Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Food-grade Pickering emulsions stabilised with solid lipid particles
Aleksandra
Pawlik
,
Daniel
Kurukji
*,
Ian
Norton
and
Fotis
Spyropoulos
School of Chemical Engineering, University of Birmingham, Birmingham, B15 2TT, UK. E-mail: d.j.kurukji@gmail.co.uk; Tel: +44 (0)7414 861610
First published on 20th May 2016
Abstract
Aqueous dispersions of tripalmitin particles (with a minimum size of 130 nm) were produced, via a hot sonication method, with and without the addition of food-grade emulsifiers. Depending on their relative size and chemistry, the emulsifiers altered the properties of the fat particles (e.g. crystal form, dispersion state and surface properties) by two proposed mechanisms. Firstly, emulsifiers modify the rate and/or extent of polymorphic transitions, resulting in the formation of fat crystals with a range of polarities. Secondly, the adsorption of emulsifiers at the particle interface modifies crystal surface properties. Such emulsifier-modified fat particles were then used to stabilise emulsions. As the behaviour of these particles was predisposed by the kind of emulsifier employed for their manufacture, the resulting particles showed different preferences to which of the emulsion phases (oil or water) became the continuous one. The polarity of the fat particles decreased as follows: Whey Protein Isolate > Soy Lecithin > Soy Lecithin + Tween 20 > Tween 20 > Polyglycerol Polyricinoleate > no emulsifier. Consequently, particles stabilised with WPI formed oil-in-water emulsions (O/W); particles stabilised solely with lecithin produced a highly unstable W/O emulsion; and particles stabilised with a mixture of lecithin and Tween 20 gave a stable W/O emulsion with drop size up to 30 μm. Coalescence stable, oil-continuous emulsions (W/O) with drop sizes between 5 and 15 μm were produced when the tripalmitin particles were stabilised with solely with Tween 20, solely with polyglycerol polyricinoleate, or with no emulsifier at all. It is proposed that the stability of the latter three emulsions was additionally enhanced by sintering of fat particles at the oil–water interface, providing a mechanical barrier against coalescence.
Introduction
Stabilising interfaces with particles (i.e. Pickering stabilisation) is an established approach,1–5 which aims at extending the kinetic stability of simple and multiple emulsions. This is because interfaces covered with colloidal particles provide an enhanced barrier to droplet coalescence,6 mass transfer across the interface5 and lipid oxidation.4 A range of food-grade particles have been investigated for use in Pickering stabilisation. These include wax crystals,7 CaCO3,8 ethyl cellulose,9 protein-polysaccharide complexes,10 fat crystals,5 OSA modified starch11etc. Many food products (e.g. mayonnaise, margarine, whipped cream, batter, ice cream) are, in fact, entirely or partially, stabilised by the sub-micron sized particles.Amongst the several factors (i.e. particle wettability, size, shape, concentration, particle–particle interactions) determining whether the solid particles will act as an emulsifier, the most important is often argued to be wettability, which is measured by the contact angle that the particles assume at the oil–water interface.1 The contact angle is also linked to surfactant adsorption onto the particle's surface.12 Ionic surfactants adsorb at the surface of silica particles, induce their flocculation at the water–oil interface, and enhance emulsion stability.13,14 Surfactants can also displace particles from an interface,3 sometimes without destabilising the emulsion.15 In a system where both solid particles (hydrophilic silica) and a low molecular weight surfactant (monoolein) were present, Pichot et al., proposed a two-fold emulsion stabilisation mechanism.6 During emulsification a highly dynamic monoolein rapidly reduced interfacial tension, facilitating further droplet break-up and limiting coalescence. This allowed sufficient time for the less mobile silica particles to gather at the oil–water interface, displace monoolein molecules and thus provide long-term emulsion stability against coalescence. Murray et al. demonstrated a synergistic effect between a protein and starch at a liquid–liquid interface.2 Without indication of specific protein–protein interactions, it was proposed that a protein could enhance solid particle assembly at the adsorbed layer, and their subsequent jamming at the interface. The resultant increase in interfacial viscoelasticity correlated with an increase in emulsion stability against coalescence and/or Ostwald ripening. Using monoglycerides Frasch-Melnik et al. (2010)5 altered the polarity of inherently hydrophobic triglyceride crystals16 and seeded their formation at the interface17 during the manufacture of the water-in-oil emulsions. By precisely selecting the ratio of a surface-active crystalline monoglyceride to a network-forming triglyceride as well as the processing conditions (cooling and shearing rates), a sintered “shell” of fat crystals at an interface surrounded by a network of fat crystals in the bulk continuous phase was formed. The interplay of the two mechanisms of fat crystal stabilisation: surface-active (Pickering stabilisation), surface-inactive (network stabilisation), or a combination of both, on the sedimentation and coalescence stability of emulsions, was investigated by Ghosh et al.18 They proposed that Pickering crystals were more effective than the network crystals for emulsion stabilisation.
Other surfactants (e.g. polyglycerol polyricinoleate (PGPR) and lecithin) have also been employed to increase aqueous wetting of triglycerides.16,19,20 The general approach was to either pre-crystallise the fat within the oil phase followed by emulsion formation or to crystallise the fat post emulsification. In the flash-cooling process Garti et al.20 produced the α polymorph of tristearin crystals in a liquid oil, which were more hydrophilic than the β polymorph, thus more easily drawn to the interface.16 Nevertheless, production of non-flocculated, submicron α fat crystals required addition of a suitable surfactant (PGPR).
In this work, solid lipid particles with tailored properties were manufactured first and then assessed for use as emulsion stabilisers. The aim was to produce aqueous dispersions of fat particles (via a hot emulsification step) designed to possess the required size, morphology, and surface characteristics necessary to function as Pickering stabilisers. To achieve this, food-grade emulsifiers with a range of chemistries (protein, phospholipid, low and high HLB surfactants, and their mixtures) were utilised and investigated for subsequent potential to: (i) stabilise fat particles in an aqueous dispersion and (ii) alter the microstructure and thus the tendency for the particles to form water- or oil-continuous emulsions.
Experimental
Materials
Tripalmitin (with purity ≥85%) and Polyoxyethylene(20)sorbitanmonolaurate (Tween 20) (HLB = 16.7) were purchased from Sigma Aldrich (UK). Whey Protein Isolate (WPI) was kindly donated by DAVISCO Foods International, Inc. (Switzerland) and Lipoid S45 was kindly provided by Lipoid GmbH (Germany). The composition of Lipoid S45 was reported from manufacturer as 45–50% soybean phosphatidylcholine and 10–18% phosphatidylethanolamine; the typical FA composition is 58–65% linoleic acid, 12–17% palmitic acid, 8–12% oleic acid and other fatty acids. Polyglycerol polyricinoleate (PGPR, HLB = 1.5 ± 0.5) was obtained from Kerry Bioscience (UK) and sunflower oil was purchased from the local supermarket. Distilled water (pH = 6.8, 1.63 μS cm−1) was used for the experiments. All materials were used without further purification or modification, and all samples were formulated and reported on a weight-by-weight basis (g/g).Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. To avoid shear-induced heating of the sample and melting of the fat particles, the mixture was cooled in an ice bath, while emulsified. The emulsions were analysed directly after preparation and then stored at 4 ± 2 °C.
000 rpm. To avoid shear-induced heating of the sample and melting of the fat particles, the mixture was cooled in an ice bath, while emulsified. The emulsions were analysed directly after preparation and then stored at 4 ± 2 °C.
Analytical methods
Results and discussion
Production and characterisation of solid lipid particles
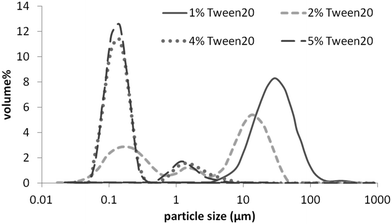 | ||
| Fig. 1 Particle size distribution of solid lipid dispersions; 5% tripalmitin in water, stabilised with 1, 2, 4 and 5% Tween 20. | ||
Fig. 1 shows that TP average particle size decreases from ∼25 μm to ∼150 nm as the concentration of the surfactant increases from 1 to 4%; it then remains constant on a further Tween 20 increase to 5%. Fig. 2A shows that discrete particles as well as larger aggregates coexist in the formulation with 1% Tween 20, which explains the increase in the measured average particle size with decreasing concentration of Tween 20. During cooling of the molten emulsion, gelation21 of formulations containing 1 and 2% Tween 20 was observed (weak gels, reversible on stirring). On the contrary, formulations with 4 and 5% Tween 20 were fully liquid, milky with a “bluish” tint. This suggests that below 4% Tween 20 there was insufficient surfactant (Tween 20) to fully cover the interfacial area, consequently leading to coalescence of liquid tripalmitin prior to crystallisation. In addition, due to incomplete coverage and/or an insufficient concentration of free surfactant molecules in the bulk, the tripalmitin droplets could have aggregated during the cooling process, when crystal growth or/and polymorphic transformations caused a further increase in the interfacial area.21 Such aggregation could result in gelling (i.e. attractive interactions between the hydrophobic chains of triglyceride crystals, reversible on stirring) or partial coalescence of solid fat particles, where the crystal growing in one particle can pierce the neighbouring one, bridging them into an irreversible aggregate. The case of the partial coalescence is supported by observations of Westesen & Siekmann,21 who, using a TEM technique, visualised needle-shaped tripalmitin crystals. It has also been reported22 that polymorphic transitions in fat particles lead to a change in the crystal shape, subsequent increase in the interfacial area, and particle aggregation even in the presence of a relatively high concentration of Tween 20. This is likely to be caused by the relatively poor steric properties of a low molecular weight surfactant such as Tween 20. As a result, particle–particle contact during (re)crystallisation, or particle collisions, may potentially lead to aggregation. In order to overcome these limitations, a range of food grade emulsifiers were investigated for their potential to stabilise tripalmitin fat particles in aqueous dispersion. Soy lecithin, WPI (globular and heat-sensitive protein), PGPR (oil-soluble emulsifier) and their mixtures with low molecular weight Tween 20 (i.e. lecithin-Tween 20 and WPI-Tween 20) were investigated with the goal of providing: (i) fast interfacial coverage during emulsification, and (ii) a thick interfacial film that imparts post production stability. Solid fat particles without any emulsifier were also investigated.
