Food matrix and processing influence on carotenoid bioaccessibility and lipophilic antioxidant activity of fruit juice-based beverages
María Janeth
Rodríguez-Roque
a,
Begoña
de Ancos
b,
Rogelio
Sánchez-Vega
a,
Concepción
Sánchez-Moreno
b,
M. Pilar
Cano
c,
Pedro
Elez-Martínez
a and
Olga
Martín-Belloso
*a
aDepartment of Food Technology, University of Lleida, Agrotecnio Center, Av. Alcalde Rovira Roure 191, 25198, Lleida, Spain. E-mail: omartin@tecal.udl.es; Fax: +34 973 702596; Tel: +34 973 702593
bDepartment of Characterization, Quality and Safety, Institute of Food Science, Technology and Nutrition (ICTAN), Spanish National Research Council (CSIC), C/José Antonio Novais 10, 28040 Madrid, Spain. Fax: +34-91-5493627; Tel: +34-91-5492300
cDepartment of Food Biotechnology and Microbiology, Institute of Food Science Research (CIAL, SIC-UAM), C/Nicolás Cabrera 9, Campus de la Universidad Autónoma de Madrid, 28049 Madrid, Spain. Fax: +34-91-0017905; Tel: +34-91-0017900
First published on 12th October 2015
Abstract
The biological activity of carotenoids depends on their bioaccessibility and solubilization in the gastrointestinal tract. These compounds are poorly dispersed in the aqueous media of the digestive tract due to their lipophilic nature. Thus, it is important to analyze the extent to which some factors, such as the food matrix and food processing, may improve their bioaccessibility. Beverages formulated with a blend of fruit juices and water (WB), milk (MB) or soymilk (SB) were treated by high-intensity pulsed electric fields (HIPEF) (35 kV cm−1 with 4 μs bipolar pulses at 200 Hz for 1800 μs), high-pressure processing (HPP) (400 MPa at 40 °C for 5 min) or thermal treatment (TT) (90 °C for 1 min) in order to evaluate the influence of food matrix and processing on the bioaccessibility of carotenoids and on the lipophilic antioxidant activity (LAA). The bioaccessibility of these compounds diminished after applying any treatment (HIPEF, HPP and TT), with the exception of cis-violaxanthin + neoxanthin, which increased by 79% in HIPEF and HPP beverages. The lowest carotenoid bioaccessibility was always obtained in TT beverages (losses up to 63%). MB was the best food matrix for improving the bioaccessibility of carotenoids, as well as the LAA. The results demonstrate that treatment and food matrix modulated the bioaccessibility of carotenoids as well as the lipophilic antioxidant potential of beverages. Additionally, HIPEF and HPP could be considered as promising technologies to obtain highly nutritional and functional beverages.
Introduction
Functional beverages are becoming more and more popular because they help maintain well-being and health.1 These beverages are generally made from fruits in combination with or without dairy and/or soy-derived products, which naturally provide great amounts of health-promoting compounds.2,3 Fruit juices retain the physicochemical and organoleptical features of the fruits from which they are produced. As a result, fruit juices represent an easy and convenient way for increasing the consumption of bioactive compounds. In addition, mixing different fruit juices allows increasing the concentration of selected bioactive compounds, adding new nutrients or improving the flavour and appearance of these beverages. For this reason, a variety of functional beverages are available in the market to suit different lifestyles of consumers, as well as to satisfy their preferences for tasty, nutritious, healthy and convenient products.4Carotenoids are a widespread family of fat-soluble plant pigments. They have been shown to play an important role in human health because of their powerful antioxidant potential and because some of them possess provitamin A activity. These compounds have been associated with immune system enhancement, antiaging, antiinflammation, antiulcer and anticancer properties.5 The main food sources of carotenoids are yellow and orange fruits, dark green vegetables and dairy products.6 Among the most utilized ingredients for producing beverages with functional properties fruit juices and milk stand out, which are considered as wholesome and nutrient-rich foods. Therefore, functional beverages based on these foodstuff could also contribute to carotenoids intake. In many cases, soymilk is utilized as surrogate of milk by consumers who experience lactose intolerance, protein milk allergy or galactosemia.7 Although soymilk does not contain carotenoids, it is an important source of other nutrients, such as phenolic compounds and isoflavones.8,9
It has been stated that the beneficial effect of food on human health comes from the antioxidant activity of bioactive compounds contained in these products.10 Particularly, carotenoids have potential antioxidant properties because they quench singlet oxygen.11 For this purpose, it is also interesting to evaluate the lipophilic antioxidant activity of these kinds of products.
Thermal treatment (TT) has widely been used to preserve food and beverages because of its excellent performance against microorganisms. Nevertheless, nutritional and sensorial features of food are affected by the high temperatures reached during this treatment.12 In order to satisfy the increased demand of consumers for nutritious, healthy and tasty products, the food industry and food researchers are looking for processing methods that do not compromise all these important characteristics. Non-thermal food processing technologies, such as high-intensity pulsed electric fields (HIPEF) or high-pressure processing (HPP), have widely been researched in the last decade because they are alternatives to heat treatment.13–16
Bioaccesibility is defined as the portion of nutritients or bioactive compounds that is released from the food matrix into the gastrointestinal tract and thus become available for intestinal absorption.17 Therefore, although functional beverages contain important amounts of nutrients, it does not mean that all this quantity can be absorbed. In particular, the availability of lipophilic constituents is limited because the hydrophobic nature of these compounds avoids their dispersion in the aqueous media of the digestive tract.18 Carotenoids must be first released from the food matrix, dispersed in the digestive tract and solubilised into mixed micelles so that they are available for absorption. Thus, the formation of micelles is one of the most important factors that affect the absorption of carotenoids.5 Bioaccessibility of nutrients is usually evaluated by in vitro gastrointestinal digestion19 and represents a useful and fast approach previous to in vivo trials.
Processing involves changes in the microstructure of food (i.e. the disruption of cell walls and membranes), as well as in the release of carotenoids from carotenoid–protein complexes, and in their solubilisation (free and ester forms).6 All these changes may modify the bioaccessibility of these nutrients. In addition to food processing, the surrounding environment in which carotenoids are contained also impacts their bioaccessibility because the interactions between carotenoid–carotenoid and/or carotenoid–food constituents (i.e. fiber and fat) could occur.20 As a result, it is important to know the concentration of bioactive compounds that is accessible for absorption after digestion and the extent to which food processing and food matrix may change their bioaccessibility. Recently, the bioaccessibility of carotenoids from single food matrices (i.e. mango, carrot, sweet potato, tomato, pungent peppers, papaya and orange juice) has been reviewed by Lemmens et al.21 There is also some information available about the influence of food processing on the bioaccessibility of carotenoids.22–26 However, to the best of our knowledge this is the first study focused on evaluating the influence of both factors (food matrix and food processing) on the bioaccessibility of carotenoids from complex matrices. For this reason, this research aimed to analyze the influence of food matrix (milk, soymilk and water) and food processing (HIPEF, HPP and TT) on the in vitro bioaccessibility of carotenoids and on the lipophilic antioxidant activity (LAA) of blended fruit juice-based beverages.
Material and methods
Materials and reagents
Pepsin from porcine stomach (≥250 units per mg solid, P7000), pancreatin from porcine pancreas (P7545), bovine bile (B3883), carotenoid standards (α-carotene 50887 purity ≥98.0%, β-carotene C4582 purity ≥95.0%, zeaxanthin 14681 purity ≥95.0%, lutein 07168 purity ≥97.0% and β-cryptoxanthin C6368 purity ≥97.0%) and the 1,1-diphenyl-2-picrylhydrazyl (DPPH˙) radical were purchased from Sigma-Aldrich (St Louis, MO, USA). The radical 1,1-diphenyl-2-picrylhydrazyl (DPPH˙) and the cellulose dialysis membrane (molecular weight cutoff of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) were acquired from Sigma-Aldrich (St Louis, MO, USA).
000 Da) were acquired from Sigma-Aldrich (St Louis, MO, USA).
Fruit juice-based beverages
Three beverages were prepared by mixing 75% of a blended fruit juice (orange (Valencia variety), kiwi (Hayward variety), pineapple (extra sweet variety) and mango (Palmer variety)), 17.5% of milk (milk-fruit juice beverage, MB), or soymilk (soymilk-fruit juice beverage, SB), or distilled water (water-fruit juice beverage, WB), and 7.5% of sugar. The pH of the beverages was adjusted to 3.30 ± 0.20 (Crison Instruments S.A., Alella, Barcelona, Spain) with citric acid. The soluble solid content was analyzed using a refractometer Comecta S.A., Abrera (Barcelona, Spain), obtaining 18.0 ± 0.2, 18.5 ± 0.2, 19.3 ± 0.3 °Brix for WB, SB and MB, respectively. Beverage formulations were selected based on a previous study, where similar concentrations of these fruit juices resulted in a high bioaccessibility of bioactive compounds.28Fruits (orange, kiwi, pineapple and mango) were purchased at commercial maturity from a local supermarket (Lleida, Spain). These fruits were washed, peeled and the juice was extracted. The freshly-squeezed juice was filtered with a cheesecloth using a vacuum pump. A blended fruit juice was obtained by mixing 40% of orange, 33% of kiwi, 13.5% of pineapple and 13.5% of mango juices.
Whole milk (Hacendado, Córdoba, Spain) and soymilk (Yosoy, Girona, Spain) were purchased from a local supermarket. According to the manufacturers, whole milk contained 3.6% of fat, 3.0% of protein and 4.5% of carbohydrates, while 1.8% of fat, 3.6% of protein, 0.7% of carbohydrates and 1% of fiber were reported in soymilk.
Food processing technologies
This procedure consisted of two digestive stages: gastric (pH 2, containing pepsin) and small intestinal digestions (pH 7, containing a pancreatin–bile mixture).
Briefly, each beverage (200 mL) was mixed with pepsin (0.2 g) in a beaker. Afterwards, the pH was immediately adjusted to 2 by the addition of 12 M HCl, and the mixture was incubated at 37 °C, 90 rpm for 2 h (incubation chamber with orbital agitation Ovan, Badalona, Spain). A portion of 20 mL of gastric digesta was placed into a beaker and 5 mL of pancreatin (4 g L−1) and bile (25 g L−1) mixture was added. This mixture was incubated for 2 h at 37 °C and 90 rpm (incubation chamber with orbital agitation Ovan). The samples were immediately placed in a cold water bath for 10 min once digested. To quantify the amount of carotenoids transferred to the aqueous-micellar fraction, a portion of small intestinal digesta (30 mL) was centrifuged (5000 rpm for 20 min at 4 °C)34 and filtered (a membrane of 0.22 μm). All samples from the micellar fraction were frozen (−45 °C) until analysis.
Bioactive compound analyses
Non-digested or digested beverages (6 mL) were mixed with 0.01 g of magnesium hydroxide carbonate, 0.01 g of butylhydroxytoluene (BHT), and 15 mL of ethanol/hexane solution (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) in an amber round-bottom flask under a N2 atmosphere and continuously agitated for 45 min. Afterward, the mixture was filtered using a70 mm low-ash filter paper (Albert-Hahnemuehle, S.L.U., Barcelona, Spain), and the residue was washed and again filtered once with 10 mL of ethanol/hexane solution (4
3 v/v) in an amber round-bottom flask under a N2 atmosphere and continuously agitated for 45 min. Afterward, the mixture was filtered using a70 mm low-ash filter paper (Albert-Hahnemuehle, S.L.U., Barcelona, Spain), and the residue was washed and again filtered once with 10 mL of ethanol/hexane solution (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v), twice with 5 mL of ethanol, and once with 5 mL of hexane. The filtrates were combined and washed with 10 mL of distilled water and 10 mL of 10% NaCl solution in an amber decanting funnel, discarding the aqueous phase each time. The organic phase was rotoevaporated at 40 °C until dryness. Then, the residue was saponified with 5 mL of methanolic KOH 0.5 M + 0.1% of BHT (v/w) and 5 mL of diethyl ether, under a N2 atmosphere for 30 min. Later, 5 mL of diethyl ether was added, and the solution was washed with 10 mL of distilled water and 10 mL of 10% NaCl solution. The organic phase was mixed with 5 mL of ethanol and rotoevaporated at 45 °C until dryness. The residue was dissolved with 4 mL of diethyl ether and then placed in an amber glass vial. Finally, the solvent was evaporated under a N2 atmosphere and stored at −45 °C until further analysis.
3 v/v), twice with 5 mL of ethanol, and once with 5 mL of hexane. The filtrates were combined and washed with 10 mL of distilled water and 10 mL of 10% NaCl solution in an amber decanting funnel, discarding the aqueous phase each time. The organic phase was rotoevaporated at 40 °C until dryness. Then, the residue was saponified with 5 mL of methanolic KOH 0.5 M + 0.1% of BHT (v/w) and 5 mL of diethyl ether, under a N2 atmosphere for 30 min. Later, 5 mL of diethyl ether was added, and the solution was washed with 10 mL of distilled water and 10 mL of 10% NaCl solution. The organic phase was mixed with 5 mL of ethanol and rotoevaporated at 45 °C until dryness. The residue was dissolved with 4 mL of diethyl ether and then placed in an amber glass vial. Finally, the solvent was evaporated under a N2 atmosphere and stored at −45 °C until further analysis.
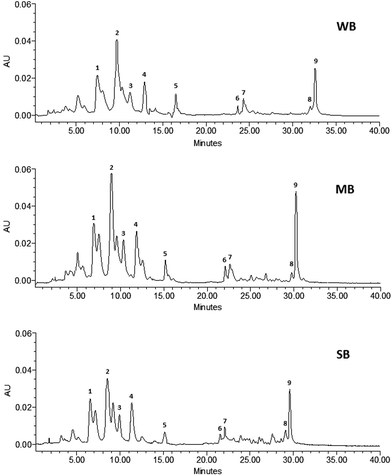
The HPLC system was equipped with a 600 controller and a 2996 diode array detector (Waters Corp.), which was set to scan from 200 to 600 nm. Carotenoids were separated using a reverse-phase C18 Spherisorb ODS2 (5 μm) stainless steel column (4.6 mm × 250 mm) operating at 30 °C at a flow rate of 1 mL min−1. A gradient elution was carried out to separate these compounds. Four eluents were employed as mobile phase: (1) 0.1 M methanol/ammonium acetate, (2) Milli-Q water, (3) methyl tert-butyl ether, and (4) methanol. Individual carotenoids were identified by comparing their retention times and spectra with the standards and/or those reported in the literature. HPLC chromatograms of the carotenoids in non-digested and untreated beverages are shown in Fig. 1. Carotenoid quantification was carried out by integrating the peak areas and using calibration curves (R2 in the range of 0.9961 to 0.9995; concentration between 0.1 and 50 mg L−1). The results were expressed in μg of carotenoid per 100 mL of sample.
Briefly, 5 mL of sample and 10 mL of tetrahydrofuran were mixed and centrifuged at 6000 rpm for 20 min at 4 °C. The supernatant was separated, whereas the residue was again mixed with 10 mL of tetrahydrofuran and centrifuged (6000 rpm for 20 min at 4 °C). Both the supernatants were combined in order to analyze the LAA. The antioxidant activity was evaluated using the colorimetric method reported by Brand-Williams et al.,35 which is based on the 1,1-diphenyl-2-picrylhydrazyl (DPPH˙) assay. Aliquots of 0.2 mL of lipophilic extracts were mixed with 3.8 mL of DPPH methanolic solution (0.025 g L−1). The homogenate was shaken vigorously and kept in the dark for 30 min. Afterward, the absorbance was measured at 515 nm against a blank of methanol. The results were expressed as a percentage of DPPH˙ inhibition.
Bioaccessibility calculations
Bioaccessibility was determined as the ratio of carotenoid concentration in the digested beverage (BCdigested) with respect to non-digested beverage (BCnon-digested) (eqn (1)). The results were expressed in percentage.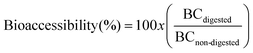 | (1) |
Statistical analysis
The food processing technologies and the in vitro gastrointestinal digestion were conducted in duplicate. Each bioactive compound was extracted and analyzed two times (n = 8). Analysis of variance (ANOVA) of the results followed by the least significant difference test (LSD) were carried out to determine significant differences (p < 0.05) in the concentration and bioaccessibility of the bioactive compounds from the beverages in relation to the factors studied in this research (food matrix and food processing). Multifactorial analysis of variance (ANOVA) was performed to study separately the main effects (food matrix and treatment) and the interaction effect (food matrix × treatment). As a significant interaction effect was observed in most of the variables, ANOVA, comparing the means within the same food matrix for different treatments and within the same treatment for different food matrix, was performed. All statistical analyses were performed with the program Statgraphics Plus 5.1 (Statistical Graphics Corporation, Inc., Rockville, MD, USA). The results were reported as mean ± standard deviation.Results and discussion
Carotenoids
The carotenoid profile in untreated, HIPEF, HPP and TT fruit juice-based beverages is presented in Tables 1 and 2. The concentration of total carotenoids (determined as the sum of individuals) was in the range of 322 to 426 μg per 100 mL in the untreated beverages, xanthophylls being up to 3.3 times higher than carotenes (Table 2). A similar concentration of carotenoids (between 223 and 540 μg per 100 mL) was reported in mixed fruit juices and beverages, where xanthophylls were also the predominant forms.28,29,33,36| Beverages | Treatments | Carotenoid concentration (μg per 100 mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cis-Violaxanthin + neoxanthin | cis-Antheraxanthin | Antheraxanthin | Lutein | Zeaxanthin | α-Cryptoxanthin | β-Cryptoxanthin | α-Carotene | β-Carotene | ||
| a Values are expressed as the mean ± standard deviation (n = 8). Different lower case letters in the same column and beverage indicate significant differences (p < 0.05) within treatments. Different capital letters in the same column and treatment indicate significant differences (p < 0.05) within beverages. WB, water-fruit juice beverage; SB, soymilk-fruit juice beverage; MB, milk-fruit juice beverage. HIPEF, high-intensity pulsed electric fields; HPP, high-pressure procesing; TT, thermal treatment. | ||||||||||
| WB | Untreated | 57.0 ± 2.2aA | 82 ± 4aA | 12.6 ± 0.5cA | 43 ± 3aA | 25.9 ± 1.3dA | 8.2 ± 0.3cC | 12.1 ± 0.8bA | 4.7 ± 0.3cA | 77 ± 5cA |
| HIPEF | 62 ± 4bB | 89 ± 3bB | 11.1 ± 0.4bA | 37.4 ± 1.5bB | 20.5 ± 1.0bA | 7.3 ± 0.5baA | 11.9 ± 0.7bA | 3.59 ± 0.12bA | 67.5 ± 1.6bA | |
| HPP | 63 ± 3abB | 85 ± 5abB | 11.5 ± 0.4bA | 40.8 ± 0.7aB | 24.1 ± 1.2cA | 7.9 ± 0.4bcA | 12.3 ± 0.5bA | 3.8 ± 0.3bA | 66.5 ± 2.1bA | |
| TT | 58.6 ± 1.8abB | 81.3 ± 2.5aB | 9.8 ± 0.4aA | 35.5 ± 1.4bB | 17.4 ± 1.2aA | 6.7 ± 0.3aA | 10.9 ± 0.6aA | 3.20 ± 0.10aA | 60 ± 3aA | |
| MB | Untreated | 66 ± 4aB | 122 ± 3cB | 18.3 ± 1.1aB | 57 ± 4aB | 34.3 ± 1.8aB | 9.2 ± 0.3aB | 15.3 ± 0.7aB | 7.5 ± 0.3bcB | 96 ± 4aB |
| HIPEF | 76.7 ± 2.2bC | 109.2 ± 1.8bC | 20.2 ± 1.0bB | 70 ± 4bC | 44 ± 3bB | 8.7 ± 0.5aB | 16.0 ± 0.6abC | 7.1 ± 0.4abC | 89 ± 4aC | |
| HPP | 80 ± 4bC | 110 ± 7bC | 20.5 ± 1.4bB | 75 ± 3bC | 47 ± 3bB | 9.2 ± 0.4aB | 16.3 ± 0.5abC | 7.9 ± 0.4cC | 102 ± 4bC | |
| TT | 70 ± 4aC | 99 ± 4aC | 18.9 ± 0.6abC | 57.4 ± 2.4aC | 32.3 ± 2.1aB | 7.7 ± 0.3bB | 15.7 ± 0.6abC | 6.88 ± 0.12aC | 85 ± 5aB | |
| SB | Untreated | 58 ± 3bA | 87 ± 5cA | 13.3 ± 0.9cA | 48 ± 3dC | 28.3 ± 1.9cA | 7.2 ± 0.3bcA | 14.2 ± 0.6bcB | 5.1 ± 0.3abA | 72 ± 2aA |
| HIPEF | 53 ± 3bA | 71 ± 3bA | 11.3 ± 0.5abA | 29.7 ± 1.0bA | 20.8 ± 1.4bA | 6.69 ± 0.21abA | 14.0 ± 0.7bB | 4.8 ± 0.3aB | 76 ± 3aB | |
| HPP | 56 ± 4bA | 75 ± 4bA | 11.9 ± 0.3bA | 33.9 ± 1.4cA | 21.4 ± 1.1bA | 7.3 ± 0.5cA | 14.9 ± 0.6cB | 5.5 ± 0.4abB | 78 ± 5aB | |
| TT | 43 ± 3aA | 57.5 ± 1.7aA | 10.8 ± 0.4aB | 25.2 ± 1.2aA | 15.3 ± 0.7aA | 6.5 ± 0.3aA | 12.4 ± 0.5aB | 5.30 ± 0.23abB | 61 ± 4aA | |
| Beverages | Treatments | Carotenoid concentration (μg per 100 mL) | ||
|---|---|---|---|---|
| Total xanthophylls | Total carotenes | Total carotenoids | ||
| a Values are expressed as the mean ± standard deviation (n = 8). Different lower case letters in the same column and beverage indicate significant differences (p < 0.05) within treatments. Different capital letters in the same column and treatment indicate significant differences (p < 0.05) within beverages. WB, water-fruit juice beverage; SB, soymilk-fruit juice beverage; MB, milk-fruit juice beverage. HIPEF, high-intensity pulsed electric fields; HPP, high-pressure procesing; TT, thermal treatment. Total xanthophylls and total carotenes were determined as the sum of individual carotenoids of each family quantified by HPLC (see Table 1). Total carotenoids corresponded to the sum of total xanthophylls and total carotenes determined by HPLC. | ||||
| WB | Untreated | 240 ± 6bA | 81 ± 4cA | 322 ± 4dA |
| HIPEF | 238 ± 5bB | 71.1 ± 1.7bA | 309 ± 3bB | |
| HPP | 244.4 ± 1.7bB | 70.4 ± 2.2bA | 315 ± 3cA | |
| TT | 220 ± 4aB | 63 ± 3aA | 283 ± 3aB | |
| MB | Untreated | 322 ± 10bC | 104 ± 4bB | 426 ± 12bB |
| HIPEF | 345 ± 4cC | 97 ± 4aC | 441.8 ± 1.3cC | |
| HPP | 358 ± 7dC | 110 ± 4bC | 467 ± 7dB | |
| TT | 302 ± 5aC | 92 ± 5aB | 393 ± 10aC | |
| SB | Untreated | 256 ± 11dB | 77.5 ± 2.1cA | 334 ± 10dA |
| HIPEF | 206.7 ± 1.7bA | 80 ± 3acB | 287 ± 4bA | |
| HPP | 220 ± 6cA | 83 ± 5bB | 303 ± 11cA | |
| TT | 170 ± 4aA | 66 ± 4aA | 237 ± 6Aa | |
Processing exerted a significant influence on the concentration of carotenoids contained in the three beverages analyzed in this study (p < 0.05). The concentration of some carotenoids increased after HIPEF treatment with respect to untreated beverages, such as cis-violaxanthin + neoxanthin from both WB (9%) and MB (16%); cis-anteraxanthin from WB (8%); anteraxanthin (10%), lutein (23%) and zeaxanthin (28%) from MB. In the same way, HPP improved the concentration of cis-violaxanthin, anteraxanthin, lutein and zeaxanthin from MB (between 12 and 37%) compared with the untreated ones. An explanation of this trend could be attributed to the greater stability of these products due to food processing, the inactivation of both hydrolytic and oxidative enzymes, as well as the disruption of cell membranes and proteins, releasing some individual carotenoids.6,12 Torregrosa et al.27 also observed a rise (in the range of 111 to 160%) in the concentration of 9-cis-violaxanthin + neoxanthin, antheraxanthin, lutein, zeaxanthin, and β-cryptoxanthin, when an orange-carrot juice was HIPEF-treated at 35 kV cm−1 for 150 μs. Similarly, Cilla et al.37 reported that lutein, zeaxanthin, and neoxanthin + 9-cis-violaxanthin improved their concentration (between 53 and 99%) in beverages made with fruit juices and milk or soymilk treated by HPP (400 MPa/40 °C/5 min).
Other carotenoids did not change their concentration in HIPEF- (mainly β-cryptoxanthin of the three samples), HPP- (α- and β-cryptoxanthin of all samples) and TT-beverages (some xanthophylls) compared with untreated ones. However, loss of some of these compounds was observed in beverages treated by any of the three technologies (HIPEF, HPP and TT), the greatest reductions were obtained in TT processing (between 8 and 48%). Carotenoids denaturalization depends on their chemical structure38 and most of them are molecules that are easily oxidized and isomerized due to the double bonds in their chemical structure.39 Thus, carotenoids could undergo several changes during processing, resulting in the degradation of these constituents.29 Zulueta et al.40 reported that treatment may affect the carotenoids concentration and their isomeric features. In addition, similar results in orange juice, orange-carrot juice, and fruit juices and milk/soymilk beverages processed by these technologies were reported.13,27,29,31,37,41
On the other hand, it was observed that the food matrix exerted a significant influence (p < 0.05) on the concentration of carotenoids extracted from beverages. MB displayed the highest concentration of all individual carotenes and xanthophylls, indicating that this beverage contained a higher total carotenoid concentration than WB and SB (Tables 1 and 2). The concentration of total carotenoids from WB and SB was very similar in untreated and HPP beverages and no statistically significant differences were found. However, SB displayed the lowest concentration of total carotenoids in HIPEF and TT samples. Therefore, these results indicated that the composition of the food matrix exerted an important effect on the stability and concentration of carotenoids extracted from blended fruit juice-based beverages. In fact, it has been reported that the presence of dietary fiber, as well as the amount and type of fat are among the main dietary factors that may affect the carotenoids extraction and in consequence, the carotenoids profile of food.5,20
| Beverages | Treatments | Bioaccessibility of carotenoids (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cis-Violaxanthin + neoxanthin | cis-Antheraxanthin | Antheraxanthin | Lutein | Zeaxanthin | α-Cryptoxanthin | β-Cryptoxanthin | α-Carotene | β-Carotene | ||
| a Values are expressed as the mean ± standard deviation (n = 8). Different lower case letters in the same column for each beverage show significant differences (p < 0.05) within treatments. Different capital letters in the same column and treatment indicate significant differences (p < 0.05) within beverages. WB, water-fruit juice beverage; SB, soymilk-fruit juice beverage; MB, milk-fruit juice beverage. HIPEF, high-intensity pulsed electric fields; HPP, high-pressure processing; TT, thermal treatment. The bioaccessibility of each carotenoid was determined as the ratio between the concentration of individual compounds in the digested beverage (micellar fraction) and that of non-digested products (see Table 1). | ||||||||||
| WB | Untreated | 15.8 ± 0.8bB | 14.0 ± 0.6bB | 9.2 ± 0.6cA | 16.0 ± 0.9dA | 17.5 ± 1.2dA | 17.5 ± 1.1bB | 17.8 ± 1.1cA | 17.7 ± 0.9cA | 16.9 ± 0.7cA |
| HIPEF | 17.2 ± 0.5cA | 10.4 ± 0.7aA | 7.05 ± 0.17bA | 14.8 ± 0.6cA | 13.5 ± 0.6cA | 12.1 ± 0.7bB | 9.2 ± 0.4bA | 13.2 ± 0.8bA | 12.2 ± 0.8bA | |
| HPP | 19.0 ± 1.2dA | 10.0 ± 0.4aA | 7.5 ± 0.3bA | 13.8 ± 0.4bA | 12.3 ± 0.4bcA | 13.4 ± 0.8bB | 9.8 ± 0.5bA | 12.9 ± 0.5bA | 13.1 ± 0.7bA | |
| TT | 8.9 ± 0.5aA | 9.8 ± 0.3aB | 6.5 ± 0.4aA | 12.1 ± 0.5aA | 11.8 ± 0.7aA | 10.4 ± 0.7aB | 7.8 ± 0.3cA | 8.5 ± 0.3aA | 10.2 ± 0.3aA | |
| MB | Untreated | 21.6 ± 1.4bC | 17.5 ± 0.8cC | 14.6 ± 1.0cC | 28.9 ± 1.4cC | 30.0 ± 0.8cC | 29,8 ± 1.3cC | 20.0 ± 1.1cB | 31.2 ± 1.4cC | 31.4 ± 2.2cC |
| HIPEF | 38.7 ± 2.5dB | 15.5 ± 0.6bC | 13.0 ± 0.6bC | 38.1 ± 1.7dC | 29.1 ± 1.6bcC | 30.1 ± 1.2cC | 19.8 ± 1.0cC | 28.5 ± 1.8bC | 29.6 ± 2.0bC | |
| HPP | 33.8 ± 1.8cC | 15.8 ± 1.1bC | 13.9 ± 0.9bcC | 25.2 ± 1.0bB | 27.6 ± 1.7bC | 26.8 ± 1.8bC | 13.7 ± 0.6bB | 30.4 ± 1.6bcC | 29.0 ± 1.3bC | |
| TT | 15.9 ± 0.8aB | 12.2 ± 0.5aC | 10.6 ± 0.7aB | 19.8 ± 0.9aB | 23.3 ± 1.6aC | 14.3 ± 1.0aC | 12.8 ± 0.7aC | 23.1 ± 0.7aC | 22.9 ± 1.2aC | |
| SB | Untreated | 13.9 ± 0.4bA | 12.2 ± 0.8cA | 11.1 ± 0.6dB | 22.7 ± 1.4aB | 24.1 ± 1.6dB | 15.1 ± 1.0cA | 18.6 ± 0.8dAB | 22.0 ± 0.7dB | 20.1 ± 1.3cB |
| HIPEF | 17.1 ± 1.2cA | 13.5 ± 0.6bB | 9.4 ± 0.4bB | 26.3 ± 1.4bB | 17.3 ± 1.1bB | 7.84 ± 0.22bA | 11.9 ± 0.6bB | 16.5 ± 1.1cB | 15.6 ± 1.1bB | |
| HPP | 21.5 ± 0.9dA | 14.0 ± 0.8bB | 9.66 ± 0.07cB | 37.6 ± 1.5cC | 20.7 ± 0.7cB | 8.5 ± 0.6bA | 15.9 ± 0.9cC | 15.3 ± 0.5bB | 16.1 ± 0.5bB | |
| TT | 9.2 ± 0.5aA | 7.8 ± 0.4aA | 7.2 ± 0.4aA | 23.6 ± 1.0aC | 14.5 ± 0.5aB | 5.6 ± 0.4aA | 10.5 ± 0.6aB | 11.8 ± 0.6aB | 12.9 ± 0.9aB | |
| Beverages | Treatments | Bioaccessibility of carotenoids (%) | ||
|---|---|---|---|---|
| Total xanthophylls | Total carotenes | Total carotenoids | ||
| a Values are expressed as the mean ± standard deviation (n = 8). Different lower case letters in the same column for each beverage show significant differences (p < 0.05) within treatments. Different capital letters in the same column and treatment indicate significant differences (p < 0.05) within beverages. WB, water-fruit juice beverage; SB, soymilk-fruit juice beverage; MB, milk-fruit juice beverage. HIPEF, high-intensity pulsed electric fields; HPP, high-pressure processing; TT, thermal treatment. The bioaccessibilities of total xanthophylls and total carotenes were determined as the ratio between the sum of the concentrations of individual compounds of each family quantified by HPLC in the digested beverage (micellar fraction) and that of non-digested products (see Table 2). The bioaccessibility of total carotenoids was determined as the ratio between the sum of the concentrations of total xanthophylls and total carotenes in the digested beverage (micellar fraction) and that of non-digested products. | ||||
| WB | Untreated | 15.19 ± 0.12cA | 17.0 ± 0.7cA | 15.63 ± 0.17dA |
| HIPEF | 12.93 ± 0.19bA | 12.3 ± 0.8bA | 12.8 ± 0.3bA | |
| HPP | 13.12 ± 0.19bA | 13.1 ± 0.7bA | 13.12 ± 0.21cA | |
| TT | 9.85 ± 0.21aA | 10.1 ± 0.3aA | 9.91 ± 0.12aA | |
| MB | Untreated | 22.0 ± 0.7bC | 31.4 ± 2.0bC | 24.3 ± 0.6bC |
| HIPEF | 27.4 ± 0.5dC | 29.5 ± 1.8bC | 27.8 ± 0.3cC | |
| HPP | 23.4 ± 1.1cC | 29.1 ± 1.1bC | 24.8 ± 0.7bC | |
| TT | 15.68 ± 0.17aC | 22.6 ± 1.1aC | 17.3 ± 0.3aC | |
| SB | Untreated | 16.3 ± 0.6bB | 20.2 ± 1.3cB | 17.2 ± 0.7cB |
| HIPEF | 16.0 ± 0.4bB | 15.7 ± 1.1bB | 15.9 ± 0.3bB | |
| HPP | 19.89 ± 0.20cB | 16.1 ± 0.5bB | 18.84 ± 0.22dB | |
| TT | 11.21 ± 0.23aB | 12.8 ± 0.8aB | 11.65 ± 0.06aB | |
Both food matrix and food processing exerted a significant influence (p < 0.05) on the bioaccessibility of carotenoids. Overall, the bioaccessibility of individual carotenoids diminished after applying any type of treatment, mainly in TT beverages where the biaccessibility declined up to 63%. HIPEF treatment decreased the bioaccessibility of carotenoids in the range of 7.6 to 48.2%, compared to untreated beverages. In the same way, carotenoids were less bioaccessible in HPP beverages (between 8.2 and 45.1%) than in the untreated ones. The carotenoids that showed the lowest bioaccessibility after applying each processing technology analyzed herein were: β-cryptoxanthin from WB after HIPEF or HPP and α-cryptoxanthin from SB after TT. As far as we know, very few reports have evaluated the influence of non-thermal (HIPEF and HPP) or thermal (TT) processing technologies on the bioaccessibility of carotenoids. In one such report, Cilla et al.37 observed that some carotenoids were around 15 and 58% less bioaccessible in fruit juice-milk based beverages treated by HPP than in the untreated beverages. However, these authors observed greater reductions in the bioaccessibility of carotenoids from fruit juice-milk or soymilk-based beverages treated by heat (between 30 and 90%).37 Stinco et al.43 reported that pasteurization reduced the bioacessibility of α-carotene and β-cryptoxanthin in orange juice as compared with fresh industrially squeezed juice.
In some cases, HIPEF processing improved the bioaccessibility of carotenoids in comparison with their respective untreated beverages, such as cis-violaxanthin + neoxanthin from the three beverages (between 9 and 79%), cis-antheraxanthin from SB (10%), and lutein from both MB (32%) and SB (16%). The bioaccessibility of total xanthophylls and total carotenoids from MB also increased by 24.5 and 15%, respectively, when HIPEF treatment was applied. A similar trend was observed in beverages treated by HPP, where cis-violaxanthin + neoxanthin from the three beverages; cis-antheraxanthin and lutein from SB, total xanthophylls from both MB and SB, and total carotenoids from SB were more bioaccessible in HPP beverages (in the range of 6.5 to 65%) than in untreated samples. On the contrary, significant reductions in the bioaccessibility of carotenoids were observed in TT beverages (between 22 and 63%). The improvement in the bioaccessibility of some carotenoids in HIPEF and HPP beverages could be justified by the changes in the structure of the food matrix due to processing effects, such as the breakdown of cell walls and membranes in which carotenoids are embedded. Thus, carotenoids could be released from the food matrix enhancing their interactions with digestive enzymes and their solubilisation into micelles. This hypothesis is supported by Stinco et al.,43 who reported that the food matrix structure is one of the most important factors that affect the bioaccessibility of carotenoids. Additionally, Maiani et al.6 found that some types of food processing can improve the carotenoid bioavailability. Cilla et al.37 reported increases between 39 and 264% in the bioaccessibility of neoxanthin + 9-cis-violaxanthin, lutein, zeaxanthin, β-cryptoxanthin and β-carotene from milk- or soymilk-based beverages treated by HPP with respect to untreated products.
The food matrix exerted a significant influence (p < 0.05) on the bioaccessibility of carotenoids. Total carotenoids from MB displayed the highest bioaccessibility with an average value of 23.5%, followed by SB (15.9%) and WB (12.9%). These results suggest that the greater fat content of milk (3.6%) compared with soymilk (1.6%) and water (0%) could favour the incorporation of carotenoids into micelles and thus, increase their bioaccessibility in MB. In accordance with this hypothesis, it has been reported that dietary fat enhances the bioaccessibility of carotenoids from food.5,20 Granado-Lorencio et al.44 also found that the addition of milk to blended fruit juices improves the bioaccessibility of carotenoids.
Fiber is another food constituent that could affect the bioaccessibility of carotenoids. Dietary fiber could increase the viscosity of the intestinal content45 entrapping bioactive compounds and decreasing the activity of digestive enzymes. Thus, the micellization and bioaccessibility of carotenoids are reduced due to the fiber content in food. In this sense, it could be expected that SB beverages contain a larger amount of dietary fiber than MB, explaining why the bioaccessibility of carotenoids diminished in SB beverages. In contrast to these results, Cilla et al.37 did not find significant differences in the bioaccessibility of carotenoids in fruit juice-based beverages containing milk or soymilk.
Considering the effect of both food matrix and processing, it was observed that a milk matrix (MB) in combination with HIPEF processing increased the bioaccessibility of total carotenoids (15%) compared with untreated beverages. Carotenoids from MB were equally bioaccesible in HPP and untreated beverages. In SB, the technology that improved the bioaccessibility of total carotenoids was HPP (10%), whereas HIPEF slightly decreased them (7%). Both non-thermal technologies (HIPEF and HPP) decreased the bioaccessibility of total carotenoids in WB (around 17%). The lowest bioaccessibility was achieved in the three beverages treated by TT (losses up to 37%), showing that TT was not adequate for improving the bioaccessibility of carotenoids contained in these beverages.
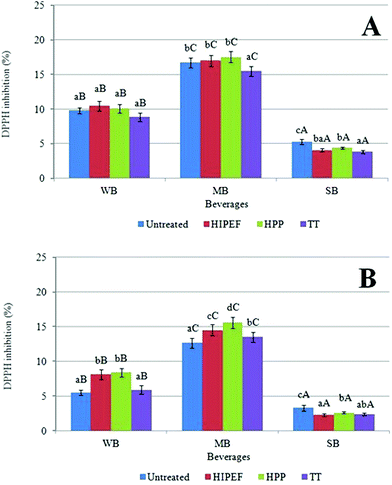
Thermal treatment (TT) exerted a significant influence (p < 0.05) on the LAA of MB and SB beverages, where the percentage of DPPH˙ inhibition diminished between 7 and 27% when compared with untreated beverages. SB beverages treated by HIPEF and HPP also exhibited a decrease of 22 and 17%, respectively, in LAA compared with untreated products. In contrast, the LAA from WB and MB treated by both non-themal technologies (HIPEF and HPP) remained unchanged with respect to untreated samples (p > 0.05). When the three treatments (HIPEF, HPP, TT) were compared, it was observed that the lowest LAA was obtained in thermally-treated beverages. On the contrary, the highest LAA was observed in products treated by HIPEF (for WB) and HPP (for MB). To our knowledge, this is the first study addressing the influence of non-thermal and thermal technologies on the lipophilic antioxidant activity of beverages. However, there is available information about the influence of HIPEF, HPP and TT on total antioxidant activity of liquid food. In this sense, Morales-de la Peña et al.46 observed that HIPEF treatment (35 kV cm−1, 4μs bipolar pulses at 200 Hz for 1400 μs) did not affect the total antioxidant activity of a blended fruit juice-soymilk beverage in comparison with untreated juice. Elez-Martínez et al.47 did not find significant differences in the antioxidant activity of HIPEF (15–35 kV cm−1, 20–10 μs mono or bipolar pulses at 50–450 Hz for 100–1000 μs), TT (90 °C/1 min) and untreated orange juice. Plaza et al.48 also showed that the antioxidant activity of orange juice was not affected by HIPEF (35 kV cm−1, 4μs bipolar pulses at 800 Hz for 750 μs) and thermal treatment (70 °C for 30 s) compared with the untreated juice. On the other hand, Patras et al.49 reported that TT (70 °C/2 min) and HPP (400 MPa/20 °C/15 min) decrease the anti-radical power of strawberry pure (25 and 19%, respectively), but not in blackberry pure treated by these technologies. Significant reductions in the antioxidant activity (between 7.5 and 11.5%) of an orange juice-milk beverage thermally treated (90 or 98 °C for 21 s) were observed.13 However, the antioxidant activity remained unchanged in HPP samples (400 MPa/5 min) as compared with the untreated.13
Considering the food matrix influence, it was observed that the LAA of all beverages were statistically different (p < 0.05), where SB displayed the lowest percentage of DPPH˙ inhibition (4%) and MB the highest (17%). It is likely that the higher fat content of milk with respect to SB and WB matrices could improve the antioxidant activity of the lipophilic constituents. Additionally, these results are in accordance with those found in carotenoids, where the greatest concentration of these compounds was obtained in MB (see previous sections). On the other hand, some protein and fiber types could mask the antioxidant activity of food50 and soymilk contains fiber and greater amounts of proteins (up to 20%), explaining why the lowest LAA was found in SB. In fact, a strong correlation between the LAA and total xanthophyll concentration (r2 = 0.8495, p = 0.0000) from SB, as well as between LAA and total carotenoid concentration (r2 = 0.7257, p = 0.0015) was observed.
All treatments (HIPEF, HPP and TT) increased the LAA of digested MB between 7 and 17% with respect to untreated beverages. Non-thermal technologies (HIPEF and HPP) also enhanced the LAA of digested WB (in the range of 47 to 53%), while non-significant differences were observed in the digested fraction of WB-TT. In contrast, the LAA of digested SB was reduced by any type of treatment, with losses between 21 and 30% as compared with untreated products. LAA correlates well with the bioaccessibility of cis-violaxanthin + neoxanthin from MB (r2 = 0.7533 p = 0.0047) and WB (r2 = 0.6487, p = 0.0225), which was the carotenoid that increased its bioaccessibility after non-thermal processing. Therefore, the increment in the LAA of non-thermally treated beverages could be linked to the improvement in the solubilisation, digestibility and bioaccessibility of some lipophilic compounds with antioxidant activity, such as carotenoids.
The food matrix exerted a significant influence on the LAA of digested beverages. The lowest LAA was observed in digested SB, with around 2.30 and 3.3% of DPPH˙ inhibition. On the other hand, digested MB displayed the highest LAA (between 12.67 and 15.6%). An explanation of these results could be attributed to the fact that the bioaccessibility of carotenoids was improved in matrices containing a certain amount of fat (such as milk), as well as in beverages treated by non-thermal technologies (in the case of certain carotenoids). Therefore, the antioxidant potential and the bioaccessibility of these compounds could be modulated by both food matrix and food processing.
Conclusion
Food matrix and food processing exerted a significant influence on the bioaccessibility of carotenoids, as well as on the lipophilic antioxidant activity (LAA) of beverages. Non-thermal technologies (HIPEF and HPP) were more effective than TT to preserve the concentration and bioaccessibility of carotenoids and other lipophilic compounds with antioxidant activity from beverages based on a blend of fruit juices (orange, pineapple, kiwi and mango) and water, milk or soymilk. The beverage with the highest bioaccessibility of total carotenoids (determined as the sum of individual compounds) was that containing milk (MB), followed by that made with soymilk (SB) and finally that of water (WB). A milk matrix (MB) in combination with HIPEF processing increased the bioaccessibility of carotenoids by 15% as compared with the untreated products. In SB beverages, HPP increased the bioaccessibility of these compounds by 10%, while all technologies (HIPEF, HPP and TT) decreased it in WB. The results demonstrate that both, food matrix and food processing, are able to modulate the bioaccessibility of carotenoids as well as the antioxidant potential of beverages, therefore these issues should be taken into consideration when developing functional food and beverages. In addition, HIPEF and HPP could be considered as promising technologies to obtain highly nutritional and functional beverages. Further studies should be carried out in order to evaluate the influence of food matrix and processing on the in vivo bioavailability of carotenoids.Acknowledgements
This research was supported by the Ministerio de Ciencia e Innovación (Spain), reference AGL2006-12758-C02-02/ALI and AGL2006-12758-C02-01/ALI. María Janeth Rodríguez-Roque thanks the Comissionat per a Universitats i Recerca, del Departament d'Innovació, Universitats i Empresa de la Generalitat de Catalunya and European Social Fund for the predoctoral grant. Dr Begoña de Ancos thanks the Ministerio de Economía y Competitividad (Spain) for its support through the proyect AGL2013-46326-R. Prof. Olga Martín-Belloso thanks the Institució Catalana de Recerca i Estudis Avançats (ICREA) for the Academia Award 2008.Notes and references
- J. Howlett, Int. Life Sci. Institute. ILSI Eur. Concise Monogr. Ser, 2008 Search PubMed.
- F. C. Prado, J. L. Parada, A. Pandey and C. R. Soccol, Food Res. Int., 2008, 41, 111–123 CrossRef CAS PubMed.
- A. Zulueta, M. J. Esteve, I. Frasquet and A. Frígola, Food Chem., 2007, 103, 1365–1374 CrossRef CAS PubMed.
- M. J. Rodríguez-Roque, In vitro bioaccessibility of health-related compounds from beverages based on fruit juice, milk or soymilk: Influence of food matrix and processing, University of Lleida, 2014 Search PubMed.
- E. Fernández-García, I. Carvajal-Lérida, M. Jarén-Galán, J. Garrido-Fernández, A. Pérez-Gálvez and D. Hornero-Méndez, Food Res. Int., 2012, 46, 438–450 CrossRef PubMed.
- G. Maiani, M. J. P. Castón, G. Catasta, E. Toti, I. G. Cambrodón, A. Bysted, F. Granado-Lorencio, B. Olmedilla-Alonso, P. Knuthsen, M. Valoti, V. Böhm, E. Mayer-Miebach, D. Behsnilian and U. Schlemmer, Mol. Nutr. Food Res., 2009, 53, 194–218 Search PubMed.
- B. Xu and S. K. C. Chang, J. Agric. Food Chem., 2009, 57, 4706–4717 CrossRef CAS PubMed.
- N. Jinapong, M. Suphantharika and P. Jamnong, J. Food Eng., 2008, 84, 194–205 CrossRef PubMed.
- M. J. Rodríguez-Roque, M. A. Rojas-Graü, P. Elez-Martínez and O. Martín-Belloso, Food Chem., 2013, 136, 206–212 CrossRef PubMed.
- İ. Gülçin, Arch. Toxicol., 2012, 86, 345–391 CrossRef PubMed.
- J. J. Yahia and M. E. Ornelas-Paz, in Fruit and vegetable phytochemicals, ed. L. A. de la Rosa, New York, USA, 2010, pp. 177–222 Search PubMed.
- I. Odriozola-Serrano, I. Aguiló-Aguayo, R. Soliva-Fortuny and O. Martín-Belloso, Trends Food Sci. Technol., 2013, 29, 98–107 CrossRef CAS PubMed.
- F. J. Barba, C. Cortés, M. J. Esteve and A. Frígola, Food Bioprocess Technol., 2012, 5, 2222–2232 CrossRef CAS.
- C. Sanchez-Moreno, B. De Ancos, L. Plaza, P. Elez-Martinez and M. P. Cano, Crit. Rev. Food Sci. Nutr., 2009, 49, 552–576 CrossRef CAS PubMed.
- R. Soliva-Fortuny, A. Balasa, D. Knorr and O. Martín-Belloso, Trends Food Sci. Technol., 2009, 20, 544–556 CrossRef CAS PubMed.
- R. Sánchez-Vega, P. Elez-Martínez and O. Martín-Belloso, Innovative Food Sci. Emerging Technol., 2015, 29, 70–77 CrossRef PubMed.
- E. Fernández-García, I. Carvajal-Lérida and A. Pérez-Gálvez, Nutr. Res., 2009, 29, 751–760 CrossRef PubMed.
- A. Nagao, E. Kotake-Nara and M. Hase, Biosci., Biotechnol., Biochem., 2013, 77, 1055–1060 CrossRef CAS PubMed.
- C. Failla and M. L. Chitchumroonchokchai, Harvest. Tech. Monogr., 2005, vol. 3, 32 pp Search PubMed.
- K. H. Van Het Hof, C. E. West, J. A. Weststrate and J. G. A. J. Hautvast, J. Nutr., 2000, 130, 503–506 CAS.
- L. Lemmens, I. Colle, S. Van Buggenhout, P. Palmero, A. Van Loey and M. Hendrickx, Trends Food Sci. Technol., 2014, 38, 125–135 CrossRef CAS PubMed.
- I. Colle, L. Lemmens, S. Van Buggenhout, A. Van Loey and M. Hendrickx, J. Food Sci., 2010, 75, C753–C759 CrossRef CAS PubMed.
- I. J. P. Colle, L. Lemmens, S. Van Buggenhout, K. Met, A. M. Van Loey and M. E. Hendrickx, Food Res. Int., 2013, 51, 32–38 CrossRef CAS PubMed.
- G. Knockaert, A. De Roeck, L. Lemmens, S. Van Buggenhout, M. Hendrickx and A. Van Loey, Food Chem., 2011, 125, 903–912 CrossRef CAS PubMed.
- G. Knockaert, L. Lemmens, S. Van Buggenhout, M. Hendrickx and A. Van Loey, Food Chem., 2012, 133, 60–67 CrossRef CAS PubMed.
- G. Knockaert, S. K. Pulissery, I. Colle, S. Van Buggenhout, M. Hendrickx and A. Van Loey, Food Chem., 2012, 135, 1290–1297 CrossRef CAS PubMed.
- F. Torregrosa, C. Cortés, M. J. Esteve and A. Frígola, J. Agric. Food Chem., 2005, 53, 9519–9525 CrossRef CAS PubMed.
- M. J. Rodríguez-Roque, M. A. Rojas-Graü, P. Elez-Martínez and O. Martín-Belloso, Food Res. Int., 2014, 62, 771–778 CrossRef PubMed.
- M. Morales-de la Peña, L. Salvia-Trujillo, M. A. Rojas-Graü and O. Martín-Belloso, Food Chem., 2011, 129, 982–990 CrossRef PubMed.
- L. Salvia-Trujillo, M. Morales-de la Peña, M. A. Rojas-Graü and O. Martín-Belloso, Food Control, 2011, 22, 1639–1646 CrossRef CAS PubMed.
- C. Sánchez-Moreno, L. Plaza, P. Elez-Martínez, B. De Ancos, O. Martín-Belloso and M. P. Cano, J. Agric. Food Chem., 2005, 53, 4403–4409 CrossRef PubMed.
- M. Muñoz, B. De Ancos, C. Sánchez-Moreno and M. P. Cano, J. Food Prot., 2007, 70, 1587–1593 Search PubMed.
- M. J. Rodríguez-Roque, M. A. Rojas-Graü, P. Elez-Martínez and O. Martín-Belloso, J. Agric. Food Chem., 2013, 61, 1859–1867 CrossRef PubMed.
- F. Granado-Lorencio, B. Olmedilla-Alonso, C. Herrero-Barbudo, I. Blanco-Navarro, B. Pérez-Sacristán and S. Blázquez-García, Food Chem., 2007, 102, 641–648 CrossRef CAS PubMed.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, LWT – Food Sci. Technol., 1995, 28, 25–30 CrossRef CAS.
- M. J. Rodríguez-Roque, M. A. Rojas-Graü, P. Elez-Martínez and O. Martín-Belloso, J. Funct. Foods, 2014, 7, 161–169 CrossRef PubMed.
- A. Cilla, A. Alegría, B. De Ancos, C. Sánchez-Moreno, M. P. Cano, L. Plaza, G. Clemente, M. J. Lagarda and R. Barberá, J. Agric. Food Chem., 2012, 60, 7282–7290 CrossRef CAS PubMed.
- G. Bitton and D. Hornero-Méndez, in Phytochemistry of fruit and vegetables, ed. F. A. Tomás-Barberán and R. J. Robins, Oxford Science Publication, New York, USA, 2001, pp. 11–29 Search PubMed.
- E. Hedrén, V. Diaz and U. Svanberg, Eur. J. Clin. Nutr., 2002, 56, 425–430 CrossRef PubMed.
- A. Zulueta, M. J. Esteve and A. Frígola, J. Food Sci., 2007, 72, C457–C463 CrossRef CAS PubMed.
- C. Cortés, F. Torregrosa, M. J. Esteve and A. Frígola, J. Agric. Food Chem., 2006, 54, 6247–6254 CrossRef PubMed.
- C. Dhuique-Mayer, P. Borel, E. Reboul, B. Caporiccio, P. Besancon and M.-J. Amiot, Br. J. Nutr., 2007, 97, 883–890 CrossRef CAS PubMed.
- C. M. Stinco, R. Fernández-Vázquez, M. L. Escudero-Gilete, F. J. Heredia, A. J. Meléndez-Martínez and I. M. Vicario, J. Agric. Food Chem., 2012, 60, 1447–1455 CrossRef CAS PubMed.
- F. Granado-Lorencio, C. Herrero-Barbudo, I. Blanco-Navarro, B. Pérez-Sacristán and B. Olmedilla-Alonso, Br. J. Nutr., 2009, 101, 576–582 CrossRef CAS PubMed.
- J. Hoffmann, J. Linseisen, J. Riedl and G. Wolfram, Eur. J. Nutr., 1999, 38, 278–285 CrossRef CAS.
- M. Morales-de la Peña, L. Salvia-Trujillo, M. A. Rojas-Graü and O. Martín-Belloso, LWT – Food Sci. Technol., 2010, 43, 872–881 CrossRef PubMed.
- P. Elez-Martínez and O. Martín-Belloso, Food Chem., 2007, 102, 201–209 CrossRef PubMed.
- L. Plaza, C. Sánchez-Moreno, P. Elez-Martínez, B. De Ancos, O. Martín-Belloso and M. P. Cano, Eur. Food Res. Technol., 2006, 223, 487–493 CrossRef CAS.
- A. Patras, N. P. Brunton, S. Da Pieve and F. Butler, Innovative Food Sci. Emerging Technol., 2009, 10, 308–313 CrossRef CAS PubMed.
- V. Sharma, H. Vijay Kumar and L. Jagan Mohan Rao, Food Res. Int., 2008, 41, 124–129 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |