by using the Knoevenagel condensation of benzaldehyde with ethyl cyanoacetate as a probe reaction (Fig. 3). Since both the reactant molecules are much larger than the aperture (0.38 × 0.38 nm) of the CHA framework, the condensation reaction most probably occurs only on the external surface of chabazite particles. Such catalytic systems were found to be highly advantageous for the selective production of ethyl trans-α-cyanocinnamate (>99%) without cis-isomers.20,22,23 As given in Table 2, a dramatic increase in the yield of the desired product was confirmed, which rises from 35% to 80% as a result of the recrystallization treatment. Further, the catalytic performance of RC1 and RC4 was also investigated and was found to be less than RC5 (Table S1, ESI†), which indicates that conditions used for recrystallizing RC5 as optimum for enhanced catalytic activity. In order to unequivocally assign this surge in the catalytic performance to the particle size reduction and the degree of crystallinity, it was necessary to subject these samples to more sophisticated physico-chemical analysis, such as specific-surface-area measurement and solid-state MAS NMR characterization, and the relevant results are summarized in Table 2 and Fig. 4, respectively. The surface area, listed in Table 2, presumably is external as observed from the N2 adsorption–desorption isotherms of raw, milled and recrystallized K-CHA samples shown in Fig. S1 of the ESI,† which dramatically increases from 11 m2 g−1 to 96 m2 g−1 as a result of milling the raw K-CHA sample, while it decreases to 65 m2 g−1 after recrystallization (Table 2). Further, the variation in the amount of N2 adsorbed relative to surface area is clearly evident from Fig. S1 (ESI†) and is consistent with the particle size variations observed from the FESEM images (Fig. 2). Despite possessing a very high surface area (96 m2 g−1), the milled K-CHA shows only an 11% increase in the yield, specifically, 46% as compared to the 35% obtained for the raw K-CHA.
| Sample | Composition | BET surface area (m2 g−1) | Product yield in the catalytic reactiona (%) | |||
|---|---|---|---|---|---|---|
| Si/Al [XRF] | Al | K | K/Al [ICP] | |||
| (mmol g−1) [ICP] | ||||||
| a Reaction conditions: catalyst, 30 mg; temperature, 353 K; time, 60 min; benzaldehyde, 1.2 mmol (130 mg); ethyl cyanoacetate, 1.4 mmol (160 mg); solvent, EtOH (ca. 3.0 mL). | ||||||
| Raw | 3.24 | 3.34 | 3.08 | 0.92 | 11 | 35 |
| Milled | 3.46 | 3.50 | 3.24 | 0.93 | 96 | 46 |
| RC5 | 3.11 | 3.47 | 3.40 | 0.98 | 65 | 80 |
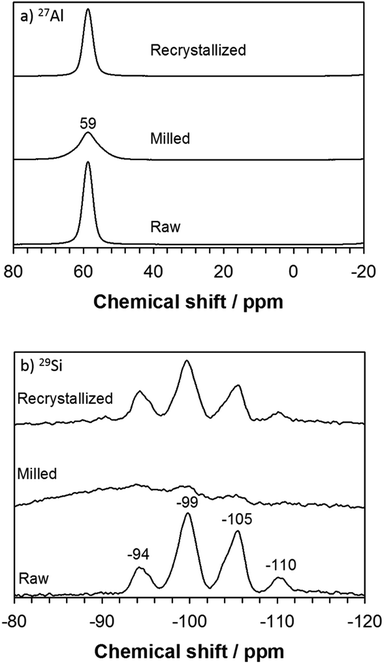 | ||
| Fig. 4 Solid-state MAS NMR spectra of raw, milled, and recrystallized K-CHA (RC5) samples: (a) 27Al DE MAS NMR and (b) 29Si DD MAS NMR. | ||
In contrast to the catalytic performance of the milled K-CHA, the recrystallized K-CHA (RC5) nanocrystals exhibit 80% yield, even though their surface area of 65 m2 g−1 is comparatively low. In an attempt to correlate this contradiction with bead-milling (i.e., particle size reduction) and postmilling-recrystallization (i.e., the degree of crystallinity), it was necessary to determine the compositions and respective chemical environments in the raw, milled, and recrystallized (RC5) samples. The former was found to be essentially consistent with the observed molar Si/Al and K/Al ratios shown in Table 2, and the latter was subsequently revealed by the solid-state MAS NMR results depicted in Fig. 4. The 27Al MAS NMR spectra feature a single peak at δ ≈ 59 ppm, which corresponds to tetrahedral coordination of Al in the framework. To some extent the diminished peak intensity of the milled and recrystallized samples is related to crystallinity variation (Fig. 4a). Similarly, the 29Si MAS NMR spectra of raw K-CHA and RC5 feature peaks at δ = −110 ppm, −105 ppm, −99 ppm, and −94 ppm, which could be assigned to ![[S with combining low line]](https://www.rsc.org/images/entities/char_0053_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif) (OSi)4,
(OSi)4, ![[S with combining low line]](https://www.rsc.org/images/entities/char_0053_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif) (OAl)(OSi)3,
(OAl)(OSi)3, ![[S with combining low line]](https://www.rsc.org/images/entities/char_0053_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif) (OAl)2(OSi)2, and
(OAl)2(OSi)2, and ![[S with combining low line]](https://www.rsc.org/images/entities/char_0053_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif) (OAl)3(OSi), respectively (Fig. 4b).24–26 However, for the milled sample, all four peaks seem to be merged with a very broad peak, which is attributable to structural collapse induced by mechanical milling (Fig. 4b).
(OAl)3(OSi), respectively (Fig. 4b).24–26 However, for the milled sample, all four peaks seem to be merged with a very broad peak, which is attributable to structural collapse induced by mechanical milling (Fig. 4b).
By carefully considering these results, we surmise that the extent of structural deformation generated as a result of milling was deleterious in the milled sample, whereas the RC5 sample regained the higher crystallinity as a result of suitable recrystallization treatment. Thus the catalytic activity exhibited by the recrystallized K-CHA nanocrystals was superior to that of the milled sample despite the former's surface area being inferior to the latter's. Therefore, combining the two steps of bead-milling and postmilling-recrystallization is crucial for the successful size reduction, structural reformation, and the enhancement of the application potential of zeolites in this top-down approach. Despite the overall contributions from the KOH content, time and temperature in the recrystallization process for downsizing the K-CHA zeolite with high crystallinity and enhanced catalytic potentials, the individual roles of these parameters are clearly evident from Table 1. Increasing the KOH content from 0.4 (RC1) to 1.2 g (RC5) directly influences the crystallinity, which increased from 46 to 80% in 5 hours at 413 K. Similarly, increasing the recrystallization time from 2 (RC2) to 5 (RC3) hours at a fixed KOH (0.8 g) content and temperature (393 K) raised the crystallinity 4 folds higher from 20 to 80%. However, deducing the specific role of the postmilling-recrystallization temperature from Table 1 is quite complex as it seems to work in coordination with either time or KOH content and sometimes both.
Conclusions
The base-catalytic activity of a K-CHA zeolite was enhanced via particle size reduction using a bead-milling and postmilling-recrystallization approach. The recrystallizing agent was 2 M KOH, and the recrystallization conditions were suitably optimized by varying the amount of KOH, temperature and the duration for the recrystallization and by assessing the influences of these parameters on the degree of crystallinity as well as on the final yield. Despite enhancing the surface area and downsizing the particles, milling inflicted deleterious structural collapse resulting in a drastic crystallinity reduction. However, the higher crystallinity has been achieved by using the optimum amount of 2 M KOH as a recrystallizing agent at 413 K for 5 h. Particles with an average size of 60 nm were thereby obtained. Combination of an appropriate amount of KOH, temperature, and duration for the recrystallization was found to be essential to achieve nanocrystalline K-CHA particles. The catalytic activity of the K-CHA nanocrystals was investigated by applying them in the Knoevenagel condensation of benzaldehyde with ethyl cyanoacetate and comparing the results with those achieved by the raw and milled samples. The K-CHA nanocrystals exhibited a twofold increase in catalytic activity, increasing the desired product yield from 35% to 80%. The N2 adsorption measurements and solid-state MAS NMR results indicated that the enhanced catalytic activity of RC5 was indeed due to the bead-milling and postmilling-recrystallization, which led to size reduction and crystallinity restoration, respectively. This approach may serve as an effective tool to design nanocrystalline zeolites with enhanced base-catalytic performance.Acknowledgements
This work has been supported partially by a “Grant for Advanced Industrial Technology Development” in 2011 from the New Energy and Industrial Technology Development Organization (NEDO) of Japan.References
- J. G. de Vries and S. D. Jackson, Catal. Sci. Technol., 2012, 2, 2009 CAS
.
- F. Zaera, Chem. Soc. Rev., 2013, 42, 2746 RSC
.
- C. Anand and A. Vinu, JSM Nanotechnol. Nanomed., 2013, 1, 1007 Search PubMed
.
- K. Hussar, S. Teekasap and N. Somsuk, Am. J. Environ. Sci., 2011, 7, 35 CrossRef CAS
.
- E. M. Flanigen, Pure Appl. Chem., 1980, 52, 2191 CrossRef CAS
.
- S. J. Kulkarni, Stud. Surf. Sci. Catal., 1998, 113, 151 CrossRef CAS
.
- F. A. Mumpton, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 3463 CrossRef CAS
.
- K. Ramesh and D. D. Reddy, Adv. Agron., 2011, 113, 219 Search PubMed
.
- N. Burriesci and S. Valente, Zeolites, 1984, 4, 373 CrossRef CAS
.
- M. Moradzadeh, H. Moazed and G. Sayyad, Acta Ecol. Sin., 2014, 34, 342 CrossRef
.
- S. Kesraoui-ouki, C. R. Cheeseman and R. Perry, J. Chem. Tech., Biotechnology, 1994, 59, 121 CAS
.
- C. Colella, Stud. Surf. Sci. Catal., 2007, 168, 999 CrossRef CAS
.
- H. Qi, W. Xu, J. Wang, L. Tong and T. Zhu, J. Environ. Sci., 2015, 28, 110 CrossRef PubMed
.
- H. Hattori, Chem. Rev., 1995, 95, 537 CrossRef CAS
.
- M. Wallau and U. Schuchardt, J. Braz. Chem. Soc., 1995, 6, 393 CrossRef CAS
.
- T. Wakihara, K. Sato, K. Sato, J. Tatami, S. Kohara, K. Komeya and T. Meguro, J. Ceram. Soc. Jpn., 2012, 120, 341 CrossRef CAS
.
- T. Wakihara, R. Ichikawa, J. Tatami, A. Endo, K. Yoshida, Y. Sasaki, K. Komeya and T. Meguro, Cryst. Growth Des., 2011, 11, 955 CAS
.
- H. Awala, J.-P. Gilson, R. Retoux, P. Boullay, J.-M. Goupil, V. Valtchev and S. Mintova, Nat. Mater., 2015, 14, 447–451 CrossRef CAS PubMed
.
- L. Tosheva and V. P. Valtchev, Chem. Mater., 2005, 17, 2494 CrossRef CAS
.
- S. Inagaki, K. Thomas, V. Ruaux, G. Clet, T. Wakihara, S. Shinoda, S. Okamura, Y. Kubota and V. Valtchev, ACS Catal., 2014, 4, 2333 CrossRef CAS
.
-
M. Bourgogne, J.-L. Guth and R. Wey, US Pat., 4503024, 1985 Search PubMed
.
- Y. Kubota, Y. Nishizaki, H. Ikeya, M. Saeki, T. Hida, S. Kawazu, M. Yoshida and H. Fujii, and Y. Sugi, Microporous Mesoporous Mater., 2004, 70, 135 CrossRef CAS
.
- Y. Kubota, Y. Sugi and T. Tatsumi, Catal. Surv. Asia, 2007, 11, 158 CrossRef CAS
.
- M. A. Camblor, P. A. Barrette, M.-J. Diaz-Cabanas, L. A. Villaescusa, M. Puche, T. Boix, E. Perez and H. Koller, Microporous Mesoporous Mater., 2001, 48, 11 CrossRef CAS
.
- G. Ikuhiro, I. Masaya, S. Syohei, H. Koutaro, I. Yusuke, S. Masahiro and T. Sano, Micropor. Mesopor. Mater., 2012, 158, 117 CrossRef
.
- B. Liu, Y. Zheng, N. Hu, T. Gui, Y. Li, F. Zhang, R. Zhou, X. Chen and H. Kita, Microporous Mesoporous Mater., 2014, 196, 270 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Separate file provided. See DOI: 10.1039/c5nj02052b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |