Isolation of prolyl endopeptidase inhibitory peptides from a sodium caseinate hydrolysate
Cheng-Hong
Hsieh†
a,
Tzu-Yuan
Wang†
b,
Chuan-Chuan
Hung†
cd,
You-Liang
Hsieh
a and
Kuo-Chiang
Hsu
*acd
aDepartment of Health Nutrition and Biotechnology, Asia University, 500 Lioufeng Road, Wufeng, Taichung 41354, Taiwan. E-mail: kchsu@mail.cmu.edu.tw; Fax: +886-4-22062891; Tel: +886-4-22053366 ext. 7522
bDivision of Endocrine and Metabolism, China Medical University Hospital, 2 Yude Road, Taichung, 40447, Taiwan
cFood Safety and Inspection Center, Asia University, 500 Lioufeng Road, Wufeng, Taichung 41354, Taiwan
dDepartment of Nutrition, China Medical University, 91 Hsueh-Shih Road, Taichung 40402, Taiwan
First published on 5th November 2015
Abstract
Prolyl endopeptidase (PEP) has been associated with neurodegenerative disorders, and the PEP inhibitors can restore the memory loss caused by amnesic compounds. In this study, we investigated the PEP inhibitory activity of the enzymatic hydrolysates from various food protein sources, and isolated and identified the PEP inhibitory peptides. The hydrolysate obtained from sodium caseinate using bromelain (SC/BML) displayed the highest inhibitory activity of 86.8% at 5 mg mL−1 in the present study, and its IC50 value against PEP was 0.77 mg mL−1. The F-5 fraction by RP-HPLC (reversed-phase high performance liquid chromatography) from SC/BML showed the highest PEP inhibition rate of 88.4%, and 9 peptide sequences were identified. The synthetic peptides (1245.63–1787.94 Da) showed dose-dependent inhibition effects on PEP as competitive inhibitors with IC50 values between 29.8 and 650.5 μM. The results suggest that the peptides derived from sodium caseinate have the potential to be PEP inhibitors.
Introduction
Prolyl endopeptidase (prolyl oligopeptidase, PEP, POP, PREP, EC 3.4.21.26) is a large intracellular serine protease, which cleaves short-length peptides (<30 amino acids) at the carboxyl side of an internal proline.1 Previous studies indicated that PEP activity is involved in key physiological functions such as learning and memory,2,3 cell division and differentiation,4 signal transduction5 and protein secretion,6 as well as in some psychiatric disorders. PEP also plays an important role in the degradation of biologically active peptide hormones and neuropeptides that contain proline residues such as oxytocin, vasopressin, substance P, neurotensin and angiotensin,7,8 which has been linked to a variety of neurological disorders, e.g. Alzheimer's disease, amnesia, depression and schizophrenia.9 A study demonstrated the increased PEP expression in the hippocampus of adult transgenic mice before the appearance of β-amyloid plaques but in parallel with the development of memory deficits.10 Altered serum PEP activity has also been reported in many psychiatric disorders, and abnormal levels of PEP activity have been found to be significantly higher in the brains of Alzheimer's patients than normal individuals.11–13 Furthermore, lowered serum PEP activity was observed in bulimia nervosa and anorexia nervosa patients.14 PEP-like immunoreactivity has also been detected in the hippocampus of senescence-accelerated mice.15,16 Therefore, PEP inhibitors are expected to be used as therapeutic agents for progressive memory deficits and cognitive dysfunction related to aging and neurodegenerative diseases of the central nervous system.Several specific PEP inhibitors have recently been developed, and those may prove valuable to treat various clinical conditions of the brain, as indicated by the neuroprotective and cognition-enhancing effects of PEP inhibitors in experimental animals.17–19 The PEP inhibitors include pramiracetam,3 baicalin,20 JTP-4819,21 KYP-204722 and S-17092.23 Most of the published inhibitors are chemically synthesized, substrate-like inhibitors that are based on the N-acyl-L-prolyl-pyrrolidine structure. But there are only a few studies on PEP inhibitors from proteins and peptides. Proteins and peptides have a wide variety of biological activities that may benefit human health by acting like antioxidant, antihypertensive and antithrombotic agents, among other activities.24,25 Bioactive peptides are small sequences of amino acids encrypted in food proteins in an inactive form that are released and activated by proteolytic enzymes during food processing or gastrointestinal digestion.26 These peptides are present in food sources such as milk, meat, eggs, soybean, wheat, maize, rice, and amaranth.24,27,28 A synthetic peptide, Ile-Tyr-Pro-Phe-Val-Glu-Pro-Ile from human β-casein could inhibit PEP activity in vitro (IC50 = 8.0 μM).29 Two γ-zein-related synthetic peptides, His-Leu-Pro-Pro-Pro-Val and His-Leu-Pro-Pro-Pro-Val-His-Leu-Pro-Pro-Pro-Val, have been shown to inhibit PEP with the IC50 values of 80 and 30 μM, respectively.30 A peptide, Met-Pro-Pro-Pro-Leu-Pro-Ala-Arg-Val-Asp-Ala-Leu-Asn, from bovine brain was determined to have great PEP inhibitory activity, and its IC50 value was 38.4 μM.31 A previous study has revealed that 6 peptides isolated from the sake cake and sake act as PEP inhibitors with IC50 values between 11.8 and 42.8 μM.32 Two peptides isolated from a red wine, and their amino acid sequences and IC50 values were Val-Glu-Ile-Pro-Glu (17 μM) and Tyr-Pro-Ile-Pro-Phe (87.8 μM).33 These peptides comprised at least one proline residue in their sequences, and the peptide length ranged from 5 to 13 amino acid residues. Therefore, the aim of this study was to investigate the PEP inhibitory activity of the enzymatic hydrolysates from various proteins rich in proline content, then isolate and identify the PEP inhibitory peptides.
Materials and methods
Materials and reagents
Wheat gluten, soy protein isolate and sodium caseinate (from cow milk) were purchased from Gemfont Corporation (Taipei, Taiwan). Tilapia fish skins, the processing byproduct recovered from fresh skin-off fillets were supplied by Fortune Life Enterprise Co. Ltd (Kaohsiung, Taiwan); the milkfish skins were donated by Simmy Seafood Co. Ltd (Long An Province, Vietnam); the halibut and Atlantic salmon (Salmo salar) skins were supplied by Albion Fisheries Ltd (Vancouver, BC, Canada). The tuna cooking juice was donated by a canned tuna processor in Chiayi County (Taiwan). Gelatin (from porcine skin) was purchased from Sigma-Aldrich, Inc. (St. Louis, Mo, USA). Bromelain (from pineapple) was purchased in dry powder form from St Bio, Inc. (Taipei, Taiwan). Thermolysin (from Bacillus thermoproteolyticus rokko), pepsin (from porcine gastric mucosa), pancreatin (from porcine pancreas), trypsin (from porcine pancreas) and Protease XXIII (from Aspergillus melleus) were obtained in dry powder form from Sigma-Aldrich, Inc. (St. Louis, Mo, USA). Prolyl endopeptidase (O9515, recombinant, expressed in E. coli), Z-Gly–Pro–4-nitroanilide (96286, Z-Gly–Pro–pNA) and bacitracin (PHR1590) were from Sigma-Aldrich, Inc. Alcalase (from Bacillus licheniformis) and Flavourzyme (from Aspergillus oryzae) were purchased from Neova Technologies Inc. (Copenhagen, Denmark). Orientase 90N (from Bacillus subtilis) was obtained in dry powder form from Hankyu Bioindustry Co. (Osaka, Japan). Other chemicals and reagents used were analytical grade and commercially available.Extraction of gelatin from fish skins
The thawed fish skins were gently washed with running tap water, drained, and cut into pieces (about 5 × 10 cm). The fish skins were soaked in 0.2 M NaOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; w/v) and stirred in a cold room at 4 °C for 30 min. This procedure was repeated three times to remove noncollagenous proteins and pigments. The skins were washed with running tap water until the pH of the rinsing water was neutral. Afterward, the skins were soaked in 0.05 M acetic acid (1
10; w/v) and stirred in a cold room at 4 °C for 30 min. This procedure was repeated three times to remove noncollagenous proteins and pigments. The skins were washed with running tap water until the pH of the rinsing water was neutral. Afterward, the skins were soaked in 0.05 M acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; w/v), stirred at room temperature for 3 h, and then washed with running tap water until the pH of the rinsing water was neutral. Almost all of the scales could be removed. The gelatin of the swollen skins was extracted in distilled, deionized water (1
10; w/v), stirred at room temperature for 3 h, and then washed with running tap water until the pH of the rinsing water was neutral. Almost all of the scales could be removed. The gelatin of the swollen skins was extracted in distilled, deionized water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; w/v) at 70 °C for 3 h. The oil and aqueous layers of the extract were separated by separatory funnels, and the extract was filtered through two layers of cheesecloth, lyophilized, and stored in a desiccator at room temperature until use.
2; w/v) at 70 °C for 3 h. The oil and aqueous layers of the extract were separated by separatory funnels, and the extract was filtered through two layers of cheesecloth, lyophilized, and stored in a desiccator at room temperature until use.
Preparation of protein hydrolysates
Wheat gluten (WG), soy protein isolate (SPI) and sodium caseinate (SC) hydrolysates were prepared using thermolysin (TLN) [E/S (enzyme/substrate) = 3%; pH 8.0; 70 °C; 20 min], bromelain (BML) (E/S = 5%; pH 6.7; 45 °C; 60 min) or gastrointestinal digestion (GID) [pepsin (E/S = 5%, pH 2.0, 37 °C, 3 h) and followed by the mixture of trypsin and pancreatin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w trypsin
1 w/w trypsin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pancreatin, E/S = 5%, pH 7.5, 37 °C, 3 h)], respectively. Tilapia fish skin gelatin (TSG), milkfish skin gelatin (MSG), halibut skin gelatin (HSG) and porcine skin gelatin (PSG) hydrolysates were prepared using Flavourzyme (FLA) (E/S = 5%, pH 7.0, 50 °C, 6 h) or Alcalase (ALC) (E/S = 5%, pH 8.0, 50 °C, 6 h), respectively. Atlantic salmon skin gelatin (SSG) was hydrolyzed by Flavourzyme (FLA) (E/S = 3%, pH 7.0, 50 °C, 4 h). Tuna cooking juice (TCJ) was hydrolyzed by protease XXIII (PA) (E/S = 2.1%, pH 7.5, 37 °C, 60 min) or Orientase 90N (OR) (E/S = 2.1%, pH 7.0, 50 °C, 60 min), respectively. After hydrolysis, the hydrolysate solutions were heated in boiling water for 15 min to inactivate the enzymes and then cooled in water at room temperature for 20 min. Hydrolysates were adjusted to pH 7.0 with 2 M NaOH and centrifuged (Centrifuge 05P-21, Hitachi Ltd, Katsuda, Japan) at 10
pancreatin, E/S = 5%, pH 7.5, 37 °C, 3 h)], respectively. Tilapia fish skin gelatin (TSG), milkfish skin gelatin (MSG), halibut skin gelatin (HSG) and porcine skin gelatin (PSG) hydrolysates were prepared using Flavourzyme (FLA) (E/S = 5%, pH 7.0, 50 °C, 6 h) or Alcalase (ALC) (E/S = 5%, pH 8.0, 50 °C, 6 h), respectively. Atlantic salmon skin gelatin (SSG) was hydrolyzed by Flavourzyme (FLA) (E/S = 3%, pH 7.0, 50 °C, 4 h). Tuna cooking juice (TCJ) was hydrolyzed by protease XXIII (PA) (E/S = 2.1%, pH 7.5, 37 °C, 60 min) or Orientase 90N (OR) (E/S = 2.1%, pH 7.0, 50 °C, 60 min), respectively. After hydrolysis, the hydrolysate solutions were heated in boiling water for 15 min to inactivate the enzymes and then cooled in water at room temperature for 20 min. Hydrolysates were adjusted to pH 7.0 with 2 M NaOH and centrifuged (Centrifuge 05P-21, Hitachi Ltd, Katsuda, Japan) at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g and 4 °C for 10 min. The supernatant was lyophilized and stored at −20 °C.
000g and 4 °C for 10 min. The supernatant was lyophilized and stored at −20 °C.
PEP inhibitory activity assay
PEP activity determination in this study was performed in 96-well microplates by measuring the increase in absorbance at 410 nm using Z-Gly–Pro–pNA as the PEP substrate and according to the method described by the previous study34 with some modifications. The unit of PEP activity is defined as one unit will hydrolyze 1.0 pmol of Ala–Pro–aminomethylcoumarin per minute at pH 7.5 at 25 °C. The lyophilized samples were dissolved in 100 mM Tris-HCl buffer (pH 7.0) at various concentrations (0.1–5 mg mL−1). The hydrolysate solution (25 μL) was added with 50 μL of 2.5 mM Z-Gly–Pro–pNA (in 40% 1,4-dioxane) and 100 μL of 100 mM Tris buffer (pH 7.0). The mixture was incubated at 30 °C for 10 min, followed by the addition of 50 μL of 0.5 units per mL PEP. The reaction mixture (total volume of 225 μL) was incubated at 30 °C for up to 60 min and the absorbance of the resulting solution was measured at 410 nm with an ELISA reader (Bio Tek μ QUANT; Bio Tek Instruments, Inc., Winooski, VT, USA). Recorded data were plotted versus time, and the PEP activity was quantified from the linear part of the curve. The % PEP inhibition was defined as the percentage of PEP activity inhibited by a given concentration of the hydrolysate. The IC50 value corresponds to the concentration of the sample needed to inhibit PEP by 50%. Bacitracin was used as positive control. The mode of inhibition of all the isolated peptides was investigated using Lineweaver and Burk kinetic analysis by measuring the initial rate of the reaction at different Z-Gly–Pro–pNA concentrations between 0.5 and 2.5 mM without inhibitors and in the presence of peptides at their IC50 concentrations. Km and Vmax values were deducted from the Lineweaver and Burk double reciprocal plots. The mode of inhibition was determined by comparing Km and Vmax obtained in the presence and absence of the inhibitors.Ultrafiltration (UF)
The peptides of the hydrolysates were fractionated by ultrafiltration (model ABL085, Lian Sheng Tech. Co., Taichung, Taiwan) with spiral wound membranes having molecular mass cutoffs of 2.5 and 1.0 kDa. The fractions were collected as follows: >2.5 kDa, peptides retained without passing through 2.5 kDa membrane; 1.0–2.5 kDa, peptides permeating through the 2.5 kDa membrane but not the 1.0 kDa membrane; <1.0 kDa, peptides permeating through the 1.0 kDa membrane. All fractions collected were lyophilized and stored in a desiccator until use.Purification of PEP inhibitory peptides
The fractionated hydrolysates were purified using reversed-phase high performance liquid chromatography (RP-HPLC) (Model L-2130 HPLC, Hitachi Ltd, Katsuda, Japan). The lyophilized hydrolysate fraction (2 mg) was dissolved in 1 mL of ddH2O, and 60 μL of the mixture was then injected into a column (ZORBAX Eclipse Plus C18, 4.6 mm × 250 mm, Agilent Tech. Inc., CA, USA) using a linear gradient of acetonitrile (5–40% in 120 min) in 0.1% TFA under a flow rate of 0.7 mL min−1. The peptides were detected at 215 nm. Each collected fraction was then lyophilized and stored in a desiccator until use. The fractions were dissolved in 100 mM Tris buffer (pH 7.0) up to the concentration of 1 mg solid per mL, and then the solution was used to determine the PEP inhibitory activity. For obtaining a sufficient amount of samples to perform the PEP inhibition assay, this process was done repeatedly.Identification of amino acid sequence by MALDI-TOF/TOF MS/MS
The purified peptides were analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS), using a delayed extraction source and a 335 nm pulsed nitrogen laser. This analysis was carried out using a MALDI-TOF/TOF (UltraFlexIII, Bruker Daltonics Inc., Billerica, MA, USA). The peptide solution (0.6 μL) was mixed with 0.6 μL of saturated α-cyano-4-hydroxycinnamic acid, and a droplet of the resulting solution was placed on the sample target mass spectrometer. The droplet was dried by evaporation at room temperature and then loaded into the mass spectrometer for analysis. The instrument was operated in positive ion reflection mode with the source voltage set at 20 kV. All spectra were the results of signal averaging of 200 shots. Measurements were determined in the mass range m/z 200–4000 Da, while the peptide sequencing was determined by MS/MS spectra processing, using BioTools (Version 3.2; Bruker Daltonics Inc., Billerica, MA, USA).Peptide synthesis
Peptides were prepared by the conventional Fmoc solid-phase synthesis method with an automatic peptide synthesizer (Model CS 136, CS Bio Co. San Carlos, CA, USA), and their purity was verified by analytical RP-HPLC-MS/MS.Statistical analysis
Each data point represents the mean of three samples and was subjected to analysis of variance (ANOVA) using SAS software version 9.1 (SAS Institute Inc., Cary, NC, USA). A test of comparison of two means was analysed by Duncan's test, and the significance level of P < 0.05 was employed.Results and discussion
PEP inhibitory activity of protein hydrolysates
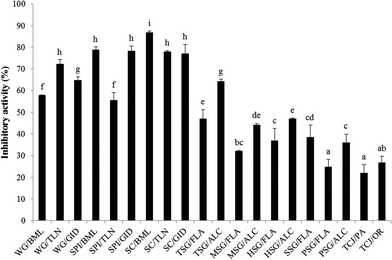
The PEP inhibitory activity of the hydrolysates from wheat gluten (WG/BML; WG/TLN; WG/GID), soy protein isolate (SPI/BML; SPI/TLN; SPI/GID), sodium caseinate (SC/BML; SC/TLN; SC/GID), fish skin gelatin (TSG/FLA; TSG/ALC; MSG/FLA; MSG/ALC; HSG/FLA; HSG/ALC; SSG/FLA), porcine skin gelatin (PSG/FLA; PSG/ALC) and tuna cooking juice (TCJ/PA; TCJ/OR) at the concentration of 5 mg mL−1 is shown in Fig. 1. The PEP inhibition rates of the wheat gluten, soy protein isolate and sodium caseinate hydrolysates were 57.9–72.4%, 55.5–78.8% and 77.1–86.8%, respectively; the inhibitory activity of fish skin gelatin, porcine skin gelatin and tuna cooking juice hydrolysates were 32.2–64.4%, 24.8–36.1% and 22.1–26.8%, respectively. To our knowledge, the proteins rich in proline content may have the potential to be the source of PEP inhibitory peptides. Therefore, the hydrolysates from wheat gluten, sodium caseinate, fish and porcine skin gelatin which contain >10 mol% of proline were expected to possess great PEP inhibitory activity.35–37 From the results shown in Fig. 1, WG/TLN, SPI/BML, SPI/GID, SC/BML, SC/TLN and SC/GID showed relatively high PEP inhibitory activity; but surprisingly, all the skin gelatin hydrolysates performed fair action on PEP inhibition. Furthermore, the hydrolysates from the same protein hydrolyzed by different proteases showed various PEP inhibitory activities. We inferred that the peptide structures determined the PEP-inhibitory activity of hydrolysates. The sodium caseinate hydrolyzed with bromelain (SC/BML) showed significantly higher PEP inhibitory activities (86.8%, P < 0.05) than the other hydrolysates. Therefore, SC/BML was used for further purification.PEP inhibitory activity of UF fractions of hydrolysates
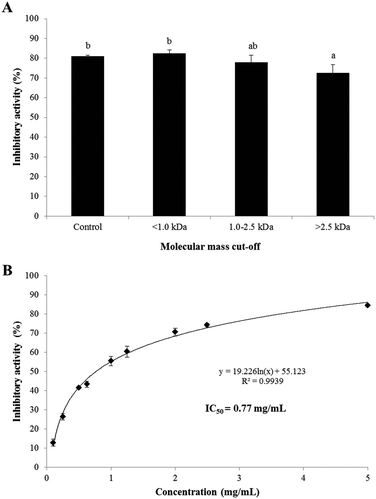
The PEP inhibitory activities of the UF fractions (>2.5 kDa, 1.0–2.5 kDa, and <1.0 kDa) of SC/BML at the concentration of 2.5 mg mL−1 are shown in Fig. 2. The result showed that the <1.0 kDa, 1.0–2.5 kDa fractions and SC/BML (control) had insignificantly different (P > 0.05) and higher PEP inhibition rates between 77.8 and 82.5% than the >2.5 kDa fraction which displayed the inhibition rates of 72.6% (Fig. 2A). To our knowledge, the majority of the kinetic studies performed on PEP enzymes have been limited to short, synthetic substrates of 2–6 residues or slightly longer neuropeptides of <15 residues with only one or two internal proline residues.38,39 To date, it is generally thought that the chain length specificity of most PEPs is capped at 30 amino acid residues, which shows that PEP can degrade peptides of molecular weight less than 3.0 kDa.8,38 As reviewed in the previous studies, the length of the PEP inhibitory peptides ranged from 3–18 amino acid residues, and these peptides comprised at least one and up to 6 proline residues in their sequences.29,31,33 Therefore, the PEP inhibitory peptides are proposed to be determined majorly by the appearance of the proline residue in the sequence rather than the peptide length. Based on the PEP inhibitory activity of the UF fractions not showing greater than the hydrolysate, SC/BML was used for further purification by RP-HPLC and identification of peptide sequences. The IC50 value of the SC/BML was determined and shown in Fig. 2B. The PEP inhibitory activities of SC/BML at various concentrations (0.1–5 mg mL−1) ranged from 12.8 to 84.4% in a dose-dependent manner, and the IC50 value was 0.77 mg mL−1.Purification of PEP inhibitory peptides by RP-HPLC
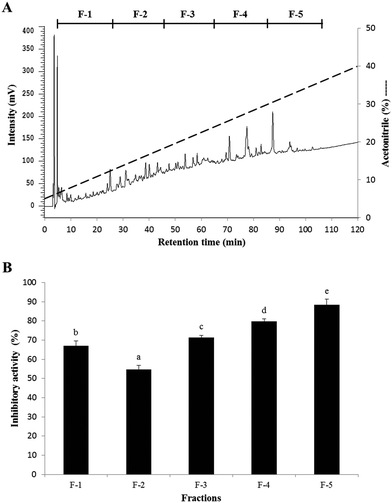
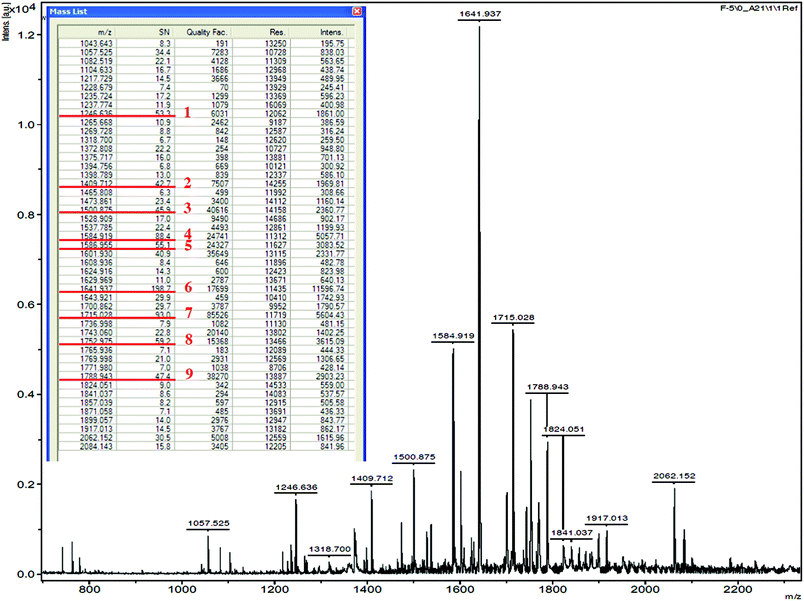
The elution profile and PEP inhibitory activity of the fractions from SC/BML separated by RP-HPLC are shown in Fig. 3. To obtain a sufficient amount of purified peptide, chromatographic separations were performed repeatedly. Five fractions (F-1 to F-5) were obtained upon RP-HPLC separation of SC/BML (Fig. 3A), and they were lyophilized and then used to determine their PEP inhibitory activities at the concentration of 1 mg solid per mL. The result showed that F-5 had the highest PEP inhibition rate of 88.4%, as compared to the others which showed the inhibition rates between 54.7 and 79.8% (Fig. 3B). Therefore, F-5 was used to identify the amino acid sequences of the peptides.The fraction F-5 was used to identify the amino acid sequences of peptides by MALDI-TOF/TOF MS. Forty-seven peaks were obtained, and the nine major peaks (m/z ranged from 1246.636 to 1788.943) with strong intensity were selected for MS/MS analysis (Fig. 4). After the analysis by MS/MS spectra processing with BioTools database, the amino acid sequences of the 9 peptides are listed in Table 1. These peptides obtained in this study comprised 13 to 20 amino acid residues, and moreover, they were composed of at least one and up to 5 internal proline residues in their sequence. The result therefore is consistent with the hypothesis that the PEP inhibitory peptides exhibit at least one proline demonstrated in the previous study.38
| No. | Peptide sequence | No. of proline | Calculated mass/observed mass | Origin | IC50 (μM) | Type of inhibition |
|---|---|---|---|---|---|---|
| 1 | CLVAVALARPKHPIKHQGLP | 3 | 1787.86/1787.94 | β-Casein | 265.6 | Competitive |
| 2 | QKEDVPSERYLGYLEQL | 1 | 1751.85/1751.97 | α-S1-Casein | 368.7 | Competitive |
| 3 | AVPYPQRDMPIQAFLLY | 3 | 1713.93/1714.02 | β-Casein | 48.2 | Competitive |
| 4 | PIHNSLPQNIPPLTQTPV | 4 | 1640.85/1640.93 | β-Casein | 29.8 | Competitive |
| 5 | PVQPFTESQSLTLTDVE | 2 | 1585.80/1585.95 | β-Casein | 236.3 | Competitive |
| 6 | TIASAEPTVHSTPTTEAIV | 2 | 1583.90/1583.91 | κ-Casein | 123.6 | Competitive |
| 7 | VPLGTQYTDAPSFSDIP | 3 | 1499.81/1499.87 | α-S1-Casein | 650.5 | Competitive |
| 8 | SIITSTPETPTVAVPTT | 3 | 1408.60/1408.71 | κ-Casein | 79.8 | Competitive |
| 9 | HPHPHLSFMAIPP | 4 | 1245.67/1245.63 | κ-Casein | 34.8 | Competitive |
| 10 | Bacitracin (positive control) | 124.6 |
PEP inhibitory activity of synthetic peptides
The molecular mass, origin and IC50 values against PEP of the 9 synthetic peptides are shown in Table 1. Based on the specificity of bromelain with the preference for cleaving the C-terminus of Lys, Ala and Tyr,40 the peptide no. 3, AVPYPQRDMPIQAFLLY, was obtained by bromelain cleavage; however, the other peptides might be obtained by other proteases as the impurity of bromelain used in the present study. The identification by MALDI-TOF/TOF MS/MS of these peptides showed homology with the α-S1, β-, and κ-casein molecules as compared to BIOPEP database (http://www.uwm.edu.pl). The molecular mass of these peptides ranged from 1245.63 to 1787.94, and the IC50 values against PEP varied from 29.8 to 650.6 μM. The PEP inhibitory activities of these peptides were not correlated to their molecular mass; peptides with more proline residues, however, showed lower IC50 values. For example, HPHPHLSFMAIPP (1245.63 Da) comprising 4 proline residues showed the IC50 value of 34.8 μM, while QKEDVPSERYLGYLEQL (1751.97 Da) with 1 proline residue exhibited the IC50 value of 368.7 μM. But VPLGTQYTDAPSFSDIP (1499.87 Da) with 3 proline residues had a higher IC50 value of 650.5 μM than TIASAEPTVHSTPTTEAIV (1583.91 Da) with only 2 proline residues (123.6 μM). We suggested that the PEP inhibitory activity of peptides might be determined by the number of proline residues and also the next amino acid residue binding to the C-terminal of proline. These peptides all behaved as competitive inhibitors of PEP (Table 1 and Fig. 5). The bacterial PEPs were reported to prefer the cleavage sites on Pro–Gln, Pro–Tyr and Pro–Phe bonds.38,41 In previous studies, Ile-Tyr-Pro-Phe-Val-Glu-Pro-Ile from human β-casein,29 Tyr-Pro-Ile-Pro-Phe from a red wine,33 Ser-Pro-Phe-Trp-Asn-Ile-Asn-Ala, Leu-Ser-Pro-Phe-Trp-Asn-Ile-Asn-Ala and Leu-Leu-Ser-Pro-Phe-Trp-Asn-Ile-Asn-Ala from the sake cake32 were reported to show PEP inhibitory activities with IC50 values ranging from 8.0 to 87.8 μM, and all the peptides comprised Pro–Phe in their sequences. While other PEP inhibitory peptides having Pro–Pro, Pro–Val, Pro–Leu, Pro–Ala, Pro-Glu-Pro-Ile, Pro–Ser, Pro–Asn and Pro–Arg in their sequences also showed low IC50 values between 11.8 and 80.0 μM.30–33 In the present study, three peptides AVPYPQRDMPIQAFLLY, PIHNSLPQNIPPLTQTPV and HPHPHLSFMAIPP, having Pro–Tyr, Pro–Gln, Pro–Ile, Pro–Pro, Pro–Val, Pro–His in their sequence, showed greater PEP inhibitory activities than the others with their IC50 values below 50 μM. A previous study has demonstrated that the substrate accessibility to the PEP active site, including substrate flexibility and inter-domain dynamics, appears to be the primary factor that limits PEP specificity.38 In the present study, we have successfully isolated, novel and potent PEP inhibitory peptides, which act as substrate-like inhibitors, from sodium caseinate.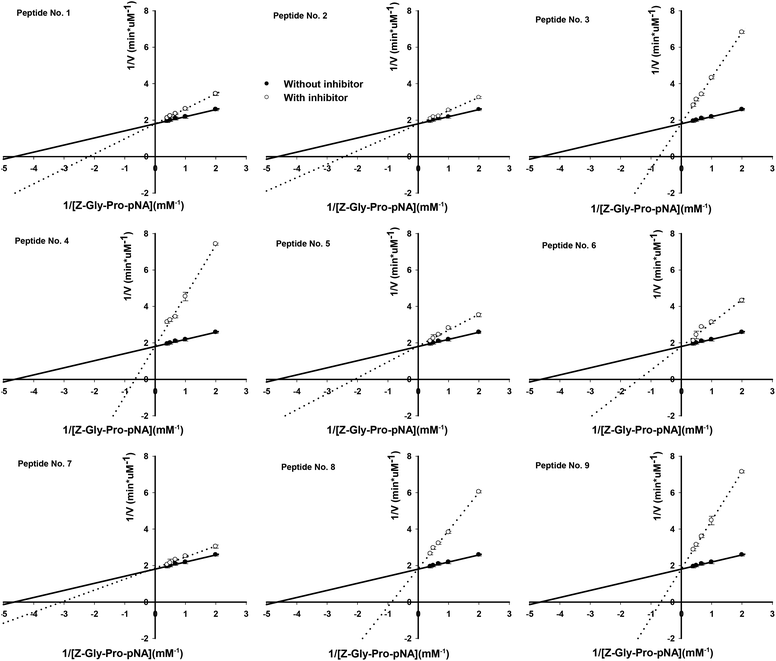 | ||
| Fig. 5 Lineweaver and Burk double reciprocal plots for PEP inhibition with the 9 synthetic peptides. Values are the mean of three determinations ± SD. | ||
Although the nine peptides isolated in the present study present in vitro PEP inhibitory activities, a challenge of the bioavailability of these peptides is to resist the degradation of digestive enzymes followed by passing through the gastrointestinal (GI) epithelium.42 The long-chain peptides are supposed to be hydrolysed to small peptides or free amino acids and then absorbed by the GI tract. In addition, the blood–brain barrier (BBB) is another formidable challenge. Therefore, further studies on the bioavailability of these peptides and/or the bioavailability improvement of the peptides modified by chemical methods43 are needed to evaluate the development of these peptides as therapeutic agents of neurological disorders.
Conclusions
Enzymatic hydrolysates from various protein sources were used to determine their PEP inhibitory activity in the present study. SC/BML from sodium caseinate had a superior inhibitory effect on PEP. Nine novel peptides were successfully isolated and identified, and they all acted as competitive inhibitors of PEP. This study indicates that sodium caseinate has the potential to be the source of the functional food for prevention of neurological disorders. A future study for the bioavailability of the peptides is needed to evaluate their therapeutic application.Acknowledgements
This study was financially supported by China Asia Associate University (Project no. CMU102-ASIA-13).Notes and references
- R. Walter, W. H. Simmons and T. Yoshimoto, Proline specific endo-and exopeptidases, Mol. Cell. Biochem., 1980, 30, 111–127 CrossRef CAS.
- D. F. Cunningham and B. O'Connor, Proline specific peptidases, Biochim. Biophys. Acta, 1997, 1343, 160–186 CrossRef CAS.
- T. Yoshimoto, K. Kado, F. Matsubara, N. Koriyama, H. Kaneto and D. Tsuru, Specific inhibitors for prolyl endopeptidase and their anti-amnesic effect, J. Pharmacobiodyn., 1987, 10, 730–735 CrossRef CAS PubMed.
- T. T. Myöhänen, J. I. Venäläinen, J. A. Garcia-Horsman, M. Piltonen and P. T. Männisto, Distribution of prolyl oligopeptidase in the mouse whole-body sections and peripheral tissues, Histochem. Cell Biol., 2008, 130, 993–1003 CrossRef PubMed.
- M. J. Moreno-Baylach, K. A. Puttonen, J. Tenorio-Laranga, J. I. Venäläinen, M. Storvik, M. M. Forsberg and J. A. García-Horsman, Prolyl endopeptidase is involved in cellular signaling in human neuroblastoma SH-SY5Y cells, Neurosignals, 2011, 19, 97–109 CrossRef CAS PubMed.
- I. Schulz, U. Zeitschel, T. Rudolph, D. Ruiz-Carrillo, J. U. Rahfeld, B. Gerhartz, V. Bigl, H.-U. Demuth and S. Roβner, Subcellular localization suggests novel functions for prolyl endopeptidase in protein secretion, J. Neurochem., 2005, 94, 970–979 CrossRef CAS PubMed.
- D. Rennex, B. A. Hemmings, J. Hofsteenge and S. R. Stone, cDNA cloning of porcine brain prolyl endopeptidase and identification of the active-site seryl residue, Biochem., 1991, 30, 2159–2203 Search PubMed.
- T. Yoshimoto, T. Nishimura, T. Kita and D. Tsuru, Post-proline cleaving enzyme (prolyl endoeptidase) from bovine brain, J. Biochem., 1983, 94, 1179–1190 CAS.
- L. Polgar, Structure-function of prolyl oligopeptidase and its role in neurological disorders, Curr. Med. Chem., 2002, 2, 251–257 CAS.
- S. Roβner, I. Schulz, U. Zeitschel, R. Schliebs, V. Bigl and H. U. Demuth, Brain prolyl endopeptidase expression in aging, APP transgenic mice and Alzheimer's disease, Neurochem. Res., 2005, 30, 695–702 CrossRef PubMed.
- T. Aoyagi, T. Wada, M. Nagai, F. Kojima, S. Harada, T. Takeuchi, K. Hirokawa and T. Tsumita, Deficiency of kallikrein-like enzyme activities in cerebral tissue of patients with Alzheimer's disease, Experientia, 1990, 46, 94–97 CrossRef CAS PubMed.
- C. Ichai, N. Chevallier, P. Delaere, P. Dournaud, J. Epelbaum, J. J. Hauw, J.-P. Vincent and F. Checler, Influence of region specific alterations of neuropeptidase content on the catabolic fates of neuropeptides in Alzheimer's disease, J. Neurochem., 1994, 62, 645–655 CrossRef CAS PubMed.
- M. Maes, F. Goossens, S. Scharpe, J. Calabrese, R. Desnyder and H. Y. Meltzer, Alteration in plasma prolyl endopeptidase activity in depression, mania, and schizophrenia: Effect of antidepressants, mood stabilizers, and antipsychotic drugs, Psychiatry Res., 1995, 58, 217–225 CrossRef CAS PubMed.
- M. Maes, P. Monteleone, R. Bencivenga, F. Goossens, M. Maj, D. van West, E. Bosmans and S. Scharpe, Lower serum activity of prolyl endopeptidase in anorexia and bulimia nervosa, Psychoneuroendocrinology, 2001, 26, 17–26 CrossRef CAS PubMed.
- A. Fukunari, A. Kato, Y. Sakai, T. Yoshimoto, S. Ishiura, K. Suzuki and T. Nakajima, Colocalization of prolyl endopeptidase and amyloid b-peptide in brains of senescence-accelerated mouse, Neurosci. Lett., 1994, 176, 201–204 CrossRef CAS PubMed.
- J. Wilson, M. Hayes and B. Carney, Angiotensin-I-converting enzyme and prolyl endopeptidase inhibitory peptides from natural sources with a focus on marine processing by-products, Food Chem., 2011, 129, 235–244 CrossRef CAS.
- K. Toide, Y. Iwamoto, T. Fujiwara and H. Abe, JTP-4819: a novel prolyl endopeptidase inhibitor with potential as a cognitive enhancer, J. Pharmacol. Exp. Ther., 1995, 274, 1370–1378 CAS.
- M. Shinoda, A. Matsuo and K. Toide, Pharmacological studies of a novel prolyl endopeptidase inhibitor, JTP-4819, in rats with middle cerebral artery occlusion, Eur. J. Pharmacol., 1996, 305, 31–38 CrossRef CAS PubMed.
- Y. Shishido, M. Furushiro, S. Tanabe, S. Shibata, S. Hashimoto and T. Yokokura, Effects of prolyl endopeptidase inhibitors and neuropeptides on delayed neuronal death in rats, Eur. J. Pharmacol., 1999, 372, 135–142 CrossRef CAS PubMed.
- T. Tarragó, N. Kichik, B. Claasen, R. Prades, M. Teixidó and E. Giralt, Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor, Bioorg. Med. Chem., 2008, 16, 7516–7524 CrossRef PubMed.
- K. Toide, M. Shinoda and A. Miyazaki, A novel prolyl endopeptidase inhibitor, JTP-4819–its behavioral and neurochemical properties for the treatment of Alzheimer's disease, Rev. Neurosci., 1998, 9, 17–29 CAS.
- A. J. Jalkanen, K. A. Puttonen, J. I. Venäläinen, V. Sinervä, A. Mannila, S. Ruotsalainen, E. M. Jarho, A. A. Wallén and P. T. Männistö, Beneficial effect of prolyl oligopeptidase inhibition on spatial memory in young but not in old scopolamine-treated rats, Basic Clin. Pharmacol. Toxicol., 2007, 100, 132–138 CAS.
- P. Morain, P. Lestage, G. De Nanteuil, R. Jochemsen, J. L. Robin, D. Guez and P.-A. Boyer, S 17092: a prolyl endopeptidase inhibitor as a potential therapeutic drug for memory impairment. Preclinical and clinical studies, CNS Drug Rev., 2002, 8, 31–52 CrossRef CAS PubMed.
- K. Erdmann, B. W. Y. Cheung and H. Schröder, The possible roles of foodderived bioactive peptides in reducing the risk of cardiovascular disease, J. Nutr. Biochem., 2008, 19, 643–654 CrossRef CAS PubMed.
- A. G. P. Samaranayaka and E. C. Y. Li-Chan, Food-derived peptidic antioxidantas: A review of their production, assessment, and potential applications, J. Funct. Foods, 2011, 3, 229–254 CrossRef CAS.
- N. P. Möller, K. E. Scholz-Ahrens, N. Roos and J. Schrezenmeir, Bioactive peptides and proteins from foods: indication for health effects, Eur. J. Nutr., 2008, 47, 171–182 CrossRef PubMed.
- B. F. Gibbs, A. Zougman, R. Masse and C. Mulligan, Production and characterization of bioactive peptides from soy hydrolysate and soy fermented food, Food Res. Int., 2004, 37, 123–131 CrossRef CAS.
- C. Silva-Sánchez, A. P. Barba de la Rosa, M. F. León-Galván, B. O. de Lumen, A. De León-Rodríguez and E. G. de Mejía, Bioactive peptides in amaranth (Amaranthus hypochondriacus L.) seeds, J. Agric. Food Chem., 2008, 56, 1233–1240 CrossRef PubMed.
- M. Asano, N. Nio and Y. Ariyoshi, Inhibition of prolyl endopeptidase by synthetic peptide fragments of human β-casein, Agric. Biol. Chem., 1991, 55, 825–828 CrossRef CAS PubMed.
- S. Maruyama, S. Miyoshi, T. Osa and H. Tanaka, Prolyl endopeptidase inhibitory activity of peptides in the repeated sequence of various Proline-rich proteins, J. Biosci. Bioeng., 1992, 74, 145–148 CrossRef CAS.
- T. Ohmori, T. Nakagami, H. Tanaka and S. Maruyama, Isolation of prolylendopeptidase-inhibiting peptides from bovine brain, Biochem. Biophys. Res. Commun., 1994, 202, 809–815 CrossRef CAS PubMed.
- Y. Saito, S. Ohura, A. Kawato and K. Suiginami, Prolyl endopeptidase inhibitors in sake and its byproducts, J. Agric. Food Chem., 1997, 45, 720–724 CrossRef CAS.
- T. Yanai, Y. Suzuki and M. Sato, Prolyl endopeptidase inhibitory peptides in wine, Biosci., Biotechnol., Biochem., 2003, 67, 380–382 CrossRef CAS PubMed.
- T. Yoshimoto, K. Ogita, R. Walter, M. Kodia and D. Tsuru, Post-proline cleaving enzyme, synthesis of a new fluorogenic substrate and distribution of the endopeptidase in rat tissues and body fluids of man, Biochim. Biophys. Acta, 1979, 569, 184–192 CrossRef CAS.
- I. Rombouts, L. Lamberts, I. Celus, B. Lagrain, K. Brijs and J. A. Delcour, Wheat gluten amino acid composition analysis by high-performance anion-exchange chromatography with integrated pulsed amperometric detection, J. Chromatogr., A, 2009, 1216, 5557–5562 CrossRef CAS PubMed.
- E. C. Y. Li-Chan, S. L. Huang, C. L. Jao, K. P. Ho and K. C. Hsu, Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors, J. Agric. Food Chem., 2012, 60, 973–978 CrossRef CAS PubMed.
- G. M. Ellinger and E. B. Boyne, Amino acid composition of some fish products and casein, Br. J. Nutr., 1965, 19, 587–592 CrossRef CAS PubMed.
- J. Gass and C. Khosla, Biomedicine & diseases: review prolyl endopeptidases, Cell. Mol. Life Sci., 2007, 64, 345–355 CrossRef CAS PubMed.
- R. Walter and T. Yoshimoto, Postproline cleaving enzyme: kinetic studies of size and stereospecificity of its active site, Biochemistry, 1978, 17, 4139–4144 CrossRef CAS PubMed.
- J. Alder-Nissen, Enzyme hydrolysis of food proteins, Elsevier Applied Science Publisher, New York, 1986 Search PubMed.
- L. Shan, T. Marti, L. M. Sollid, G. M. Gray and C. Khosla, Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for coeliac sprue, Biochem. J., 2004, 383, 311–318 CrossRef CAS PubMed.
- J. Renukuntla, A. D. Vadlapudi, A. Patel, S. H. S. Boddu and A. K. Mitra, Approaches for enhancing oral bioavailability of peptides and proteins, Int. J. Pharm., 2013, 447, 75–93 CrossRef CAS PubMed.
- M. Segura-Campos, L. Chel-Guerrero, D. Betancur-Ancona and M. Hernandez-Escalante, Bioavailability of bioactive peptides, Food Rev. Int., 2011, 27, 213–226 CrossRef CAS.
Footnote |
| † Equal contribution to this study. |
| This journal is © The Royal Society of Chemistry 2016 |