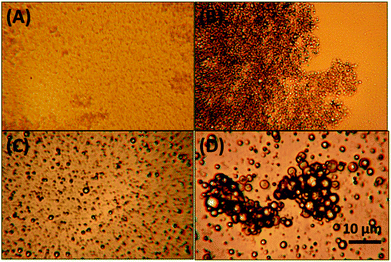 | ||
| Fig. 2 Micrographs of solid lipid dispersions; 5% tripalmitin in water, stabilised with: (A) 1% Tween 20, (B) 1% PGPR, (C) 5% WPI and (D) no emulsifier. | ||
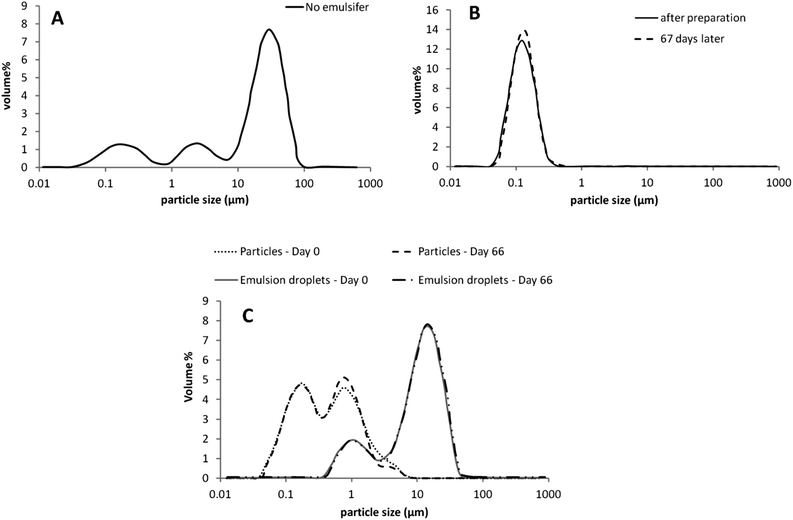
Without an emulsifier present during emulsification, a 5% tripalmitin-in-water system was milky white with no macroscopic phase separation or bulk gelation occurring during cooling of the molten emulsion. The particle size distribution of the dispersion was complex (Fig. 3A), with three populations ca. 0.15, 2 and 25 μm. Microscopic images showed near-spherical, partially flocculated particles (Fig. 2D); however, a few bigger “lumps” of fat (1–2 mm) were also present. To investigate the potential of breaking down these flocculated crystals, the suspension was treated via a mild sonication process (to avoid excessive heat production); however, no significant reduction in particle size was achieved. To further reduce the size of solid fat particles in water, a cold high-pressure homogenisation (NS 1001L Panda) was also performed. However, the hydrophobic nature of these particles caused a blockage inside the processing equipment. The hydrophobic character of the particles also manifested in their propensity for adsorption at air–water interface, resulting in formation of a leathery wrinkled film. A similar film of particles was observed by Do et al. for water-insoluble ethyl cellulose particles investigated for use as viscosity modifiers in low fat chocolate.23 The fact an emulsifier was not required to produce a stable dispersion of fat particles in water is perhaps surprising, as one could expect coalescence of fat during sonication and cooling. The stability of fat particles in water could be enhanced by surface-active impurities (e.g. monoglycerides) present in the tripalmitin, which might cover the interface during emulsification and subsequent storage. Additionally, during the cooling step, α crystals of tripalmitin that form first have been reported16 to be more polar than the β crystals. This increase in polarity may ensure that the energy penalty for fat particles in the dispersed state is smaller due to their relatively increased propensity for water.
When soy lecithin (5%) was used to stabilise 5% tripalmitin-in-water, the particle dispersion gelled on cooling and, as such, a laser diffraction particle size measurement was impossible. Gelation in the lecithin-tripalmitin system can be explained by the specific behaviour of lecithin in water, i.e. formation of lamellar phases, which exhibit lower mobility than highly dynamic surfactant micelles. Therefore, when the fat dispersion was cooled down after the emulsification, there was no shearing force to disrupt liquid crystals of lecithin and so its adsorption to the newly formed interfaces (during crystal growth on cooling) was slow. We have shown, on the example with the fat particles with no emulsifier (Fig. 3A), that an uncovered interface does not necessarily lead to particle aggregation and gelation of bulk tripalmitin. Hence, gelation of the tripalmitin dispersion in the presence of lecithin is more likely caused by an attractive particle interactions induced by the phospholipid. Lecithin has a complex composition and on the molecular level its structure is somewhat similar to that of a triglyceride.21 By being incorporated into the tripalmitin network, lecithin may bridge between the neighbouring particles thus leading to aggregation. We have shown (Fig. 3B) that particle aggregation in the formulation with lecithin can be avoided by using a co-emulsifier, a small molecular surfactant (Tween 20, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with lecithin). Such formulation design ensures production of small tripalmitin particles (120 nm, Fig. 3B) resulting from fast adsorption of Tween 20 via its highly dynamic micellar structure. Moreover, fast coverage of the interface may also limit the bridging effect of the adsorbing lecithin, which nevertheless introduces a steric interfacial barrier against crystals protruding towards the neighbouring particles. As a result, fat dispersions stabilised by the mixture of a lecithin and Tween 20 were stable over the 9 week observation period.
1 with lecithin). Such formulation design ensures production of small tripalmitin particles (120 nm, Fig. 3B) resulting from fast adsorption of Tween 20 via its highly dynamic micellar structure. Moreover, fast coverage of the interface may also limit the bridging effect of the adsorbing lecithin, which nevertheless introduces a steric interfacial barrier against crystals protruding towards the neighbouring particles. As a result, fat dispersions stabilised by the mixture of a lecithin and Tween 20 were stable over the 9 week observation period.
Producing solid tripalmitin particles with 5% WPI resulted in a bimodal particle size distribution (Fig. 3C), with two size populations, ca. 150 and 700 nm, which did not change significantly over the storage period of 9 weeks. The bimodal size distribution could be caused by a heat-induced partial denaturation and unfolding of the protein (during sonication),23 subsequently lead to aggregation of such protein-stabilised fat particles. Microscopic images (Fig. 2C), however, showed no apparent flocs. Moreover, partial coalescence or gelling of particles during cooling is likely to be hindered by a relatively thick layer of the adsorbed protein and electrostatic repulsions induced by them. Instead, the reason behind the bimodal size distribution is more likely as result of coalescence of the liquid fat droplets during emulsification. This may be result of the high molecular weight of WPI (18 kg mol−1) and the fact in the region pH = 5–8 WPI exists as a dimer.24
WPI was also combined with a co-emulsifier (Tween 20) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and used to stabilise tripalmitin dispersions. However, in this case, the fat phase separated from water during cooling and stayed as a solid layer on the top of the bulk water phase. A displacement of WPI by Tween 20 is highly questionable owing to a multiple point protein anchoring at the interface. At the moment, the exact reason for this behaviour is unknown but is probably governed by the nature of interaction between WPI and tripalmitin.
1 ratio and used to stabilise tripalmitin dispersions. However, in this case, the fat phase separated from water during cooling and stayed as a solid layer on the top of the bulk water phase. A displacement of WPI by Tween 20 is highly questionable owing to a multiple point protein anchoring at the interface. At the moment, the exact reason for this behaviour is unknown but is probably governed by the nature of interaction between WPI and tripalmitin.
Tripalmitin suspensions were also stabilised with 1% PGPR. These dispersions were mildly gelled (thus laser diffraction particle size data could not be obtained) and the microscopic images showed relatively small particles of uniform size (Fig. 2B). Gelation is often caused by an insufficient surfactant concentration in the system.
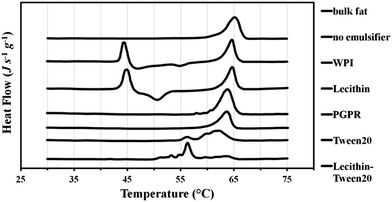
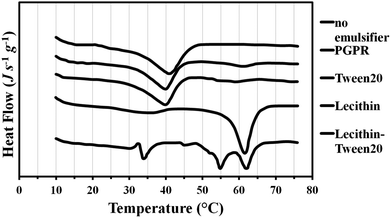
The peak melting temperature of bulk tripalmitin was 65 °C (Fig. 4); this is within the range of the values reported by Helgason et al., Bunjes et al., and Knoester et al.:22,25,26 63, 64 and 66 °C, respectively. The difference between the reported values is most likely a consequence of varying tripalmitin purity across the experiments. The melting curves in Fig. 4 show that when no emulsifier is present to stabilise the tripalmitin in dispersion, first the meta-stable α crystalline form melts at 44 °C (45 °C in Helgason et al.22), followed by an exothermic peak at 55 °C (i.e. recrystallisation into a more stable β′ or β) and then melting of a stable β form at 65 °C. This is identical to the melting temperature of a β form in the bulk tripalmitin. Similar melting behaviour was exhibited by the WPI stabilised tripalmitin particles; however, the recrystallisation peak (α to β′ or β) occurred at 50 °C. The α crystals were no longer present in formulations containing lecithin or PGPR, where one endothermic peak at 64 °C indicated the presence of the stable β crystals. This suggests that that both lecithin and PGPR promote crystal lattice zone refinement, facilitating crystal movements and thus their transition to the most energetically favourable state. This can be explained by the fact that both emulsifiers have large protruding hydrophobic tails, which sufficiently penetrate into the particle's fat matrix. Bulky hydrophobic tails provide large spacing between fat molecules, creating grain boundaries and easing molecular reorganisation within the particle. Alternatively, the molecular similarity of the emulsifier chains to the fat molecules (such as lecithin to tripalmitin) may mean that they initiate (or promote) crystallisation of the more stable crystal form. Contrary to the lecithin and PGPR, WPI may adsorb on the crystal surface interface rather than penetrate into the fat crystal; this is because a globular protein first adsorbs at the interface and then unfolds over time, revealing its hydrophobic moieties. However, quick fat solidification may restrict protein unfolding at the interface, limiting the number of hydrophobic moieties penetrating the fat phase. As a result, two mechanisms are possible. Firstly, due to its position at the interface, WPI does not enter the fat matrix at all and thus the system behaves like an emulsifier free dispersion (thus unaided and slow polymorphic transformation). Secondly, the viscoelastic membrane formed by the interfacial protein, prevents the crystal movements, slowing down the polymorphic transformations. It has been postulated by Helgason et al.22 that close packing of surfactant tails may form a relatively rigid “shell” surrounding the lipid particle, which then restricts the molecular motions within the fat matrix.
When Tween 20 and its mixture with the lecithin were employed, there was a general decrease in a melting temperature of the most stable crystal form. Fig. 4 shows that Tween 20 on its own caused the crystal structure to melt first at 56 °C (most likely β′) followed by a larger peak at 62 °C (β). When lecithin was added to Tween 20, the fat melting curve is of multiple peaks with maxima at 48 °C, 56 °C (biggest peak) and 63 °C. There are several possible explanations for such thermal behaviour. Firstly, as suggested by Helgason et al.,22 the emulsifiers could significantly slow down or even stop the polymorphic transition at the metastable β′ form (melting at 56 °C). Through specific interactions occuring at the interface (leading to, for instance, an increase in viscoelasticity) crystal movement can be transiently hindered by the emulsifier film present. An additional argument supporting the presence of metastable crystals comes from the fact that the melting peaks are very broad, suggesting a high degree of imperfections (grain boundaries) in the crystal lattice27 typical for lower melting crystalline forms. Secondly, the depression of the melting point of the stable β crystals (to 56 °C) could be caused by very small sizes of fat particles.27 Such particles have very high surface curvatures, which increases their solubility and thus lowers the melting temperature. Such dampening of the melting temperature does not occur when larger particles, for example, those with no emulsifier or stabilised with WPI and lecithin (Fig. 4). The latter explanation seems more likely, as the attempts to temper the particle crystal matrix, by keeping it (up to 2 h) at the temperature slightly above the melting temperature, did not result in further polymorphic transformation.
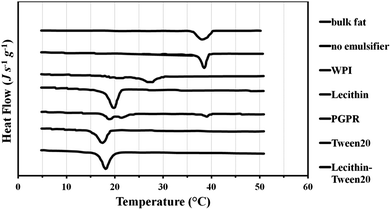
DSC curves for crystallisation of the solid lipid particles are given in Fig. 5. The crystallisation of bulk tripalmitin, via formation of α crystals, took place at 39 °C, which is between 37 °C reported by Helgason et al. and 42 °C reported by Bunjes et al. A similar crystallisation temperature of 38 °C was observed for the fat dispersion in the absence of the emulsifiers. This would be expected as prior heating of the sample (i.e. the melting curve shown in Fig. 4) causes fat coalescence, phase separation and thus subsequent bulk fat crystallisation behaviour. Fig. 5 shows that Tween 20, lecithin and the corresponding mixture caused a significant decrease in the recrystallisation temperature to 17, 20 and 18 °C, respectively. This is due to the requirement for substantial super-cooling of particles with a smaller average size. Using WPI, recrystallisation of tripalmitin occurred at 27 °C due to larger sizes of the particles (up to 700 nm, Fig. 3) and thus a reduced degree of supercooling required. Recrystallisation of tripalmitin particles stabilised with PGPR show a first small peak at 39 °C followed by a multi-peak with a maximum at 19 °C. This demonstrates that PGPR (being an oil soluble emulsifier) was not particularly efficient at stabilising the molten tripalmitin dispersion and/or the PGPR concentration employed was too low. This could lead to coalescence of a significant number of liquid fat droplets, which then recrystallised in a bulk fat manner (at 39 °C), while the rest of stable particles recrystallised at the temperature (at 19 °C) similar to the dispersions with lecithin and Tween 20, where no coalescence occurred.
Thermal analysis was repeated on all samples after one week and also one year of storage and no significant change in the thermal behaviour was observed. This means that polymorphic transformations facilitated by certain emulsifiers happen relatively fast (up to 24 h). However, when such emulsifiers are not present or do not penetrate the fat network (i.e. WPI), the metastable forms of tripalmitin crystals exist for at least a year.
Production & characterisation of emulsions
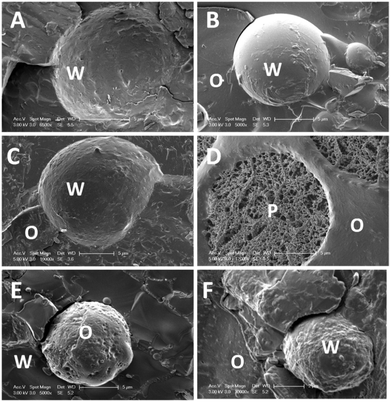
The droplet size evolution of W/O emulsions with time, measured using a NMR, is given in Fig. 6. It shows that the emulsions stabilised with tripalmitin particles without the emulsifiers, with Tween 20, and with PGPR had similar droplet sizes (ca. 15 μm) and did not change significantly over the storage period. All emulsions, however, as expected, displayed sedimentation on storage, with a distinct layer of clear oil on the top of the storage vessel. When particles were stabilised with a lecithin-Tween 20 mixture, the resulting emulsion had a larger average droplet size (25–30 μm) and were also stable of the storage period. Optical and SEM micrographs of the emulsion with the lecithin-Tween 20 stabilised tripalmitin (Fig. 7B and 8F) show spherical water droplets covered with particles of different sizes. The emulsions stabilised with tripalmitin-lecithin particles visibly flocculated and after the initial 2 weeks of storage, large water droplets sedimented on the bottom of the container. This was accompanied by an increase in the droplet size from ∼27 μm (after preparation) to ∼42 μm (day 30) followed by a decrease to ∼29 μm (day 72) (Fig. 6).
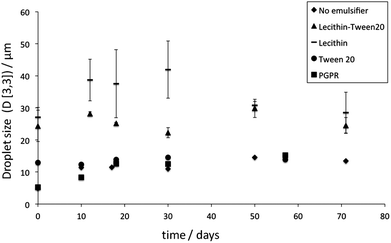 | ||
| Fig. 6 W/O emulsions stabilised with fat particles; droplet size evolution with time. Where error bars cannot be visualised they are covered by the symbol. | ||
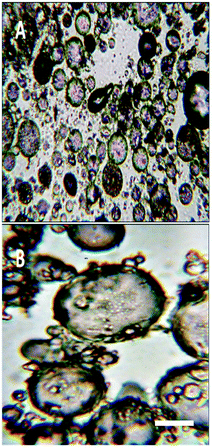 | ||
Fig. 7 Micrographs of: (A) O/W emulsion stabilised with fat particles made with 5% WPI and (B) W/O emulsion stabilised with fat particles made with lecithin-Tween 20 mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Scale bar 20 μm. 1). Scale bar 20 μm. | ||
The latter droplet size is a result of “free water” in the sample. “Free water” is any water compartment that is not encapsulated within the ∼50 μm diameter droplet. Therefore, in water droplets larger than ∼50 μm the instrument detects an unrestricted diffusion and identifies them as “free water”. As a result of such a limitation, the average calculated droplet size is reduced. The droplet size data shows (Fig. 6), that even though the particles stabilised with Tween 20 and a mixture of lecithin and Tween 20 are of a similar size (i.e. ∼130 nm, Fig. 1 and 3B), the emulsion obtained with the tripalmitin-Tween 20 particles had markedly smaller droplets. This means that: (i) not only the size of the particles determines the curvature of the interface, but also their surface properties and/or (ii) a higher concentration of free (unbounded to fat particles) Tween 20 molecules in the system with tripalmitin. Tween 20 allows for a faster decrease in the interfacial tension between sunflower oil and water during the emulsification.
Tripalmitin particles produced with WPI showed a preference for the formation of O/W emulsions. This is most probably due to their hydrophilic character, which originates from the α crystalline form of tripalmitin (Fig. 4) and a relatively thick layer of the protein at the particle interface. The droplet size distribution of such emulsions measured directly after their preparation and during storage, is shown in Fig. 3C. It is evident that the emulsion is stable against coalescence over a period of 50 days. Moreover, microscopic analyses (Fig. 7A and 8E) show that the oil droplets are covered by particles, which suggests the Pickering stabilisation mechanism. We propose that the produced emulsions are stabilised by Pickering particles rather than purely emulsifiers used for the particle preparation. This is supported by the fact that: i. the emulsion stabilised with the tripalmitin particles without any emulsifier was stable, ii. all emulsifiers used (except PGPR) are water-soluble and do not stabilise oil-continuous emulsions. For example, 5% Tween 20 in the formulation with water and oil (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) caused a phase inversion to a water-continuous emulsion and iii. Microscopic analyses showed emulsion interfaces were covered by particles.
4 ratio) caused a phase inversion to a water-continuous emulsion and iii. Microscopic analyses showed emulsion interfaces were covered by particles.
When tripalmitin-lecithin particles were used for emulsion stabilisation, the low temperature peak was small and broad and, as such, the melting temperature was difficult to determine. The broad peak was followed by melting of crystalline material at 62 °C (represented by a single peak in Fig. 9) similar to the melting temperature of the β crystals in the respective particle dispersion (64 °C shown in Fig. 4). When particles were prepared with the lecithin-Tween 20 mixture, the melting curve of the emulsion they stabilise was very complex (Fig. 9), with multiple peaks across the whole range of scanning temperatures (broad peak below 31 °C followed by peaks at 34, 44, 55 and 61 °C). These peak temperatures correspond to the melting of the respective particle dispersion in water (48, 56 and 63 °C, Fig. 4). Thermal properties of the O/W emulsions stabilised with the tripalmitin-WPI particles were identical to these of a tripalmitin-WPI dispersion (Fig. 4). This is caused by the large amount of free fat particles residing in the water-continuous phase. Melting of the bulk particles superimposes the melting of the interfacial particles, thus it is impossible to discriminate them.
The explanations for the differences between the emulsions stabilised with tripalmitin combined or not with an emulsifier, are as follows. When no emulsifier is used to stabilise fat particles, they become solubilised by the liquid oil at the emulsion's interface, which is manifested by reduction of the tripalmitin melting temperature (Fig. 9). The solubilisation process also promotes rearrangements of the fat molecules at the interface and consequently the crystals sinter into a shell-like structure5 around the water droplet (Fig. 8A).
A similar mechanism occurs when the tripalmitin particles are covered with Tween 20 and PGPR, which both facilitate zone refinement of the fat crystal network, promoting its reorganisation into the most energetically favourable state (Fig. 4). A relatively small hydrophilic group of the oil-soluble PGPR does not significantly influence the polarity of the particle surface. And so the particles stay hydrophobic, become gradually solubilised by the liquid oil at the emulsion interface, and then sinter to form a relatively smooth fat shell (Fig. 8B). This is marginally less so for Tween 20 (Fig. 8C), which has larger hydrophilic head groups, making the particles more polar and thus a portion of such particles stays within the water phase. This is confirmed by a small melting peak around 59 °C (Fig. 9) and microscopic observations (Fig. 8D). Manipulation of the pressure and temperature during the SEM imaging, revealed the inside of the water droplet, shown in Fig. 8D. Forcing the water out of the system exposed a network of Tween 20-stabilised fat particles, suggesting their affinity for the polar phase. The above data suggests that, the larger the surfactant hydrophilic head group is (Tween 20 > PGPR), the bigger the steric energy barrier against particles sintering at the interface. In emulsions, highly dynamic, non-ionic surfactants do not usually form strong repulsive interactions at the interface, hence do not create a significant steric barrier against the emulsion instability. However, when surfactant molecules at the interface are immobilised within the fat matrix, their ability to provide steric protection against aggregation is increased.
We propose that lecithin molecules either promote zone refinement of the fat matrix by aiding the polymorphic transitions or, due to a similarity in the molecular structure, facilitate fat crystallisation in the most stable crystal form (Fig. 4). Thermal characterisation of the tripalmitin-lecithin stabilised W/O emulsions, reveals large amounts of the crystalline material with the similar properties to these of the respective particle dispersion (melting at 62 °C). This suggests a high particle affinity for water and thus small number of particles in contact with the liquid oil. The reason behind this could be that the adsorbed lecithin molecules make the fat surface more polar29 and so the fat solubility in the liquid oil decreases. This potential increase in the polar behaviour of the tripalmitin-lecithin particles results in the particles preference to reside within the aqueous phase, followed by the instability of the oil-continuous emulsion. This emulsion breakdown during pre-SEM-imaging preparation made it impossible to visualise the sample. When Tween 20 and lecithin were used in conjunction, the reduced amount of the lecithin in the overall formulation resulted in a decrease in particle polarity. They still mainly reside in the water phase (demonstrated by a presence of the high melting crystals in Fig. 9), but there is also a significant percentage of particles adsorbed at the interface and so in the contact with the liquid oil (low melting peaks below 35 °C). SEM images (Fig. 8F) reveal a rough interface, suggesting that interfacial crystal sintering is limited, most likely due to steric hindrance of large head groups of lecithin and Tween 20.
WPI, as discussed earlier, does not significantly penetrate into the tripalmitin particles. Through formation of a thick protein interfacial membrane and combined with the α form of the fat crystals (Fig. 4), the particles exhibit more hydrophilic character and thus stabilise water-continuous emulsions. When the particles are in contact with the liquid oil (i.e. in emulsion) their visco-elastic protein layer provides a sufficient steric and electrostatic barrier against sintering. This is confirmed by the SEM images of O/W emulsion stabilised with the tripalmitin-WPI particles (Fig. 8E). Except for the tripalmitin-PGPR stabilised, all other emulsions phase separated when heated up to 80 °C during the DSC cycle, and as such, we decided recrystallisation was not relevant.
Conclusions
We have shown that by using a variety of food grade emulsifiers, tripalmitin particles with different characteristics with respect to their emulsion-forming tendencies can be produced. We propose that two mechanisms are involved. Firstly, emulsifiers can affect the rate and/or the extent of fat polymorphic transitions and result in a formation of fat crystals with modified polarity. Secondly, adsorption of an emulsifier to the particle interface may also serve to modify its surface properties. We propose that the polarity of the fat particles increases in the following order: no emulsifier < PGPR < Tween 20 < lecithin + Tween 20 < lecithin < WPI. As a consequence, highly polar tripalmitin particles produced with WPI stabilised water-continuous emulsions; particles with lecithin formed unstable oil-continuous emulsions; and with lecithin-Tween 20 mixtures, an oil-continuous emulsion was produced with a comparatively large drop size. Tripalmitin particles prepared with Tween 20, PGPR, or no emulsifier, formed stable oil-continuous emulsions with sintered fat shells around the water droplets. Further analytical approaches to study the formation of sintered shells would be a worthwhile extension to this work. Emulsion droplets with defined interfacial permeability may be produced using this approach and this could prove useful for controlled delivery of food/pharmaceutical ingredients. However, further understanding of the sintering mechanism is required, both in terms of chemistry and process.There is still much to learn about designing new edible particles. Such particles have enormous appeal in foods and other emulsion-based products, with key technical drivers including improvements in product shelf life and/or controlled and targeted release of functional ingredients. We have shown how to produce emulsions stabilised via a Pickering mechanism, wherein the Pickering particle functionality (towards its w/o or o/w emulsion forming potential) is controlled by the emulsifier chosen in particle manufacture.
Acknowledgements
We would like to thank the EPSRC for financial support as well as Dr Phil Taylor for useful discussions.References
- B. P. Binks, Curr. Opin. Colloid Interface Sci., 2002, 7, 21 CrossRef CAS.
- B. S. Murray, K. Durga, A. Yusoff and S. D. Stoyanov, Food Hydrocolloids, 2011, 25, 627 CrossRef CAS.
- R. Pichot, F. Spyropoulos and I. T. Norton, J. Colloid Interface Sci., 2010, 352, 128 CrossRef CAS PubMed.
- M. Kargar, K. Fayazmanesh and M. Alavi, J. Colloid Interface Sci., 2012, 366, 209 CrossRef CAS PubMed.
- S. Frasch-Melnik, I. T. Norton and F. Spyropoulos, J. Food Eng., 2010, 98, 437 CrossRef CAS.
- R. Pichot, F. Spyropoulos and I. T. Norton, J. Colloid Interface Sci., 2009, 329, 284–291 CrossRef CAS PubMed.
- B. P. Binks and A. Rocher, J. Colloid Interface Sci., 2009, 335, 94–104 CrossRef CAS PubMed.
- W. Z. Zhou, J. Cao, W. C. Liu and S. Stoyanov, Angew. Chem., Int. Ed., 2009, 48, 378–381 CrossRef CAS PubMed.
- A. L. Cambell, S. D. Stoyanov and V. N. Paunov, Soft Matter, 2009, 5, 1019–1023 RSC.
- S. Schmitt and S. L. Turgeon, Adv. Colloid Interface Sci., 2011, 167, 63–70 CrossRef PubMed.
- A. Timgren, M. Rayner, M. Sjoo and P. Dejmek, Procedia Food Sci., 2011, 1, 95–103 CrossRef CAS.
- D. E. Tambe and M. M. Sharma, Adv. Colloid Interface Sci., 1994, 52, 1–63 CrossRef CAS.
- B. P. Binks, J. A. Rodrigues and W. J. Frith, Langmuir, 2007, 23, 3626–3636 CrossRef CAS PubMed.
- B. P. Binks and J. A. Rodrigues, Langmuir, 2007, 23, 7436–7439 CrossRef CAS PubMed.
- C. Vashisth, C. P. Whitby, D. Fornasiero and J. Ralston, J. Colloid Interface Sci., 2010, 349, 537–543 CrossRef CAS PubMed.
- D. Johansson, B. Bergenstahl and E. Lundgren, J. Am. Oil Chem. Soc., 1995, 72, 921–931 CrossRef CAS.
- J. W. Mullin, Crystallisation, Butterworth-Heineman, 3 edn, 1993 Search PubMed.
- S. Ghosh, T. Tran and D. Rousseau, Langmuir, 2011, 27, 6589–6597 CrossRef CAS PubMed.
- S. M. Hodge and D. Rousseau, J. Am. Oil Chem. Soc., 2005, 82, 159–164 CrossRef CAS.
- N. Garti, H. Binyamin and A. Aserin, J. Am. Oil Chem. Soc., 1998, 75, 1825–1831 CrossRef CAS.
- K. Westesen and B. Siekmann, Int. J. Pharm., 1997, 151, 35–45 CrossRef CAS.
- T. Helgason, T. S. Awad, K. Kristbergsson, D. J. McClements and J. Weiss, J. Colloid Interface Sci., 2009, 334, 75–81 CrossRef CAS PubMed.
- T. A. L. Do, J. Vieira, J. M. Hargreaves, J. R. Mitchell and B. Wolf, Food Sci. Technol., 2011, 44, 1207–1211 CAS.
- J. M. Rodriguez Patino and A. M. R. Pilosof, Food Hydrocolloids, 2011, 25, 1925–1937 CrossRef CAS.
- H. Bunjes, K. Westesen and M. H. J. Koch, Int. J. Pharm., 1996, 129, 159–173 CrossRef CAS.
- M. Knoester, P. De Bruijne and M. Van den Tempel, Chem. Phys. Lipids, 1972, 9, 309–319 CrossRef CAS.
- B. Siekmann and K. Westesen, Colloids Surf., B, 1993, 3, 159–175 CrossRef.
- I. T. Norton, C. D. Lee-Tuffnell, S. Ablett and S. M. Bociek, J. Am. Oil Chem. Soc., 1985, 62, 1237–1244 CrossRef CAS.
- D. Johansson and B. Bergenstahl, J. Am. Oil Chem. Soc., 1995, 72, 205–215 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |