Open Access Article
Open Access ArticleSynthesis and in vitro evaluation of the antitumor potential and chemo-sensitizing activity of fluorinated ecdysteroid derivatives†‡
J.
Csábi
a,
A.
Martins§
b,
I.
Sinka
c,
A.
Csorba
 a,
J.
Molnár
b,
I.
Zupkó
cd,
G.
Tóth
ae,
L. M. V.
Tillekeratne
f and
A.
Hunyadi
*ad
a,
J.
Molnár
b,
I.
Zupkó
cd,
G.
Tóth
ae,
L. M. V.
Tillekeratne
f and
A.
Hunyadi
*ad
aInstitute of Pharmacognosy, University of Szeged, Eötvös str. 6, 6720 Szeged, Hungary. E-mail: hunyadi.a@pharm.u-szeged.hu; Fax: +3662545704; Tel: +3662546456
bDepartment of Medical Microbiology and Immunobiology, University of Szeged, Dóm sq. 9, 6720 Szeged, Hungary
cDepartment of Pharmacodynamics and Biopharmacy, University of Szeged, Eötvös str. 6, 6720 Szeged, Hungary
dInterdisciplinary Centre for Natural Products, University of Szeged, Eötvös str. 6, 6720 Szeged, Hungary
eNMR group, Department of Inorganic and Analytical Chemistry, Budapest University of Technology and Economics, Szt. Gellért Sq. 4, H-1111, Budapest, Hungary
fDepartment of Medicinal and Biological Chemistry, College of Pharmacy and Pharmaceutical Sciences, MS 606, University of Toledo, Toledo, OH 43606, USA
First published on 26th September 2016
Abstract
Efflux pumps, like the ABCB1 transporter, play an important role in the chemo-resistance of various tumors and particularly of cancer stem cells. We have previously reported the chemo-sensitizing activity of apolar ecdysteroid derivatives on cancer cell lines of various origin and sensitivity to chemotherapeutics. Herein we report the preparation of three fluorinated derivatives and a dehydrated byproduct from 20-hydroxyecdysone 2,3;20,22-diacetonide (1) through diethylaminosulfur trifluoride (DAST)-mediated fluorination. Complete NMR assignment of the products is provided. In vitro bioactivity testing was performed on four human breast cancer cell lines, a neuroblastoma, and a mouse lymphoma cell line and its counterpart expressing the human ABCB1 efflux transporter. Fluorination increased the ABCB1 inhibitory effect of the compounds but had little effect on their limited antiproliferative action, which was, however, markedly increased by a Δ14,15 double bond. Compound 5, a new 14,25-difluoro analog of 1, exerted higher chemo-sensitizing activity to doxorubicin as compared to its parental compound.
Introduction
Ecdysteroids represent one of the most systematically and extensively documented family of natural steroids.1 They play an important hormonal role in regulating development and reproduction of arthropods. Many plant species can also biosynthesize these compounds as a possible defense mechanism against non-adapted phytophagous invertebrates.1,2 The most abundant ecdysteroid, 20-hydroxyecdysone (20E), represents the most typical structural features of these compounds: a cholest-7-en-6-one carbon skeleton (C27) with a cis junction of the A/B-, and trans junction of the C/D-rings and with several hydroxyl groups. Ecdysteroids are known to exert a wide range of non-hormonal bioactivities in mammals that have extensively been reviewed.3–5 Our research group has recently discovered that particularly the less polar ecdysteroids can exert potent chemo-sensitizing activity on various cancer cell lines. The studied cell lines included both drug susceptible (breast: MCF-7, prostate: PC3 and LNCaP, epidermal: KB-3-1 and murine lymphoma: L5178) and multi-drug resistant (MDR) ones obtained either by transfection with the human ABCB1 transporter (L5178MDR) or by stepwise adaptation to a chemotherapeutic agent (MCF-7Dox to doxorubicin, KB-C-1 to colchicine).6–9 The compounds were identified as weak to mild inhibitors of ABCB1 function, and this activity was only marginally correlating with their very strong chemo-sensitizing activity observed on ABCB1 over-expressing cancer cells such as L5178MDR.6,8 Moreover, a less pronounced, but still significant chemo-sensitization was also observed on cancer cell lines with minimal or no detectable ABCB1 expression, suggesting the involvement of mechanisms other than the inhibition of efflux pump function.9 Nevertheless, ABCB1 is a potential target particularly in cancer stem cells (CSCs): over-expression of this transporter is not only an important mechanism for the chemo-resistance of CSCs,10 but it has also been found to be closely associated to stem-likeness in non-small cell lung cancer whose stem-like properties could be overcome by ABCB1 inhibition.11An increasing number of fluorinated antitumor agents are becoming available for cancer treatment.12 Special properties of the fluorine atom, such as strong electronegativity, small size and the low polarizability of the C–F bond, can have great impact on the biological activity and frequently also on the metabolic stability of a molecule, and fluorination of natural products appears to be an increasingly attractive strategy to obtain new leads for drug discovery.13 Accordingly, our aim was to prepare fluorinated derivatives of 20E 2,3;20,22-diacetonide (1), an ecdysteroid with particularly strong chemo-sensitizing properties, and to investigate related changes in the antitumor potential as compared to that of compound 1. Fluorination of this compound was previously performed in an earlier study by Pascual et al. aiming to prepare 25-fluoroponasterone A, where the acetonide moieties served as protecting groups and were eventually removed by acidic hydrolysis. The 25-fluorinated target compound was tested in an insect bioassay, and it showed an activity on the oocyte development of the German cockroach (Blattella germanica) similar to that of the parental 20E.14 To the best of our knowledge, however, fluorinated ecdysteroids have never been reported for their activity on any bioassays related to mammals.
Results and discussion
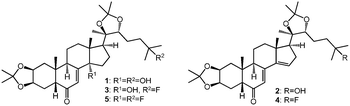
Following a two-step purification by column chromatography and preparative HPLC, four compounds, 2–5 were obtained from the diethylaminosulfur trifluoride (DAST)-mediated fluorination reaction of 1. Compounds 2, 3, 4 and 5 gave parent ion [M + H]+ peaks in their ESI-MS spectra at m/z 543, 563, 545, 565, respectively. Appearance of an [M + H–H2O]+ peak in the MS spectra of 1, 2 and 3 strongly suggested the presence of at least one remaining hydroxyl group in these compounds, while such peaks were not visible in case of compounds 4 and 5, suggesting that these latter two compounds possess no free OH-groups. The 1H and 13C NMR chemical shifts of the starting compound 1 were in perfect agreement with literature data.15 The structures of compounds 2–5 were assigned by comprehensive one- and two-dimensional NMR methods, and by also utilizing the spectra of compound 1 as reference in addition to those reported from our recent NMR study on a series of 20E dioxolanes.7The mass spectrum of compound 2 suggested that the elimination of a water molecule from 1 took place. This was confirmed by the NMR spectra indicating the presence of a Δ14,15 double bond, represented by the appearance of the chemical shifts of HC-15 (6.08/130.1 ppm) and the quaternary C-14 (150.4 ppm) observed in the 1H and/or the 13C NMR spectra. Accordingly, the HMQC spectrum revealed seven methylene groups, one less than in compound 1, while the disappearance of a hydrogen atom from position 15 was also detected in the 1H,1H COSY spectrum leaving only four members in this structural fragment of correlated protons: H-15 (6.08 ppm), H2-16 (2.38; 2.63 ppm) and H-17 (2.08 ppm). These assignments were supported by the HMBC spectrum. Moreover, C-28 and C-29 showed HMBC cross peaks with two methyl groups each analogously to the parent compound 1, providing further evidence that the acetonide groups at position 2,3 and 20,22 remained intact after the reaction. Hence, compound 2 was identified as Stachysterone B 2,3;20,22-diacetonide.15
In case of compounds 3–5, evidence for fluorine substitution was found. It is well-known that, as a result of altered substituent increments, the exchange of an sp3C connected –OH group to a –F manifests in characteristic changes in the NMR spectrum. In the α position, namely directly on the substituted carbon, a ca. 20–25 ppm paramagnetic shift and in the β position a ca. 2–3 ppm diamagnetic shift can be observed, which effect decreases below 1 ppm in the γ position. In addition to these, both the 13C and the 1H NMR spectra show signal splitting caused by the characteristic direct (∼165 Hz), geminal (22–26 Hz) and vicinal (∼5 Hz) nJ(F,C) and 3J(F,H) (4.5 Hz) couplings. Based on these, it could be evidenced that compounds 3–5 contain a fluorine atom connected to the C-25, and that compound 5 contains another fluorine substituent also at position C-14. Fluorination of alcohols catalyzed by DAST can take place through either SN1 or SN2 reaction mechanism, which also determines the stereo-specificity of the reaction:16a priori, the 14-F substituent in compound 5 can be present in either α or β position. The latter case would also involve a change of the initially trans C/D ring junction to cis. The effect of such a configurational change on the 13C chemical shifts can well be estimated by comparing the corresponding chemical shifts of 20-hydroxyecdysone and its diastereomer 14-epi-20-hydroxyecdysone, where significant, i.e. more than 2 ppm differences could be detected on the δC-9 (+2.4), δC-12 (+9.2), δC-13 (+4.0), δC-15 (+9.0), δC-16 (+3.2) and δC-17 (+6.2) (our own unpublished NMR data for 14-epi-20-hydroxyecdysone isolated from the plant Serratula wolffii17). Considering that no such changes were detected in case of compound 5, a retained C-14 configuration can be concluded. Moreover, a detailed analysis of the NOESY spectrum revealed the steric proximity of the H3C-18 and the Hβ-15 hydrogen atoms, which is only possible in case of a trans C/D ring junction, providing further evidence for a 14α-F group in compound 5. Accordingly, the SN1 reaction mechanism is suggested for this substitution, which mechanism is also more likely whenever steric effects (e.g. due to a rigid steroid skeleton, as in our case) prevent an SN2 attack.16 Altogether, compound 5 was identified as 14-deoxy-14,25-difluoroponasterone A 2,3;20,22-diacetonide, a new ecdysteroid.
Thanks to the comprehensive one- and two-dimensional NMR techniques utilized in the structure elucidation process, a complete signal assignment could be achieved for all compounds including compounds 3 and 4, whose previously published NMR data lacked the assignment of several hydrogens, and certain carbon signals were assigned as interchangeable.14 The 1H and 13C NMR data of parent compound 1 and its derivatives 2–5 are compiled in Table 1, structures of the compounds are shown in Fig. 1.
| Atom no. | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| H | C | H | C | H | C | H | C | H | C | |
| a In case of compounds 3–5 the symbol “d” after the 13C chemical shifts indicates doublet splitting (Hz) resulted from 1J(F,C), 2J(F,C) or 3J(F,C) coupling. On signal C-13 in compound 5, due to overlapping with the strong solvent signal, the determination of 3J(F,C) failed. | ||||||||||
| 1α | 1.99 | 39.0 | 1.95 | 38.8 | 1.97 | 39.0 | 1.96 | 38.8 | 1.99 | 38.5 |
| β | 1.22 | 1.24 | 1.21 | 1.23 | 1.27 | |||||
| 2 | 4.27 | 73.7 | 4.24 | 73.5 | 4.24 | 73.7 | 4.23 | 73.5 | 4.28 | 73.6 |
| 3 | 4.30 | 73.3 | 4.30 | 73.4 | 4.29 | 73.3 | 4.28 | 73.4 | 4.30 | 73.1 |
| 4α | 1.98 | 27.9 | 1.83 | 28.1 | 1.96 | 27.8 | 1.83 | 28.0 | 1.93 | 27.7 |
| β | 1.96 | 1.98 | 1.96 | 1.98 | 1.93 | |||||
| 5 | 2.24 | 52.7 | 2.22 | 52.5 | 2.22 | 52.6 | 2.22 | 52.5 | 2.31 | 52.3 |
| 6 | — | 205.8 | — | 205.3 | — | 205.8 | — | 205.3 | — | 204.7 |
| 7 | 5.80 | 122.0 | 6.06 | 121.0 | 5.78 | 122.0 | 6.06 | 121.0 | 5.92 | 124.4d 6.5 |
| 8 | — | 167.1 | — | 158.0 | — | 167.0 | — | 158.0 | — | 159.4d 20.5 |
| 9 | 2.93 | 35.9 | 2.54 | 40.0 | 2.91 | 35.9 | 2.54 | 40.0 | 2.76 | 37.3 |
| 10 | — | 38.9 | — | 39.7 | — | 38.9 | — | 39.7 | — | 38.8 |
| 11α | 1.76 | 21.8 | 1.81 | 21.7 | 1.76 | 21.7 | 1.81 | 21.7 | 1.84 | 21.8 |
| β | 1.66 | 1.73 | 1.64 | 1.75 | 1.69 | |||||
| 12α | 2.10 | 32.5 | 1.59 | 40.7 | 2.08 | 32.4 | 1.58 | 40.7 | 1.98 | 33.0d 4.3 |
| β | 1.87 | 2.25 | 1.82 | 2.25 | 1.98 | |||||
| 13 | — | 48.7 | — | 48.7 | — | 48.7 | — | 48.9 | — | 49.7da |
| 14 | — | 85.4 | — | 150.4 | — | 85.4 | — | 150.4 | — | 108.9d |
| 165.6 | ||||||||||
| 15α | 1.61 | 31.8 | 6.08 | 130.1 | 1.59 | 31.7 | 6.08 | 130.1 | 1.88 | 29.1d 24.0 |
| β | 1.95 | 1.94 | 2.13 | |||||||
| 16α | 1.84 | 22.6 | 2.38 | 32.8 | 1.84 | 22.6 | 2.38 | 32.8 | 1.86 | 22.2 |
| β | 2.04 | 2.63 | 2.02 | 2.63 | 2.12 | |||||
| 17 | 2.31 | 50.6 | 2.08 | 59.0 | 2.27 | 50.6 | 2.07 | 58.9 | 2.17 | 51.3 |
| 18 | 0.82 | 17.8 | 1.07 | 20.0 | 0.80 | 17.8 | 1.07 | 20.0 | 0.86 | 17.1d 3.5 |
| 19 | 0.96 | 24.2 | 0.94 | 23.8 | 0.95 | 24.2 | 0.94 | 23.8 | 1.00 | 22.6 |
| 20 | — | 86.0 | — | 84.8 | — | 85.9 | — | 84.8 | — | 85.3 |
| 21 | 1.18 | 22.8 | 1.21 | 22.0 | 1.15 | 22.8 | 1.21 | 22.0 | 1.19 | 24.2 |
| 22 | 3.69 | 83.5 | 3.74 | 83.2 | 3.68 | 83.0 | 3.76 | 82.7 | 3.71 | 82.8 |
| 23 | 1.52 | 24.9 | 1.56 | 24.9 | 1.52 | 24.5d | 1.491.56 | 24.6d 4.5 | 1.53 | 24.5d 4.5 |
| 1.51 | 1.49 | 1.51 | 5.1 | 1.53 | ||||||
| 24 | 1.49 | 42.4 | 1.46 | 42.1 | 1.61 | 40.2d | 1.63 | 39.9d | 1.63 | 40.0d 23.1 |
| 1.73 | 1.70 | 1.86 | 22.9 | 1.85 | 22.5 | 1.87 | ||||
| 25 | — | 71.3 | — | 71.2 | — | 96.5d | — | 96.6d | — | 96.0d 166.0 |
| 164.9 | 164.9 | |||||||||
| 26 | 1.20 | 29.1 | 1.17 | 29.0 | 1.34 | 26.8d | 1.35 | 26.7d | 1.33 | 26.8d 24.8 |
| 24.5 | 24.9 | |||||||||
| 27 | 1.21 | 29.0 | 1.19 | 29.7 | 1.31 | 27.4d | 1.31 | 27.5d | 1.36 | 27.3d 24.7 |
| 24.4 | 24.4 | |||||||||
| 28 | — | 109.6 | — | 109.6 | — | 109.6 | — | 109.6 | — | 109.6 |
| 29 | — | 108.2 | — | 108.2 | — | 108.3 | — | 108.3 | — | 108.4 |
| iPr-(2,3) β | 1.47 | 29.0 | 1.45 | 28.9 | 1.45 | 29.0 | 1.45 | 28.9 | 1.47 | 29.0 |
| iPr-(2,3) α | 1.32 | 26.8 | 1.30 | 26.7 | 1.30 | 26.8 | 1.30 | 26.8 | 1.31 | 26.7 |
| iPr-(20,22) β | 1.32 | 27.3 | 1.28 | 27.3 | 1.30 | 27.3 | 1.28 | 27.3 | 1.32 | 27.2 |
| iPr-(20,22) α | 1.39 | 29.5 | 1.38 | 29.4 | 1.37 | 29.5 | 1.39 | 29.4 | 1.40 | 29.4 |
Based on previous reports on fluorination reactions using DAST, it is not surprising, that two kinds of structural modifications took place in this setting: the OH group in position 14 was either eliminated as in compounds 2 and 4, or substituted with fluorine as in compound 5. Furthermore, fluorine substitution of the 25-OH group led to the production of compounds 3–5.
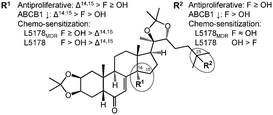
Compounds 1–5 were tested for their antiproliferative activities on a diverse set of tumor cell lines including a panel of human breast cancer cell lines (MCF-7, T47D, MDA-MB-231 and MDA-MB-361), a human neuroblastoma cell line (SH-SY5Y), and a murine lymphoma cell line (L5178) and its transfected multi-drug resistant (MDR) counterpart expressing the human ABCB1 efflux transporter (L5178MDR). The compounds were also tested for their ability to interfere with the efflux function of ABCB1 on the L5178MDR cell line, as determined by means of the intracellular accumulation of rhodamine 123, a well-known ABCB1 substrate fluorescent dye; results of these studies are compiled in Table 2. In general, fluorine substitution at C-25 or C-14 and C-25 appears to have little effect on the very mild antiproliferative activity of compound 1, as seen from a comparison of the results obtained for compounds 1, 3 and 5. Moreover, a comparison of the activities of compounds 2 and 4 leads to the same conclusion. On the other hand, a Δ14,15 double bond, formed by the elimination of the 14-OH group, markedly increased the antiproliferative activity of compounds 2 and 4, as compared to compounds 1 and 3, respectively. It is worth noting that, even though all of the above structural changes led to more lipophilic compounds, their activity did not show any correlation to the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p values, which were calculated as 4.01, 4.61, 4.91, 5.50 and 5.80 for compounds 1–5 by using ChemAxon's web based resource available at http://chemicalize.org.18 The most relevant cell line specific differences in the antiproliferative potential of the compounds were observed between the MCF-7 and T47D cells. Although receptorial status (i.e. expression of estrogenic and progestin receptors) of these two cell lines is similar, the expression levels of steroid metabolizing enzymes are substantially different.19 Interestingly, an approximately 3-times selectivity towards T47D over MCF-7 cells was observed in case of compounds with a Δ14,15 double bond (compounds 2 and 4) or a 14-F moiety (compound 5), which selectivity was not present for neither of the two 14-hydroxyecdysteroids (compounds 1 and 3). Moreover, both the fluorination (at either position) and the 14-OH elimination manifested in a significant increase in the ABCB1 inhibitory activity, as compared to the parental compound 1, particularly when both a Δ14,15 double bond and a 25-fluoride group was present as in compound 4. Concerning the chemical changes at C-14, it might be worth noting that dehydroxylation at this position is among the known primary metabolic conversions of ecdysteroids in mammals.3,20 Accordingly, one could also expect a higher metabolic stability for 14-fluorinated (5) or dehydrated compounds (2 and 4) as compared to that of the 14-hydroxyecdysteroids 1 and 3; this should certainly be clarified by future studies.
p values, which were calculated as 4.01, 4.61, 4.91, 5.50 and 5.80 for compounds 1–5 by using ChemAxon's web based resource available at http://chemicalize.org.18 The most relevant cell line specific differences in the antiproliferative potential of the compounds were observed between the MCF-7 and T47D cells. Although receptorial status (i.e. expression of estrogenic and progestin receptors) of these two cell lines is similar, the expression levels of steroid metabolizing enzymes are substantially different.19 Interestingly, an approximately 3-times selectivity towards T47D over MCF-7 cells was observed in case of compounds with a Δ14,15 double bond (compounds 2 and 4) or a 14-F moiety (compound 5), which selectivity was not present for neither of the two 14-hydroxyecdysteroids (compounds 1 and 3). Moreover, both the fluorination (at either position) and the 14-OH elimination manifested in a significant increase in the ABCB1 inhibitory activity, as compared to the parental compound 1, particularly when both a Δ14,15 double bond and a 25-fluoride group was present as in compound 4. Concerning the chemical changes at C-14, it might be worth noting that dehydroxylation at this position is among the known primary metabolic conversions of ecdysteroids in mammals.3,20 Accordingly, one could also expect a higher metabolic stability for 14-fluorinated (5) or dehydrated compounds (2 and 4) as compared to that of the 14-hydroxyecdysteroids 1 and 3; this should certainly be clarified by future studies.
| IC50 (μM) | FAR | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MCF-7 (n) | T47D (n) | MDA-MB-231 (n) | MDA-MB-361 (n) | SH-SY5Y (n) | L5178 (n) | L5178MDR (n) | 2 μM | 20 μM | |
| 1 | 75.1 ± 3.4 (5) | 84.7 ± 3.9 (5) | 106.1 ± 7.2 (4) | 69.2 ± 6.0 (4) | 126.8 ± 9.8 (3) | 82.9 ± 1.0 (2) | 106.1 ± 3.3 (2) | 3.33 | 11.28 |
| 2 | 30.1 ± 0.8 (5) | 10.9 ± 0.3 (5) | 38.53 ± 3.8 (5) | 13.8 ± 0.4 (5) | 20.8 ± 0.3 (3) | 14.6 ± 0.3 (3) | 19.3 ± 0.5 (3) | 1.28 | 57.36 |
| 3 | 63.1 ± 2.3 (5) | 70.3 ± 1.3 (5) | 48.85 ± 2.0 (5) | 30.9 ± 2.6 (4) | 70.7 ± 4.1 (3) | 48.3 ± 0.2 (3) | 50.1 ± 2.1 (3) | 1.59 | 38.62 |
| 4 | 43.8 ± 1.7 (4) | 17.4 ± 1.0 (5) | 50.18 ± 2.6 (5) | 11.8 ± 1.2 (5) | 18.8 ± 1.2 (3) | 14.1 ± 0.8 (3) | 17.4 ± 1.2 (3) | 31.15 | 109.37 |
| 5 | 127.5 ± 4.3 (5) | 49.2 ± 5.0 (5) | 98.43 ± 17.2 (4) | 53.8 ± 5.7 (5) | 125.6 ± 13.2 (2) | 88.6 ± 1.1 (2) | 82.3 ± 1.4 (3) | 11.02 | 89.78 |
| C | 5.8 ± 0.3 (5) | 9.8 ± 1.0 (5) | 19.1 ± 1.8 (5) | 3.7 ± 0.5 (5) | — | — | — | — | — |
| D | — | — | — | — | 0.14 ± 0.02 (3) | 0.23 ± 0.01 (3) | 3.54 ± 0.44 (3) | — | — |
Following the above observations, our next aim was to test the effect of these structural and bioactivity changes on the chemo-sensitizing properties of compound 1. Accordingly, the antiproliferative activity of all compounds was tested in combination with doxorubicin on the mouse lymphoma cell line pair, by using the checkerboard microplate method and calculating combination index (CI) values to characterize and quantify the interaction between the two drugs.21 Results of this study are compiled in Table 3.
| Cell line | Drug ratioa | CI values at | D m | m | r | CIavg | |||
|---|---|---|---|---|---|---|---|---|---|
| ED50 | ED75 | ED90 | |||||||
| a Molar drug ratios are given; serial dilutions of doxorubicin were initiated from a commercially available injection of 2 mg mL−1 (doxorubicin hydrochloride, Teva). | |||||||||
| 1 | L5178MDR | 10.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.31 | 0.18 | 0.11 | 8.926 | 1.902 | 0.937 | 0.17 (ref. 6) |
20.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.27 | 0.14 | 0.07 | 11.678 | 3.246 | 0.964 | 0.13 (ref. 6) | ||
81.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.39 | 0.22 | 0.12 | 26.598 | 3.859 | 0.970 | 0.20 (ref. 6) | ||
| L5178 | 81.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.74 | 0.71 | 0.68 | 6.742 | 1.614 | 0.933 | 0.70 | |
163![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.67 | 0.55 | 0.46 | 11.236 | 2.103 | 0.942 | 0.53 | ||
326![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.61 | 0.55 | 0.51 | 17.795 | 1.872 | 0.945 | 0.54 | ||
| 2 | L5178MDR | 10.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.55 | 0.45 | 0.39 | 7.300 | 2.460 | 0.992 | 0.44 |
20.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.54 | 0.44 | 0.37 | 8.338 | 2.905 | 0.989 | 0.42 | ||
81.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.09 | 0.87 | 0.69 | 19.144 | 3.664 | 0.985 | 0.82 | ||
| L5178 | 81.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.99 | 0.89 | 0.82 | 9.506 | 2.300 | 0.982 | 0.87 | |
163![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.93 | 0.89 | 0.86 | 11.103 | 2.244 | 0.970 | 0.88 | ||
326![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.90 | 0.81 | 0.73 | 12.112 | 2.720 | 0.991 | 0.78 | ||
| 3 | L5178MDR | 10.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.19 | 0.17 | 0.16 | 4.617 | 2.646 | 0.999 | 0.17 |
20.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.19 | 0.19 | 0.19 | 5.760 | 2.468 | 0.908 | 0.19 | ||
81.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.35 | 0.33 | 0.33 | 13.551 | 3.436 | 0.938 | 0.33 | ||
| L5178 | 81.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.80 | 0.66 | 0.56 | 9.945 | 2.675 | 0.982 | 0.63 | |
163![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.85 | 0.76 | 0.71 | 15.042 | 2.488 | 0.978 | 0.75 | ||
326![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.04 | 0.96 | 0.91 | 23.554 | 2.696 | 0.981 | 0.95 | ||
| 4 | L5178MDR | 10.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.56 | 0.34 | 0.22 | 9.059 | 5.479 | 1.000 | 0.32 |
20.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.48 | 0.36 | 0.28 | 9.620 | 3.338 | 0.983 | 0.34 | ||
81.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.78 | 0.73 | 0.69 | 19.112 | 2.281 | 0.935 | 0.72 | ||
| L5178 | 81.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.73 | 0.80 | 0.89 | 6.893 | 2.560 | 0.965 | 0.83 | |
163![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.80 | 0.87 | 0.95 | 8.861 | 2.850 | 0.987 | 0.90 | ||
326![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.79 | 0.84 | 0.90 | 9.569 | 3.217 | 0.993 | 0.86 | ||
| 5 | L5178MDR | 10.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.23 | 0.11 | 0.06 | 5.904 | 4.546 | 0.948 | 0.10 |
20.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.21 | 0.12 | 0.07 | 7.733 | 3.375 | 0.957 | 0.11 | ||
81.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.24 | 0.16 | 0.11 | 13.888 | 3.040 | 0.966 | 0.15 | ||
| L5178 | 81.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.70 | 0.42 | 0.27 | 6.822 | 2.765 | 0.990 | 0.39 | |
163![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.60 | 0.44 | 0.34 | 9.757 | 2.248 | 0.944 | 0.42 | ||
326![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.72 | 0.59 | 0.52 | 17.179 | 2.134 | 0.990 | 0.58 | ||
Considering that in case of chemotherapy the desirable outcome is a complete eradication of the tumor, a weighted average CI value (where CIs at higher inhibition rates count more) has been suggested as an important measure for the relevance of synergism or antagonism on cancer cell lines.21 As compared to compound 1 that acted in strong synergism with doxorubicin, the more cytotoxic dienone compounds 2 and 4 exerted a much less profound chemo-sensitizing activity on both cell lines. This is particularly interesting in view of their high ABCB1 inhibitory activity, providing further evidence to our previous assumption that a functional efflux pump inhibition is unlikely to be the key mechanism for chemo-sensitization by ecdysteroids.6,9 A 25-fluoro substitution had a small, but rather weakening effect on the chemo-sensitizing activity as compared to the corresponding 25-hydroxyecdysteroids. On the other hand, the 14,25-difluorinated compound 5 showed a stronger synergism with doxorubicin as compared to the parental compound 1 in particularly on the non-MDR cell line L5178. Structure–activity relationships observed in our studies are summarized in Fig. 2.
Experimental
General information
20-Hydroxyecdysone 2,3;20,22-diacetonide (1) was s0before;6 briefly, 20E was reacted with acetone in the presence of phosphomolybdic acid as catalyst. Reverse phase HPLC was performed on a system of two Jasco PU-2080 pumps connected to a Jasco MD-2010 Plus photodiode-array detector (Jasco Co., Tokyo, Japan), by utilizing Kinetex XB-C18 analytical (250 × 4.6 mm, 5 μm; 80% aqueous acetonitrile, 0.8 mL min−1) or preparative (250 × 21.2 mm, 5 μm; 90% aqueous acetonitrile, 10.0 mL min−1) columns. Retention times for the analytical system are given below (see compound characterization data). Mass spectra were recorded on an API 2000 triple quadrupole tandem mass spectrometer (AB SCIEX, Foster City, CA, USA) in positive mode with an electrospray ion source (ESI).Synthetic procedure
120 mg (0.214 mmol) of compound 1 was dissolved in 3 mL of anhydrous methylene chloride (Acros Organics, New Jersey, USA) at −78 °C under nitrogen atmosphere. 34 μL (1.2 equiv.) of (diethylamino)sulphur-triflouride (DAST, Acros Organics, New Jersey, USA) was added, and the reaction mixture was stirred at room temperature for 75 minutes. On completion, the mixture was quenched with 5% aqueous solution of NaHCO3 (Fisher Scientific, Pittsburgh, PA, USA), then the mixture was extracted with methylene chloride (Pharmaco-Aaper, USA). The combined organic layers were dried with anhydrous Na2SO4 (Fisher Scientific, Pittsburgh, PA, USA), filtered and evaporated in vacuo. Column chromatography on silica was applied using a gradient of n-hexane (Sigma-Aldrich, USA) and ethyl acetate (Pharmaco-Aaper, USA) with the following steps: 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7.5
2, 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 7
2.5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 6.5
3, 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5, 6
3.5, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 4
4 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v each. Ten fractions of 10 mL were collected with each composition, and the combined fractions 8–9, 10–13, 16–22 and 41–55 contained compounds 5, 4, 3 and 2, respectively. Final purification was performed by preparative HPLC as described above, and the isolated yields were as follows: 2 (41 mg, 35.3%), 3 (20 mg, 16.6%), 4 (20 mg, 17.2%), 5 (4 mg, 3.3%).
6, v/v each. Ten fractions of 10 mL were collected with each composition, and the combined fractions 8–9, 10–13, 16–22 and 41–55 contained compounds 5, 4, 3 and 2, respectively. Final purification was performed by preparative HPLC as described above, and the isolated yields were as follows: 2 (41 mg, 35.3%), 3 (20 mg, 16.6%), 4 (20 mg, 17.2%), 5 (4 mg, 3.3%).
NMR spectroscopy
1H (600 MHz) and 13C (150 MHz) NMR spectra were recorded at room temperature on a Bruker Avance III 600 MHz spectrometer equipped with a cryo probehead. Amounts of approximately 1–10 mg of compounds 1–5 were dissolved in 0.6 mL of methanol-d4 and transferred to 5.0 mm Norell XR-55-7 NMR sample tubes. Chemical shifts are given on the δ-scale and are referenced to the solvent (methanol-d4: δC = 49.1 and δH = 3.31 ppm). Pulse programs of all experiments (1H, 13C, HMQC, COSY, HMBC and NOESY) were taken from the Bruker TopSpin 3.1pl5 software library.Compound characterization data
Cancer cell lines
Breast cancer (MCF-7, MDA-MB-231, MDA-MB-361 and T47D), neuroblastoma (SH-SY5Y) and mouse T-cell lymphoma (L5178) cell lines were purchased from the European Collection of Cell Cultures (ECCAC, Salisbury, UK). L5178MDR cell line was obtained by transfecting L5178 cells with pHa MDR1/A retrovirus,23 and it was selected by culturing the infected cells with 60 μg L−1 colchicine. Breast cancer cells were cultured in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acids and antibiotic–antimycotic mixture. SH-SY5Y neuroblastoma cells were cultured in MEM supplemented with non-essential amino acids, 1 mM Na-pyruvate, 10% FBS, nystatin, 2 mM L-glutamine, 100U penicillin, and 0.1 mg streptomycin. L5178 and L5178MDR cells were cultured in McCoy's 5A medium supplemented with 10% heat inactivated horse serum, L-glutamine, and antibiotics (penicillin and streptomycin). All cell lines were cultured at 37 °C in humidified air containing 5% CO2. Media and supplements were purchased from Lonza Group Ltd., (Basel, Switzerland), Difco (Detroit, MI, USA) or Sigma.Cytotoxicity assay
The antiproliferative actions of the prepared compounds were determined by means of MTT assay.24 Briefly, near-confluent cancer cells were seeded onto a 96-well microplate at the density of 5000 cells per well except for MDA-MB-361 and SH-SY5Y, which were seeded at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per well. After overnight preincubation, 200 μL new medium, containing the tested compounds in the concentration range of 1–150 μM, was added. With respect to the non-adherent mouse lymphoma cell lines, 6 × 103 cells were added to each well containing McCoy's 5A medium and compounds at the same concentration range as mentioned above. After incubation for 72 h (48 h in case of SH-SY5Y cells), the viability of the cells was assayed by the addition of 20 μL of 5 mg ml−1 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution. MTT was metabolized by intact mitochondrial reductase and precipitated as purple crystals during a 4 h contact period. The medium was next removed and the precipitated formazan crystals were dissolved in 100 μL of DMSO during a 60 min period of shaking at 37 °C. Finally, the reduced MTT was assayed at 545 nm, using a microplate reader; wells with untreated cells served as control. IC50 values, referring to the concentration of compound exerting 50% growth inhibition, were calculated by nonlinear regression utilizing the log(inhibitor) vs. normalized response (variable slope) curve fitting model of GraphPad Prism 5.0 with an ordinary fit. Two experiments were performed with 5 parallel wells.
000 per well. After overnight preincubation, 200 μL new medium, containing the tested compounds in the concentration range of 1–150 μM, was added. With respect to the non-adherent mouse lymphoma cell lines, 6 × 103 cells were added to each well containing McCoy's 5A medium and compounds at the same concentration range as mentioned above. After incubation for 72 h (48 h in case of SH-SY5Y cells), the viability of the cells was assayed by the addition of 20 μL of 5 mg ml−1 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution. MTT was metabolized by intact mitochondrial reductase and precipitated as purple crystals during a 4 h contact period. The medium was next removed and the precipitated formazan crystals were dissolved in 100 μL of DMSO during a 60 min period of shaking at 37 °C. Finally, the reduced MTT was assayed at 545 nm, using a microplate reader; wells with untreated cells served as control. IC50 values, referring to the concentration of compound exerting 50% growth inhibition, were calculated by nonlinear regression utilizing the log(inhibitor) vs. normalized response (variable slope) curve fitting model of GraphPad Prism 5.0 with an ordinary fit. Two experiments were performed with 5 parallel wells.
ABCB1 inhibition assay
Inhibition of ABCB1 function was investigated through the intracellular retention of rhodamine 123, a fluorescent dye, evaluated by flow cytometry.6 Briefly, 2 × 106 cells per mL were treated with 2 or 20 μM of each compound. After 10 min incubation, rhodamine 123 (Sigma) was added to a final concentration of 5.2 μM and the samples were incubated at 37 °C in water bath for 20 min. Samples were centrifuged (2000 rpm, 2 min) and washed twice with phosphate buffer saline (PBS, Sigma). The samples were re-suspended in 0.5 mL of PBS and their fluorescence was measured with a Partec CyFlow flow cytometer (Partec, Münster, Germany). 20 μM of verapamil (Sanofi-Synthelabo, Budapest, Hungary) was used as positive control.Combination assay
The combined activity of doxorubicin (Teva, Hungary) and ecdysteroids was determined using the checkerboard microplate method as published before.6 Briefly, cell suspension (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) was incubated with doxorubicin and the compound to be tested for 48 h at 37 °C under 5% CO2. Cell growth rate was determined through MTT staining, as described above. The interaction was evaluated by using the CompuSyn software (CompuSyn, Inc, USA) for the constant ratios and combination index (CI) values are presented for 50, 75 and 90% of growth inhibition.
000 cells per well) was incubated with doxorubicin and the compound to be tested for 48 h at 37 °C under 5% CO2. Cell growth rate was determined through MTT staining, as described above. The interaction was evaluated by using the CompuSyn software (CompuSyn, Inc, USA) for the constant ratios and combination index (CI) values are presented for 50, 75 and 90% of growth inhibition.
Conclusions
In the present study, four compounds have been prepared from a DAST-mediated fluorination of 20-hydroxyecdysone 2,3;20,22-diacetonide (1), a previously identified chemo-sensitizer agent. Water elimination at C-14, as well as fluoride substitution at C-25 and/or C-14 took place during the reaction, leading to one new (5) and three known (2–4) ecdysteroids. NMR assignments of the known compounds have been completed herein. None of the obtained compounds have previously been studied for their antitumor properties, and valuable new structure–activity relationships were revealed concerning the ABCB1 inhibition, antiproliferative and chemo-sensitizing activity of ecdysteroid derivatives.Considering the major importance of the ABCB1 efflux pump in the chemo-resistance and stem-likeness of cancer stem cells,10,11 compounds that can sensitize ABCB1 over-expressing cancer cells to chemotherapeutics are of potential interest to the CSC paradigm. Based on its highly potent chemo-sensitizing activity on the mouse lymphoma cell line pair, compound 5, a new 14,25-difluorinated derivative of 1, is particularly promising in this regard; further studies are planned to investigate its potential use in the eradication of cancer stem cells.
Acknowledgements
This work was performed within the framework of COST Actions CM1106, “Chemical Approaches to Targeting Drug Resistance in Cancer Stem Cells”, and CM1407, “Challenging Organic Syntheses Inspired by Nature – from Natural Products Chemistry to Drug Discovery”. A. H. acknowledges support from the National Research, Development and Innovation Office, Hungary (NKFIH; K119770) and from the János Bolyai fellowship of the Hungarian Academy of Sciences. J. C. was supported by a mobility grant within the Campus Hungary Program.References
- L. Dinan, Phytoecdysteroids: biological aspects, Phytochemistry, 2001, 57, 325–339 CrossRef CAS PubMed.
- L. Dinan, The Karlson Lecture. Phytoecdysteroids: What use are they?, Arch. Insect Biochem. Physiol., 2009, 72, 126–141 CrossRef CAS PubMed.
- R. Lafont and L. Dinan, Practical uses for ecdysteroids in mammals including humans: an update, J. Insect Sci., 2003, 3, 7 CrossRef CAS PubMed.
- M. Báthori, N. Tóth, A. Hunyadi, Á. Márki and E. Zádor, Phytoecdysteroids and Anabolic-Androgenic Steroids – Structure and Effects on Humans, Curr. Med. Chem., 2008, 15, 75–91 CrossRef.
- N. Tóth, A. Hunyadi, M. Báthori and E. Zádor, Phytoecdysteroids and Vitamin D Analogues – Similarities in Structure and Mode of Action, Curr. Med. Chem., 2010, 17, 1974–1994 CrossRef.
- A. Martins, N. Tóth, A. Ványolós, Z. Béni, I. Zupkó, J. Molnár, M. Báthori and A. Hunyadi, Significant activity of ecdysteroids on the resistance to doxorubicin in mammalian cancer cells expressing the human ABCB1 transporter, J. Med. Chem., 2012, 55, 5034–5043 CrossRef CAS PubMed.
- A. Balázs, A. Hunyadi, J. Csábi, N. Jedlinszki, A. Martins, A. Simon and G. Tóth, 1H and 13C NMR investigation of 20-hydroxyecdysone dioxolane derivatives, a novel group of MDR modulator agents, Magn. Reson. Chem., 2013, 51, 830–836 CrossRef PubMed.
- A. Martins, J. Csábi, D. Kitka, A. Balázs, L. Amaral, J. Molnár, A. Simon, G. Tóth and A. Hunyadi, Synthesis and Structure–Activity Relationships of Novel Ecdysteroid Dioxolanes as MDR Modulators in Cancer, Molecules, 2013, 18, 15255–15275 CrossRef CAS PubMed.
- A. Martins, P. Sipos, K. Dér, J. Csábi, W. Miklós, W. Berger, A. Zalatnai, L. Amaral, J. Molnár, P. Szabó-Révész and A. Hunyadi, Ecdysteroids Sensitize MDR and Non-MDR Cancer Cell Lines to Doxorubicin, Paclitaxel, and Vincristine but Tend to Protect Them from Cisplatin, BioMed Res. Int., 2015, 895360 Search PubMed.
- K. Moitra, Overcoming Multidrug Resistance in Cancer Stem Cells, BioMed Res. Int., 2015, 635745 Search PubMed.
- T. Sugano, M. Seike, R. Noro, C. Soeno, M. Chiba, F. Zou, S. Nakamichi, N. Nishijima, M. Matsumoto, A. Miyanaga, K. Kubota and A. Gemma, Inhibition of ABCB1 Overcomes Cancer Stem Cell-like Properties and Acquired Resistance to MET Inhibitors in Non-Small Cell Lung Cancer, Mol. Cancer Ther., 2015, 14, 2433–2440 CrossRef CAS PubMed.
- P. Cozzi, A. Mongelli and A. Suarato, Recent anticancer cytotoxic agents, Curr. Med. Chem.: Anti-Cancer Agents, 2004, 4, 93–121 CrossRef CAS PubMed.
- D. Bonnet-Delpon and J. P. Bégué, Recent advances (1995–2005) in fluorinated pharmaceuticals based on natural products, J. Fluorine Chem., 2006, 127, 992–1012 CrossRef.
- J. Tomás, F. Camps, J. Coll, E. Melé and N. Pascual, Synthesis of 25-Fluoroponasterone A, a Fluorinated Analogue of 20-Hydroxyecdysone, Tetrahedron, 1992, 44, 9809–9818 CrossRef.
- R. Lafont, J. Harmatha, F. Marion-Poll, L. Dinan and I. D. Wilson, The ecdysone handbook, 3rd edn On-line at http://ecdybase.org/index.php?&action=browse&row=280 (accessed July 2016) Search PubMed.
- D. F. Shellhamer, A. A. Briggs, B. M. Miller, J. M. Prince, D. H. Scott and V. L. Heasley, Reaction of aminosulfur trifluorides with alcohols: inversion vs. retention, J. Chem. Soc., Perkin Trans. 2, 1996, 5, 973–977 RSC.
- A. Hunyadi, A. Gergely, A. Simon, G. Tóth, G. Veress and M. Báthori, Preparative-Scale Chromatography of Ecdysteroids of Serratula wolffii Andrae, J. Chromatogr. Sci., 2007, 45, 76–86 CAS.
- M. Swain, Chemicalize.org, J. Chem. Inf. Model., 2012, 52, 613–615 CrossRef CAS.
- N. Hevir, N. Trošt, N. Debeljak and T. L. Rižner, Expression of estrogen and progesterone receptors and estrogen metabolizing enzymes in different breast cancer cell lines, Chem.-Biol. Interact., 2011, 191, 206–216 CrossRef CAS PubMed.
- S. Kumpun, J. P. Girault, L. Dinan, C. Blais, A. Maria, C. Dauphin-Villemant, B. Yingyongnarongkul, A. Suksamrarn and R. Lafont, The metabolism of 20-hydroxyecdysone in mice: relevance to pharmacological effects and gene switch applications of ecdysteroids, J. Steroid Biochem. Mol. Biol., 2011, 126, 1–9 CrossRef CAS PubMed.
- T.-C. Chou, Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies, Pharmacol. Rev., 2006, 58, 621–681 CrossRef CAS PubMed.
- V. N. Odinokov, I. V. Galyautdinov, D. V. Nedopekin and L. M. Khalilov, Trifluoroacetylation and dehydration of 20-hydroxyecdysone acetonides. Synthesis of stachisterone B, Russ. Chem. Bull., 2003, 52, 232–236 CrossRef CAS.
- I. Pastan, M. M. Gottesman, K. Ueda, E. Lovelace, A. V. Rutherford and M. C. Willingham, A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 4486–4490 CrossRef CAS.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
Footnotes |
| † The authors declare no competing interests. |
| ‡ Electronic supplementary information (ESI) available: NMR spectra of compound 5, HPLC chromatograms of compounds 2–5. See DOI: 10.1039/c6md00431h |
| § Permanent address: Synthetic Systems Biology Unit, Institute of Biochemistry, Biological Research Centre, Temesvári krt. 62, 6726 Szeged, Hungary. |
| This journal is © The Royal Society of Chemistry 2016 |