Measure and control: molecular management is a key to the Sustainocene!
Douglas R.
MacFarlane
*,
Xinyi
Zhang
and
Mega
Kar
School of Chemistry, Monash University Clayton, Victoria 3800, Australia. E-mail: Doug.MacFarlane@monash.edu
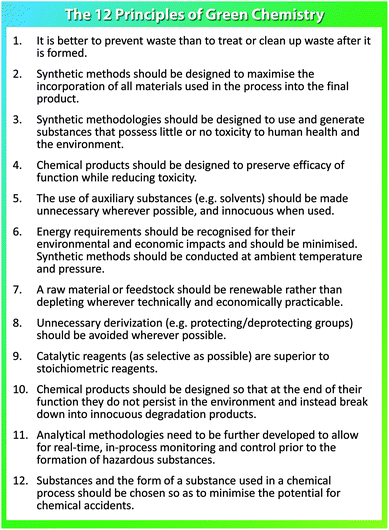 | ||
| Fig. 1 The 12 principles of green chemistry.1 | ||
Advances in analytical methodologies – towards single-molecule sensitivity
There is now an increasing demand for industrial processes to implement environmental monitoring technologies in order to improve product quality and energy efficiency and prevent environmental pollution and its associated problems. Gas sensing technologies are essential in many industries, as well as our daily life. Gas sensing is typically based on the variation of properties, such as conductivity, of semiconductors, polymers and metal–organic framework materials as a result of absorption of the target molecules.3 In order to reduce weight, power consumption and cost, as well as to enable introduction to processes in-line, a great effort has been made in the miniaturization of these gas sensors. The further development of new sensing strategies and materials is essential to the continued advancement of these technologies. Nanomaterials such as carbon nanotubes, polymer nanowires, and more recently graphene and its derivatives have been demonstrated for sensing molecules such as CO, NO2, H2S and NH3, etc.4–6 However, in high temperature industrial processes such as methanol steam reforming and coal gasification, gas sensing becomes difficult because of the harsh and complex environment; to fulfil these needs wireless analytical methods based on optics or integrated technologies have emerged.7,8One critical challenge is the monitoring of several components present in a gas system simultaneously. The integration of sensors into arrays has been shown to be a good solution to monitoring environments characterized by the simultaneous presence of several critical compounds. Microelectrode arrays (MEAs) comprised of spatially addressable ensembles of different receptors, can enable facile, simultaneous, and multiple analyte detection. Individually addressable MEAs also provide an excellent approach for improving analytical sensitivities and detection limits.9 Major applications of this analytical technology beyond industrial processes include the monitoring of polluting gases from vehicles, in situ methane detection in mining process, monitoring of greenhouse gases from power plants and indoor oxygen and carbon monoxide monitoring.
Toxic, bio-accumulating heavy metals can remain in ecological systems and in the food chain indefinitely and therefore heavy metal monitoring in processes and in the environment has become important, especially in ensuring water and food quality.10 Electrochemical sensors have become an important subclass of chemical sensors for this purpose, in which an electrode is used as the transduction element; these are well suited for meeting the size, cost, and power requirements of on-site monitoring.11 Many work on the basis of the measurement of electrochemical potential differences, but this ultimately limits their selectivity and sensitivity. Automated anodic stripping analysis is one of the more sensitive electroanalytical techniques that can be used for the detection and quantification of ultra-trace concentrations of toxic metals. To avoid the use of toxic mercury electrodes, “green” bismuth film electrodes for real time stripping voltammetric measurements have been developed.12,13 Gong et al. developed monodispersed Au-nanoparticle decorated graphene as an enhanced sensing platform for ultrasensitive stripping voltammetric detection of mercury(II), with a detection limit as low as 6 ppt (S/N = 3).14 Screen-printed electrodes have revolutionized this field, not only by reducing the manufacturing cost, but also by making possible the production of a variety of electrodes and MEAs in a highly reproducible manner.15 These extend the range of available non-toxic electrode systems and thereby the analytical possibilities of voltammetry and related methods.
Optical detection systems are useful alternatives to electrochemical detection methods; these are especially attractive analytical tools whenever continuous monitoring and real-time information is desired. Optical detection methods include optical adsorption and light emission fluorescence techniques. Compared to absorbance-based techniques, the fluorescence method has higher sensitivity with potential detection limits down to single molecule.16
Since the first report of the detection of a single molecule, surface-enhanced Raman spectroscopy (SERS) has emerged as one of the most versatile tools for sensing applications of trace organics.17 SERS-based molecular detection can be achieved by using metal (mostly silver and gold) nanostructures as plasmonic antennas to amplify Raman signals. A unique feature of these metal nanostructures is their ability to support localized surface plasmons, i.e., light-driven coherent oscillations of conduction electrons, which can generate an enormous electromagnetic field enhancement in the close vicinity of the metal surface. For example, we have designed and fabricated a new class of 3D plasmonic metamaterial-based SERS substrates, namely hierarchically-ordered porous gold membranes consisting of close-packed arrays of nano-hole channels and uniformly distributed mesopores over the bulk. These 3D plasmonic metamaterials exhibit a significantly enhanced Raman intensity and a detection limit down to 10−13 M for benzenethiol, a toxic aromatic and also a commonly used model for a broad family of similar toxic compounds.18 SERS has also been utilized for on-line monitoring of catalytic processes on plasmonic catalysts. More recently, an integrated SERS spectro-electrochemical analysis system has been developed for real-time, in situ monitoring of the process of electrochemical oxidation of the metalloporphyrin hemin on a nanostructured Au working electrode.19
Parallel to the advances in sensor and analysis technology there have been enormous developments in the software and control electronics that allow data to be (i) acquired from multiple sources at a high rate, (ii) analysed numerically in real time, (iii) stored and (iv) fed to programmable logic controllers in-line to control conditions. For example, in IR spectroscopy fast algorithms for principle component analysis allow reliable monitoring of certain infrared bands against a complex background, while in electrochemical techniques pulsed and variable frequency methods allow the continuous extraction of multiple parameters.
As an example, in the ammonia industry process analytics are critical for process quality and optimized efficiency is achieved through tight control of the H2/N2 ratio. On the other hand, where hydrogen is produced from natural gas for this process, toxic emission sources of NOx, SO2, H2S and CO need to be treated and monitored. Currently, in situ gas chromatography20 and in situ laser gas analyzers21 are used, which allow for multiple component detection in a single device; however, the further improvement of selectivity and repeatability for such systems remains a challenge. On-line mass spectrometry (MS) is another practical technique that has been used to monitor chemical reactions and investigate reaction mechanisms.22 For example, charge-transfer ionization (CTI) with O2+ as the reagent ion based on a vacuum ultraviolet (VUV) source in a time-of-flight mass spectrometer (CTI-TOFMS) has been applied for the real-time monitoring of ammonia synthesis. This high time-resolution method can not only measure the equilibrium conversion rates of NH3 rapidly, but also monitor the catalyst activity variation during the reaction.23
PAT encourages greener chemistry in pharma
In the pharmaceutical/organic chemicals industry, the FDA have approved Process Analytical Technology (PAT) as an approach for the monitoring and control of Active Pharmaceutical Ingredient (API) production via in situ analytical techniques such as IR, Raman, NMR, etc.24 As a result, such in situ analytical methods have been implemented across many pharmaceutical producers. The techniques often involve more than one analytical tool being utilized simultaneously to determine the progress of the reaction. This increases the chances of detecting all reactants, products and by-products. For example, recent work by Foley et al. focused on analysing the formation of an imine via the reaction between an amine and aldehyde in the presence of Hünig's base using on-line NMR and HPLC.25 In addition to monitoring the conversion of the reactants to the product, NMR also identified some by-products, such as hemiacetals and aminals, formed during the reaction. The presence of Hünig's base in the reaction was found to decrease the reaction rate as observed by NMR. This implies that by using such in situ methods the efficiency of subsequent reactions can be improved. In this case, a 100% conversion to the imine was achieved in 2 hours by removing Hünig's base.Aside from operating on-line, as is the case with 1H NMR, many of these analytical approaches can operate in-line; where the analysis occurs as part of the process stream, or at-line in the production step, where the sample is processed near the stream. This eliminates any exposure of reactive species during the process of transporting the material manually to the analytical instrument.26
The end product in the pharmaceutical industry is often crystalline and can vary in its polymorphism. Thus some of the techniques, such as focused beam reflectance measurement (FBRM) and particle vision measurement (PVM), are being used to monitor and control the crystallisation process. Recently, various forms of polymorphism in piracetam were detected and controlled by Barrett et al. using in situ Raman spectroscopy and FBRM.27 The various polymorphs can produce different molecular vibrations and rotations that can be distinguished using Raman spectroscopy. Additionally, the size distribution of the various crystal structures can be deduced from the chord length distribution (CLD) data using FBRM. In this case it was observed that a faster cooling rate resulted in a metastable polymorph, while a slower cooling rate resulted in a more stable polymorph since the latter resulted in a low level of super-saturation.
PAT naturally encourages more efficient and greener chemistry. Before PAT's approval, due to the uncertainty about the nature, concentration and origins of some intermediates, many products were rejected.28 With the best in situ methods implemented, process analysis and control can increase quality and yield of APIs, leading to less waste.
Looking forward…… towards the Sustainocene
Today's global challenges that emanate from the overlapping issues of energy, food and water supply fundamentally involve large scale chemical processes that are currently, or have the potential to in the future, produce damaging by-products. In many unfortunate cases the solution to one challenge produces its own challenges. For example, the use of amine CO2 carbon capture media in the coal-fired power industry currently accounts for large losses of amines into the atmosphere and the impact of this in the longer term is yet to be determined. Advances in appropriate analysis and control methodologies clearly have a continuing role to play in optimising these processes.Is this principle of Green Chemistry involving an imperative to develop analysis and control technologies still valid today? Without doubt, an emphasis on measurement, optimization and control will continue to impact positively on making chemical processes greener and more sustainable. It will also help us to avoid big mistakes such as CFCs and the ozone hole in the future. Perhaps we should broaden the original scope of this principle from being only on the generation of hazardous substances; the green and sustainable aspects of any process can be optimized with in-process monitoring and control. As atom efficiency and energy efficiency become more and more vital, these tools provide the means to achieving the most sustainable outcome in all respects, ultimately paving the way towards an era some commentators are now calling the Sustainocene.2
References
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 1998 Search PubMed.
- T. A. Faunce, Nanotechnology toward the Sustainocene, Pan Stanford Publishing Pte. Ltd., 2015 Search PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- J. Kong, N. R. Franklin, C. W. Zhou, M. G. Chapline, S. Peng, K. J. Cho and H. J. Dai, Science, 2000, 287, 622–625 CrossRef CAS PubMed.
- M. Law, H. Kind, B. Messer, F. Kim and P. D. Yang, Angew. Chem., Int. Ed., 2002, 41, 2405–2408 CrossRef CAS.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- X. Liu, S. T. Cheng, H. Liu, S. Hu, D. Q. Zhang and H. S. Ning, Sensors, 2012, 12, 9635–9665 CrossRef PubMed.
- C. J. Rawn, J. R. Leeman, S. M. Ulrich, J. E. Alford, T. J. Phelps and M. E. Madden, Rev. Sci. Instrum., 2011, 82, 024501 CAS.
- F. L. Meng, L. Zhang, Y. Jia, J. Y. Liu, Y. F. Sun, T. Luo, M. Q. Li, J. H. Liu and X. J. Huang, Sens. Actuators, B, 2011, 153, 103–109 CrossRef CAS.
- G. Aragay, J. Pons and A. Merkoci, Chem. Rev., 2011, 111, 3433–3458 CrossRef CAS PubMed.
- N. Thiyagarajan, J. L. Chang, K. Senthilkumar and J. M. Zen, Electrochem. Commun., 2014, 38, 86–90 CrossRef CAS.
- J. Wang, Acc. Chem. Res., 2002, 35, 811–816 CrossRef CAS PubMed.
- O. Mikkelsen, K. Strasunskiene, S. M. Skogvold and K. H. Schroder, Curr. Anal. Chem., 2008, 4, 202–205 CrossRef CAS.
- J. M. Gong, T. Zhou, D. D. Song and L. Z. Zhang, Sens. Actuators, B, 2010, 150, 491–497 CrossRef CAS.
- C. Dossi, D. Monticelli, A. Pozzi and S. Recchia, Biosensors, 2016, 6, 15 CrossRef PubMed.
- L. Zang, R. C. Liu, M. W. Holman, K. T. Nguyen and D. M. Adams, J. Am. Chem. Soc., 2002, 124, 10640–10641 CrossRef CAS PubMed.
- K. M. Mayer and J. H. Hafner, Chem. Rev., 2011, 111, 3828–3857 CrossRef CAS PubMed.
- X. Y. Zhang, Y. H. Zheng, X. Liu, W. Lu, J. Y. Dai, D. Y. Lei and D. R. MacFarlane, Adv. Mater., 2015, 27, 1090–1096 CrossRef CAS PubMed.
- T. Yuan, L. L. T. Ngoc, J. van Nieuwkasteele, M. Odijk, A. van den Berg, H. Permentier, R. Bischoff and E. T. Carlen, Anal. Chem., 2015, 87, 2588–2592 CrossRef CAS PubMed.
- C. C. Chen and P. C. Lin, Anal. Methods, 2015, 7, 6947–6959 RSC.
- B. L. Upschulte, D. M. Sonnenfroh and M. G. Allen, Appl. Opt., 1999, 38, 1506–1512 CrossRef CAS PubMed.
- C. T. Martha, N. Elders, J. G. Krabbe, J. Kool, W. M. A. Niessen, R. V. A. Orru and H. Irth, Anal. Chem., 2008, 80, 7121–7127 CrossRef CAS PubMed.
- Y. Xie, L. Hua, K. Hou, P. Chen, W. Zhao, W. Chen, B. Ju and H. Li, Anal. Chem., 2014, 86, 7681–7687 CrossRef CAS PubMed.
- A. Chanda, A. M. Daly, D. A. Foley, M. A. Lapack, S. Mukherjee, J. D. Orr, G. L. Reid, D. R. Thompson and H. W. Ward, Org. Process Res. Dev., 2015, 19, 63–83 CrossRef CAS.
- D. A. Foley, J. Wang, B. Maranzano, M. T. Zell, B. L. Marquez, Y. Xiang and G. L. Reid, Anal. Chem., 2013, 85, 8928–8932 CrossRef CAS PubMed.
- A. M. Thayer, Chem. Eng. News, 2014, 92, 5 Search PubMed.
- M. Barrett, H. Hao, A. Maher, K. Hodnett, B. Glennon and D. Croker, Org. Process Res. Dev., 2011, 15, 681–687 CrossRef CAS.
- C. A. Marasco, Chem. Eng. News, 2005, 83, 7 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |
