Curcumin prevents cisplatin-induced decrease in the tight and adherens junctions: relation to oxidative stress†
Joyce
Trujillo‡
a,
Eduardo
Molina-Jijón‡
b,
Omar Noel
Medina-Campos
a,
Rafael
Rodríguez-Muñoz
b,
José Luis
Reyes
b,
María L.
Loredo
c,
Diana
Barrera-Oviedo
d,
Enrique
Pinzón
e,
Daniela Saraí
Rodríguez-Rangel
a and
José
Pedraza-Chaverri
*a
aDepartment of Biology, Faculty of Chemistry, National Autonomous University of Mexico (UNAM), 04510 University City, D.F., Mexico. E-mail: pedraza@unam.mx; Fax: +52 55 5622-3878; Tel: +52 55 5622-3878
bDepartment of Physiology, Biophysics and Neurosciences, Center for Research and Advanced Studies of the National Polytechnic Institute (Cinvestav-IPN), Mexico City, 07360, Mexico
cSchool of Medicine, Panamericana University, Mexico City, 03920, Mexico
dDepartment of Pharmacology, Faculty of Medicine, National Autonomous University of Mexico (UNAM), University City, 04510, Mexico
eAnimal Care Unit, Faculty of Medicine, National Autonomous University of Mexico (UNAM), University City, 04510, Mexico
First published on 29th September 2015
Abstract
Curcumin is a polyphenol and cisplatin is an antineoplastic agent that induces nephrotoxicity associated with oxidative stress, apoptosis, fibrosis and decrease in renal tight junction (TJ) proteins. The potential effect of curcumin against alterations in TJ structure and function has not been evaluated in cisplatin-induced nephrotoxicity. The present study explored whether curcumin is able to prevent the cisplatin-induced fibrosis and decreased expression of the TJ and adherens junction (AJ) proteins occludin, claudin-2 and E-cadherin in cisplatin-induced nephrotoxicity. Curcumin (200 mg kg−1) was administered in three doses, and rats were sacrificed 72 h after cisplatin administration. Curcumin was able to scavenge, in a concentration-dependent way, superoxide anion, hydroxyl radical, peroxyl radical, singlet oxygen, peroxynitrite anion, hypochlorous acid and hydrogen peroxide. Cisplatin-induced renal damage was associated with alterations in plasma creatinine, expression of neutrophil gelatinase-associated lipocalin and of kidney injury molecule-1, histological damage, increase in apoptosis, fibrosis (evaluated by transforming growth factor β1, collagen I and IV and α-smooth muscle actin expressions), increase in oxidative/nitrosative stress (evaluated by Hsp70/72 expression, protein tyrosine nitration, superoxide anion production in isolated glomeruli and proximal tubules, and protein levels of NADPH oxidase subunits p47phox and gp91phox, protein kinase C β2, and Nrf2) as well as by decreased expression of occludin, claudin-2, β-catenin and E-cadherin. Curcumin treatment prevented all the above-described alterations. The protective effect of curcumin against cisplatin-induced fibrosis and decreased proteins of the TJ and AJ was associated with the prevention of glomerular and proximal tubular superoxide anion production induced by NADPH oxidase activity.
Introduction
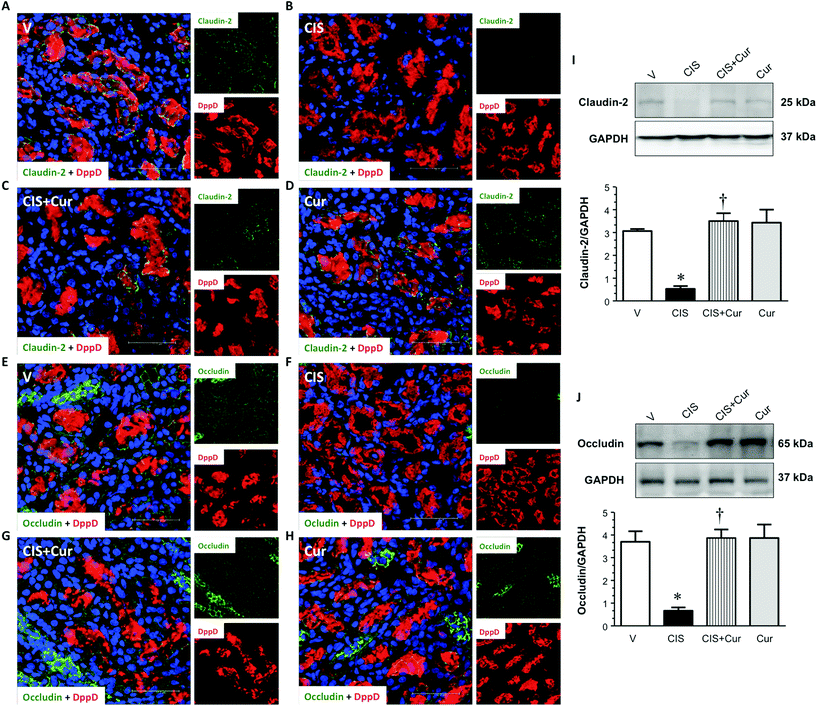
The renal tubule plays a major role in the reabsorption of water and solutes in the nephron. Tight junction (TJ) restricts paracellular transport of solutes and water, and adherens junction (AJ) regulates transcellular and paracellular transport. Several integral membrane proteins have been identified as components of TJ strands, and some of them include occludin and claudins.1,2 The mammalian nephron displays a wide spectrum of claudins, whose distribution varies in each tubular segment, thus determining the permeability properties of the renal epithelia. In the kidney, occludin is expressed along the nephron tubular segments3,4 and claudin-2 is expressed in leaky epithelia.5In vitro studies have shown that claudin-2 acts as a cation-selective paracellular pore6 which mediates water transport in the renal proximal tubule.7 E-cadherin is the major AJ protein expressed in epithelial cells and is highly expressed in the distal nephron and the collecting ducts, where it plays a role in decreasing paracellular permeability8 and disruption of E-cadherin directly mediates epithelial-mesenchymal transition (EMT) downstream of transforming growth factor-beta1 (TGFβ1) in renal tubular epithelial cells.9 Therefore, the TJ and AJ provide important adhesive contacts between neighboring epithelial cells. Previous studies have reported that TJ structure and function are sensitive to oxidative stress damage induced by several factors like heavy metals10–12 and hydrogen peroxide (H2O2).13 Also, in early diabetic nephropathy, oxidative stress decreases occludin and claudin-2 expression in proximal tubules (PT) and claudin-5 in glomeruli (GL).14Curcumin is a phenolic compound extracted from Curcuma longa rhizome and is used commonly in India, China and Southeast Asia as a spice, pigment, additive, and also in traditional medicine.15,16 Curcumin has broad biological functions, particularly antioxidant,17–21 anti-inflammatory,22 and renoprotective functions.17,23–25 Curcumin is a bifunctional antioxidant by the ability to exert both direct and indirect antioxidant effects.26 It is able to react directly with reactive oxygen and nitrogen species17 and induce the expression of several cytoprotective proteins21,26 many of them driven by nuclear factor erythroid-derived 2-like 2 (Nrf2).27
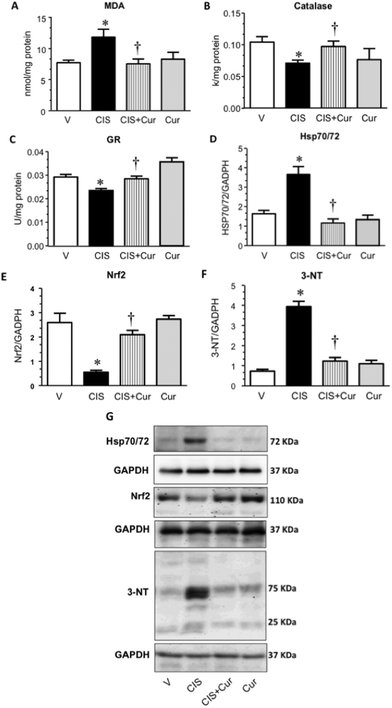
Cisplatin (CIS) is an effective anticancer drug used against lung and ovarian cancer and some lymphomas, however renal damage has limited its use.28–30 Oxidative and nitrosative stress are involved in the mechanism by which CIS induces renal damage.31,32 In this context it has been shown that curcumin administration provides protection against CIS-induced nephrotoxicity in rats33,34 and in mice.22 Curcumin administration attenuates the CIS-induced decrease of antioxidant defense, including superoxide dismutase, catalase (CAT) and glutathione (GSH).33 Renal Nrf235,36 is decreased in CIS-induced nephrotoxicity and Nrf2 inductors are able to maintain its levels.35–38 These findings suggest that Nrf2 regulation plays a role in CIS-induced nephrotoxicity and curcumin may induce Nrf2 in kidney.23,24 Rapid expression of the survival gene family heat shock protein 70 (Hsp70) was shown to be critical for mounting cytoprotection against severe cellular stress, as well as elevated temperature (Hsp72).39
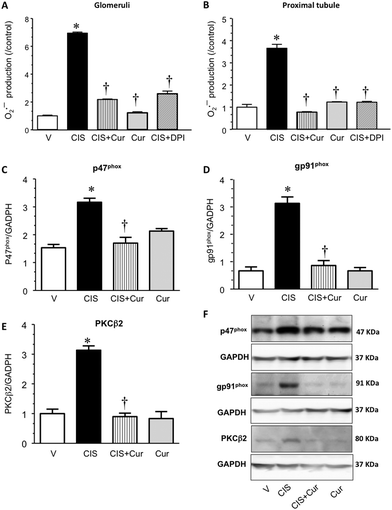
We previously reported that occludin and claudin-2 expressions are decreased in CIS-induced nephrotoxicity associated with oxidative stress derived in part due to an increased production of superoxide anion (O2˙−) by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity.32,40 The potential effect of curcumin against TGFβ1-induced EMT and fibrosis, and alterations in TJ and AJ structure and function has not been evaluated in CIS-induced nephrotoxicity. This study evaluated the potential protective effect of curcumin against renal fibrosis and alterations in TJ and AJ proteins. It was found that CIS induced renal damage, increase in profibrotic proteins such as TGFβ1, decrease in renal expression of occludin, claudin-2 and E-cadherin as well as oxidative/nitrosative stress evaluated by measuring malondialdehyde (MDA) levels, Hsp70/72 expression, 3-nitrotyrosine (3-NT) abundance, and expressions of Nrf2, NADPH oxidase subunits p47phox and gp91phox and protein kinase C (PKC) β2. All these changes were effectively prevented by curcumin pretreatment.
Experimental
Reagents and antibodies
cis-Diammineplatinum(II)dichloride [CIS (Cat. no. 479306, Lt MKBH5984 V)], curcumin [Cat. no. C1386, Lt 079K1756 V], 2,2-azobis(2-amidinopropane)dihydrochloride (AAPH), xanthine, xanthine oxidase, nitroblue tetrazolium (NBT), fluorescein, DL-penicillamine, diethylenetriaminepentaacetic acid (DTPA), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH˙), terephthalic acid (TA), ascorbic acid, Amplex Red, horseradish peroxidase (HRP), sodium pyruvate, dimethylthiourea (DMTU), lipoic acid, GSH, glutathione disulfide (GSSG), 1-chloro-2,4-dinitrobenzene (CDNB), nordihydroguaiaretic acid (NDGA), dimethyl sulfoxide (DMSO), phenylmethylsulfonyl fluoride (PMSF), sodium dodecyl sulfate (SDS), diphenylene iodonium (DPI), 1,3-diphenylisobenzofuran (DPBF), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), collagenase (from Clostridium histolyticum, type II), rabbit anti-PKCβ2 and rabbit anti-Nrf2 antibodies were from Sigma-Aldrich (St. Louis, MO, USA). Tiron (superoxide dismutase mimetic) was from Fluka (St. Louis, MO, USA). Dihydroethidium (DHE) was purchased from Molecular Probes (Eugene, OR, USA). Dihydrorhodamine 123 (DHR-123), 2′,7′-dichlorofluorescein diacetate (DCDHF-DA) and mouse anti-3-NT antibody were from Cayman Chemical Co. (Ann Arbor, MI, USA). Trolox was from EMD Millipore (Billerica, MA, USA). Ethylenediaminetetraacetic acid (EDTA), sodium hypochlorite and H2O2 were from JT Baker (Xalostoc, Edo. Mexico, Mexico). The rabbit anti-claudin-2, rabbit anti-occludin, peroxidase-conjugated anti-rabbit, peroxidase-conjugated anti-mouse, peroxidase-conjugated anti-goat, Alexa Fluor® 488 donkey anti-rabbit, Alexa Fluor® 488 donkey anti-goat and Alexa Fluor® 594 donkey anti-mouse antibodies were purchased from Invitrogen (Carlsbad, CA, USA). Mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was purchased from Millipore Corp. (Billerica, MA, USA). Goat anti-kidney injury molecule-1 (KIM-1) was purchased from R&D Systems (McKinley Place, MN, USA). Mouse anti-Hsp70/72 antibody was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). The mouse anti-dipeptidylpeptidase (DppD)-IV antibody was purchased from AbD Serotec (Raleigh, NC, USA). The mouse anti-desmoplakin (DMPK) antibody was purchased from MP Biomedicals (Solon, OH, USA). The goat anti-p47phox, goat anti-gp91phox, rabbit anti-E-cadherin, rabbit anti-neutrophil gelatinase-associated lipocalin (NGAL), mouse anti-caspase-3, mouse anti-collagen I, mouse anti-collagen IV, mouse anti-α-alpha-smooth muscle actin (α-SMA), rabbit anti-β-catenin and rabbit anti-TGFβ1 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Protease inhibitor cocktail Complete 1X was from Roche Applied Science (Mannheim, Germany). Commercial kits for the measurement of blood urea nitrogen (BUN) and plasma creatinine concentration (Sera-pak plus creatinine, Cat. no. 1001111 and urea, Cat. no. 1001325) were from Spinreact (Girona, Spain). The Micro BCA™ Protein Assay Reagent Kit was from Pierce (Rockford, IL, USA). All other reagents were of analytical grade and commercially available.Ferric reducing ability power (FRAP) assay
The total antioxidant activity of curcumin was determined by the FRAP assay (the ability to reduce Fe3+ to Fe2+) as previously described.41In vitro reactive oxygen species (ROS) scavenging assays
In all scavenging assays solutions of curcumin at different concentrations (from a stock of 3 mg ml−1 in DMSO) were used and the optical densities or fluorescence units were obtained using a Synergy HT multimode microplate reader (Biotek Instruments, Winooski, VT, USA). In each assay a tube with water instead of curcumin or reference compound was used and considered as the 0% of scavenging activity. In addition, the scavenging activity of DMSO (as a vehicle of curcumin) was tested in each assay.Superoxide anion (O2˙−) scavenging assay
O2˙− was generated by the xanthine–xanthine oxidase system. Scavenging activity of curcumin was determined by evaluating its ability to inhibit the O2˙−-induced DHR123 oxidation.42Singlet oxygen (1O2) scavenging assay
1O2 was generated from hypochlorite and H2O2 as previously described.431O2 causes a reduction in fluorescence of DPBF that was determined at excitation and emission wavelengths of 410 nm and 455 nm, respectively.H2O2 scavenging assay
The ability of curcumin to scavenge H2O2 was evaluated using the Amplex Red reagent. This compound is oxidized in the presence of H2O2 to produce resorufin, a fluorescent compound which is measured using excitation and emission filters of 530/25 and 590/35, respectively.43Hydroxyl radical (OH˙) scavenging assay
OH˙ was generated by the Fenton reaction.44 A fluorescent product that was detected at excitation and emission wavelengths of 326 nm and 432 nm, respectively, was obtained.Peroxynitrite anion (ONOO−) scavenging assay
ONOO− was synthesized according to Cervantes et al.43 DCDHF-DA was used as an indicator of the presence of this anion; in the absence of an antioxidant, DCDHF-DA is oxidized to dichlorofluorescein, a fluorescent compound that is measured at excitation and emission wavelengths of 502 and 523 nm, respectively.Hypochlorous acid (HOCl) scavenging assay
The ability of curcumin to scavenge HOCl was determined using para-aminobenzoic acid which reacts with HOCl to produce the fluorescent compound 3-chloro-4-aminobenzoic acid.45 Fluorescence was determined at excitation and emission wavelengths of 280 nm and 340 nm, respectively.Peroxyl radical (ROO˙) scavenging assay
The scavenging activity of curcumin was determined by the stability of fluorescence of fluorescein by ROO˙.46 Fluorescence was determined at excitation and emission wavelengths of 485 nm and 520 nm, respectively, for 1.5 h at 37 °C. At the end of the assay the area under the curve was obtained by using Gen 5 software (Biotek Instruments).In vivo experimental model
Twenty male Wistar rats (200–250 g) were fed with standard chow and water ad libitum. The animals were randomly distributed in 4 groups of 5 rats each: the first group received only vehicle (V, isotonic saline) by intraperitoneal injection (i.p.), the second group received a single i.p. dose of 5 mg kg−1 of CIS, the third group received three doses of curcumin by gavage (Cur + CIS, 200 mg kg−1 + 5 mg kg−1): 30 min before and 24 and 48 hours after CIS injection, and the fourth one received curcumin as described above. The doses of curcumin were based on previous studies47 and the one of cisplatin was based on experiments (see ESI Fig. 1†). Seventy-two hours after CIS administration, rats were anesthetized with sodium pentobarbital (90 mg kg−1, i.p.); blood was collected from the aorta in heparinized tubes. Both kidneys were immediately dissected and frozen by immersion in liquid nitrogen. We followed the guidelines of the Official Mexican Standard Care and Use of Laboratory Animals (NOM-062-ZOO-1999) and the Local Ethics Committee (FQ/CICUAL/069/13) approved the protocol.Determination of renal function
BUN, plasma creatinine, N-acetyl-β-D-glucosaminidase (NAG), NGAL and KIM-1 were used as markers of renal injury.32,48 BUN and plasma creatinine were determined with commercial kits from Spinreact®.49 NAG activity was measured in the kidney tissue by a colorimetric assay, as previously described.50 KIM-1 was measured by western blot and immunofluorescence and NGAL were measured by western blot as described later.Histological studies and apoptosis
Histological studies were performed as previously described.32 H&E-stained paraffin sections were assessed by an expert pathologist in a blind manner to experimental groups, with a digital camera incorporated to a Zeiss Axiophot 2 light microscope by means of an imaging software, AxioVision 4.8. The severity of tubular injury was calculated semi-quantitatively in eight random subcortical periglomerular fields (magnification ×200) per each rat for cell detaching, apoptosis, acute tubular necrosis and cast formation, using a 0–3 scale: 0 (absence); 1+ (mild or <5%); 2+ (moderate or 5 to 25%); 3+ (severe or >25%) of juxtamedullary proximal tubules. Apoptosis was evaluated by analyzing the expression of cleaved-caspase 3 by western blot as described later.Markers of oxidative stress
MDA, O2˙− production in isolated GL and PT, 3-NT, expression of Hsp70/72, expression of PKCβ2, NADPH oxidase subunits p47phox and gp91phox and Nrf2 and activity of antioxidant enzymes were measured as markers of oxidative stress. Renal MDA concentration was measured as previously described.51 The activities of antioxidant enzymes CAT and glutathione reductase (GR) were assayed in kidney homogenates as previously described.28Isolation of GL and PT
GL isolation was performed as previously described.14 PT were isolated by Percoll gradients as previously described14 and was confirmed by light microscopy observation.O2˙− production assay
Fluorescence detection of O2˙− production in GL and PT was performed as described by Trujillo et al.40 Fluorescence intensity of each sample was normalized relative to the control. Protein content was measured using the Lowry method.Extraction of proteins from renal cortex for western blot
Extraction of proteins from the renal cortex was performed as described by Molina-Jijón et al.14 Total protein quantification was performed using the Micro BCA Protein Assay Reagent Kit.Western blot
Western blot analysis was performed as previously described.14 Polyvinylidene difluoride (PVDF) membranes were incubated overnight at 4 °C with the appropriate primary antibodies against NGAL, GAPDH, KIM-1, caspase-3, TGFβ1, collagen I, collagen IV, α-SMA, Nrf2, 3-NT, Hsp70/72, p47phox, gp91phox, PKCβ2, claudin-2, occludin, β-catenin and E-cadherin (used at a dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000). Peroxidase-conjugated anti-rabbit, anti-goat and anti-mouse antibodies were incubated for 1 h at room temperature (used at a dilution 1
1000). Peroxidase-conjugated anti-rabbit, anti-goat and anti-mouse antibodies were incubated for 1 h at room temperature (used at a dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Immunoblots were developed using the ECL™ prime western blotting detection reagent (Amersham™, GE Healthcare, Buckinghamshire, UK). Chemiluminescence was detected using an EC3 Imaging System (UVP BioImaging Systems, Cambridge, UK). Protein band density was quantified by transmittance densitometry (ImageJ software, USA).
000). Immunoblots were developed using the ECL™ prime western blotting detection reagent (Amersham™, GE Healthcare, Buckinghamshire, UK). Chemiluminescence was detected using an EC3 Imaging System (UVP BioImaging Systems, Cambridge, UK). Protein band density was quantified by transmittance densitometry (ImageJ software, USA).
Immunofluorescence
Kidney samples were prepared for immunofluorescence as previously described.14 Kidney sections were incubated overnight at 4 °C with primary antibodies anti-KIM-1, anti-DppD, anti-claudin-2, anti-occludin, anti-E-cadherin (used at a dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and anti-DMPK (used at a dilution 1
100) and anti-DMPK (used at a dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). DppD and DMPK were used as markers of proximal and distal tubules respectively. Secondary antibodies Alexa Fluor® 488 donkey anti-rabbit, Alexa Fluor® 488 donkey anti-goat and Alexa Fluor® 594 donkey anti-mouse were used at a 1
50). DppD and DMPK were used as markers of proximal and distal tubules respectively. Secondary antibodies Alexa Fluor® 488 donkey anti-rabbit, Alexa Fluor® 488 donkey anti-goat and Alexa Fluor® 594 donkey anti-mouse were used at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 dilution. Immunofluorescence was evaluated using a confocal inverted microscope (TCS-SP8, Leica, Heidelberg, Germany). Immunofluorescence experiments were performed at least three times in samples from three different animals per group. Nonspecific labeling was estimated by omission of the primary antibodies.
300 dilution. Immunofluorescence was evaluated using a confocal inverted microscope (TCS-SP8, Leica, Heidelberg, Germany). Immunofluorescence experiments were performed at least three times in samples from three different animals per group. Nonspecific labeling was estimated by omission of the primary antibodies.
Statistical analysis
All the values are expressed as mean ± standard error of the mean (SEM). Results of scavenging ability were expressed as IC50 (ability of the sample to scavenge 50% of each ROS). Values were determined by interpolation using the least squares method calculated from 3 independent experiments. One-way ANOVA and Bonferroni analysis were used to compare the in vivo data of the four groups, p < 0.05 was considered significant.Results and discussion
In vitro antioxidant activity of curcumin
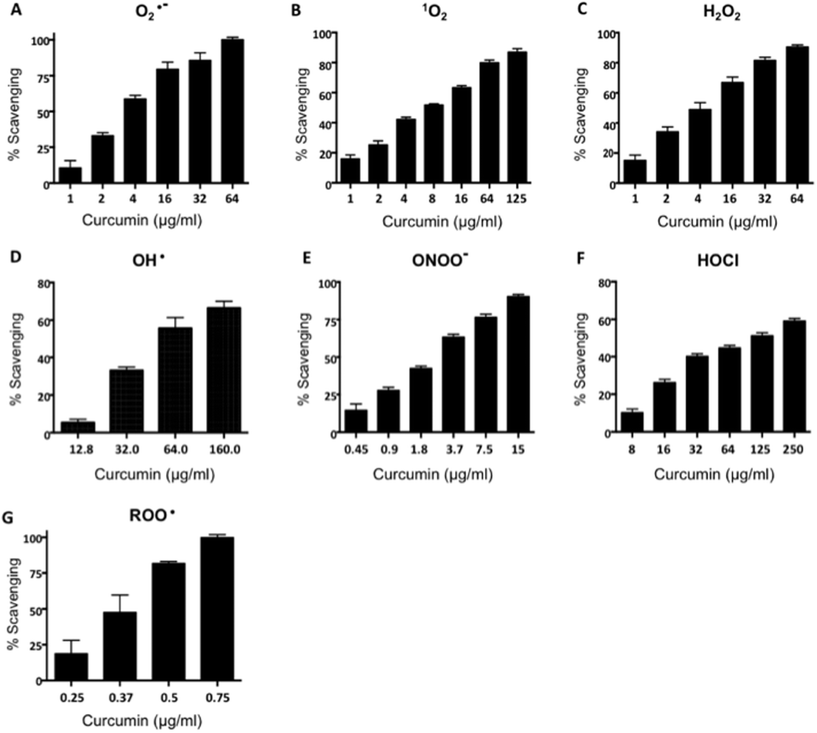
Curcumin is a strong antioxidant compound and its renal protective effects have been studied in several models of renal oxidative damage.19,22,24,25 In order to demonstrate that the curcumin used in this study is functional we determined its antioxidant properties using several antioxidant assays such as the FRAP method and ROS scavenging specific assays. The total antioxidant activity, measured by the FRAP assay, showed that curcumin has the ability to reduce Fe3+ to Fe2+ and this capacity has a value of 4548.2 ± 109.4 μmoles of Fe2SO4 equivalents per g of curcumin. As shown in Fig. 1, the specific antioxidant scavenging capacity of curcumin was tested for O2˙−, 1O2, H2O2, OH˙, ONOO−, HOCl and ROO˙, and compared with reference scavengers. The IC50 values are summarized in Table 1. The order of curcumin IC50 values were the following: HOCl > OH˙ > 1O2 > H2O2 > O2˙− > ONOO− > ROO˙. Curcumin scavenges ROO˙, ONOO−, H2O2, and 1O2 more efficiently than Trolox, penicillamine, sodium pyruvate, and lipoic acid, respectively, since the IC50 value was smaller than the mentioned reference compounds (Table 1). The scavenging activity of curcumin for OH˙ and HOCl was less efficient than their respective reference compounds, DMTU and ascorbic acid, respectively, meanwhile the scavenging activity of curcumin for O2˙− was similar to Tiron. In addition, curcumin was able to scavenge these ROS in a concentration-dependent way (for ROO˙, 0.25–75 μg ml−1, r2 = 0.95315 ± 0.0059; for ONOO−, 0.45–15 μg ml−1, r2 = 0.9489 ± 0.0279; for H2O2, 1–64 μg ml−1, r2 = 0.9734 ± 0.0069; for 1O2, 1–125 μg ml−1, r2 = 0.9795 ± 0.0068; for OH˙, 12.8–160 μg ml−1, r2 = 0.9669 ± 0.0056; for HOCl, 8–250 μg ml−1, r2 = 0.9853 ± 0.0047; for O2˙−, 1 to 64 μg ml−1, r2 = 0.9354 ± 0.0189). These data show that curcumin used in this work has efficient direct antioxidant properties.| O2˙− | 1O2 | H2O2 | OH˙ | ONOO− | HOCl | ROO˙ | |
|---|---|---|---|---|---|---|---|
| a Tiron. b Lipoic acid. c Pyruvate. d Dimethylthiourea. e Penicillamine. f Ascorbic acid. g Trolox. Data are means ± SEM n = 3 experiments. Asterisk indicates statistical significance and p value is indicated below. | |||||||
| Curcumin | 3.9 ± 0.5 | 8.6 ± 0.4 | 7.4 ± 0.3 | 56.2 ± 7.8 | 2.3 ± 0.2 | 106 ± 5 | 0.35 ± 0.03 |
| Reference compound | 3.7 ± 0.3a | 576 ± 6*b | 136.5 ± 9.5*c | 67.0 ± 7.2d | 9.9 ± 1.4*e | 75 ± 3*f | 1.2 ± 0.08*g |
| p | 0.748 | 0.0016 | 0.0002 | 0.7254 | 0.0058 | 0.006 | 0.0006 |
In vivo experimental model
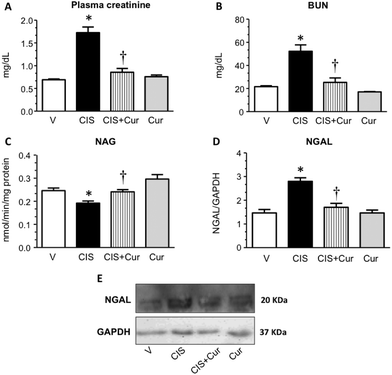
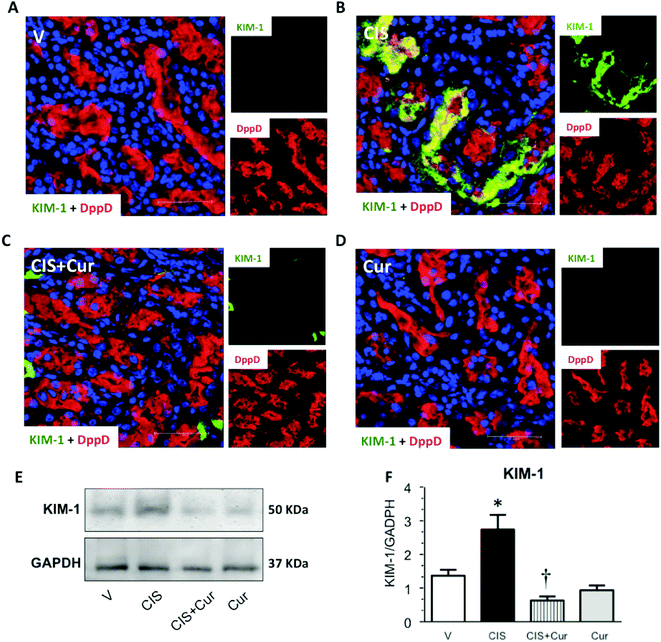
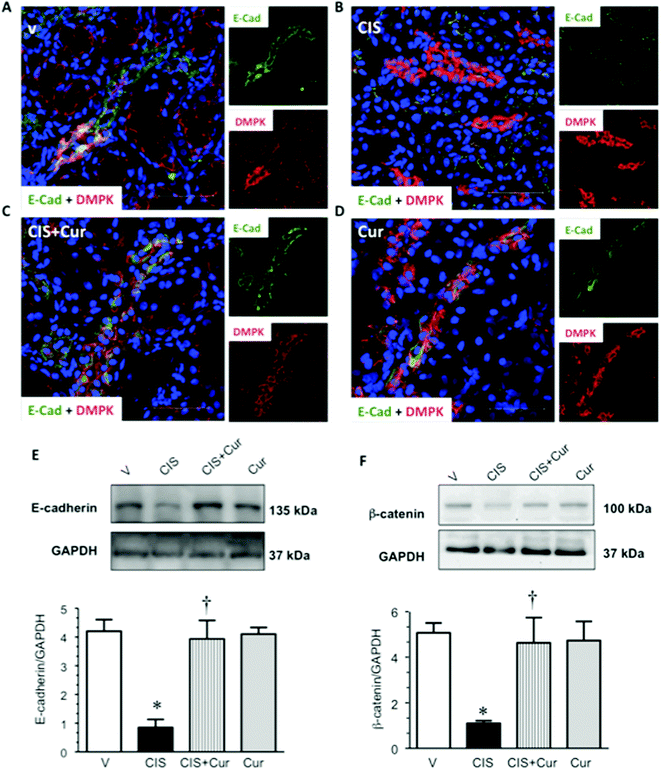
Next, we analyzed the effect of curcumin on renal and tubular dysfunction and injury induced by CIS. As shown, curcumin significantly ameliorated the CIS-induced increment in plasma creatinine (Fig. 2A), BUN (Fig. 2B) and renal expression of NGAL (Fig. 2D and E) and decrement of renal NAG (Fig. 2C). Rats treated with curcumin alone showed similar values compared to the control group. To evaluate tubular injury, KIM-1 (a sensitive marker of tubular damage that is overexpressed when proximal tubules are found under proteinuric, toxic and ischemic kidney disease54,55) expression was assessed by confocal microscopy (Fig. 3A–D) and western blot (Fig. 3E and F) in kidneys from the four experimental groups. It was found that CIS increased KIM-1 expression in proximal tubules (label of KIM-1 co-localized with DppD, a marker of proximal tubular brush border) and that curcumin significantly decreased KIM-1. The curcumin group had similar labeling of KIM-1 to that of the control group. In agreement with the functional results, KIM-1 expression increased in CIS treated rats and curcumin was able to attenuate this alteration. These data suggest that curcumin treatment exerted renoprotective effects on CIS-induced renal damage. However, Namboothiri et al. (2008)56 patented that hydrazino derivatives of curcumin possess an enhanced stability and process for preparation thereof with highly potent chemotherapeutic actions, raising the possibility that these compounds may be better for the treatment of CIS nephrotoxicity.
| Lesion | V | CIS | CIS + Cur | Cur |
|---|---|---|---|---|
| The severity of tubular injury was calculated semi-quantitatively in eight random subcortical periglomerular fields (magnification ×200) per each rat for apoptosis, acute tubular necrosis, cast formation and cell shedding using a 0 to 3 scale: 0, absence; 1+ (mild or <5%); 2+ (moderate or 5 to 25%); 3+ (severe or >25%) of juxtamedullary proximal tubules. | ||||
| Apoptosis | — | +++ | ++ | — |
| Acute tubular necrosis | — | + | — | — |
| Tubular cast | — | +++ | ++ | — |
| Cell shedding | + | +++ | +++ | + |
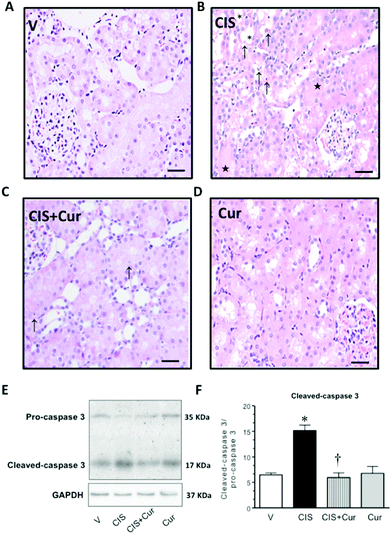
To evaluate cell death by apoptosis, western blot of caspase 3 was performed in the renal tissue (Fig. 4E and F). As shown, curcumin decreased the increment of cleaved-caspase 3 (Fig. 4E, band of 17 kDa) induced by CIS. This is in agreement with previous studies where the anti-apoptotic mechanism exerted by curcumin is associated with its ability to inhibit TGF-β signaling62 and to induce Bcl2 expression.63 However, no changes were found in the pro-caspase 3 expression in the four experimental groups studied (Fig. 4E, band of 35 kDa). Densitometric analysis of the cleaved-caspase 3/pro-caspase 3 ratio is shown in Fig. 4F. These data suggest that curcumin exerts an antiapoptotic effect in CIS-induced renal injury.
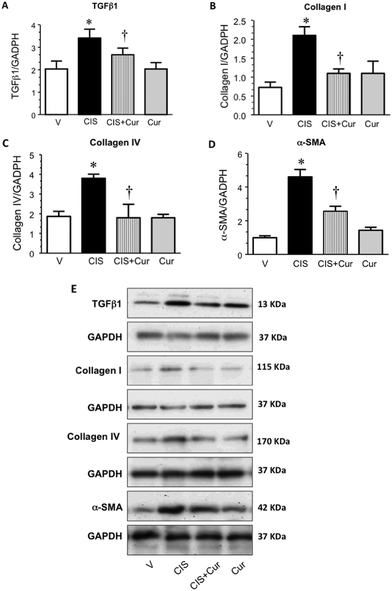
Together with TGFβ known as one of strong profibrogenic factors that mediates EMT,74,75 in this process epithelial markers such as E-cadherin and claudins are lost whilst increased expression of collagens and α-SMA are favored. Based on the findings described above it can be concluded that curcumin decreases CIS-induced EMT and fibrosis. Thus, curcumin treatment was able to prevent CIS-induced mislocalization of TJ and AJ proteins from cell borders.66 In conclusion, these data suggest that curcumin treatment prevents loss of cell–cell contacts induced by CIS.
Conclusions
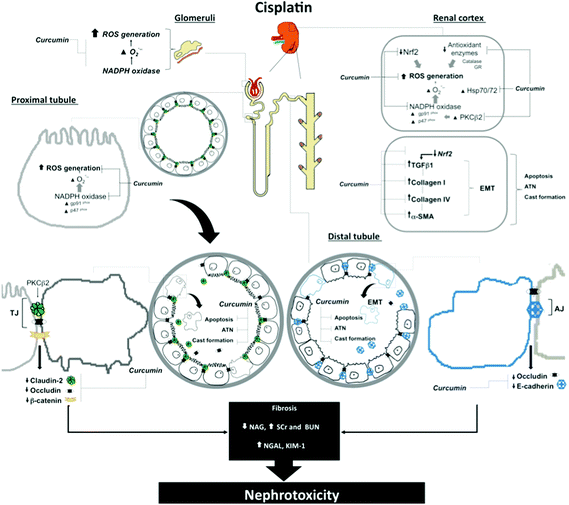
In conclusion, we propose that the pathway through which curcumin ameliorates CIS-induced EMT and fibrosis and decreased expression of the tight (claudin-2 and occludin) and adherens (E-cadherin) junction proteins might be linked to its antioxidant properties. A scheme showing the mechanism proposed in this study is shown in Fig. 10.Acknowledgements
CONACYT (Grants 220046 and 252008) and PAPIIT no. IN210713 supported this work.References
- M. Furuse, T. Hirase, M. Itoh, A. Nagafuchi, S. Yonemura, S. Tsukita and S. Tsukita, J. Cell Biol., 1993, 123, 1777–1788 CrossRef CAS.
- M. Furuse, K. Fujita, T. Hiiragi, K. Fujimoto and S. Tsukita, J. Cell Biol., 1998, 141, 1539–1550 CrossRef CAS.
- O. Kwon, B. D. Myers, R. Sibley, D. Dafoe, E. Alfrey and W. J. Nelson, J. Histochem. Cytochem., 1998, 46, 1423–1434 CrossRef CAS PubMed.
- J. L. Reyes, M. Lamas, D. Martin, M. del Carmen Namorado, S. Islas, J. Luna, M. Tauc and L. Gonzalez-Mariscal, Kidney Int., 2002, 62, 476–487 CrossRef CAS PubMed.
- A. H. Enck, U. V. Berger and A. S. Yu, Am. J. Physiol. Renal Physiol., 2001, 281, F966–F974 CrossRef CAS PubMed.
- Y. Kiuchi-Saishin, S. Gotoh, M. Furuse, A. Takasuga, Y. Tano and S. Tsukita, J. Am. Soc. Nephrol., 2002, 13, 875–886 CAS.
- R. Rosenthal, S. Milatz, S. M. Krug, B. Oelrich, J. D. Schulzke, S. Amasheh, D. Gunzel and M. Fromm, J. Cell Sci., 2010, 123, 1913–1921 CrossRef CAS PubMed.
- A. O. Perantoni, Exp. Nephrol., 1999, 7, 80–102 CrossRef CAS PubMed.
- G. Zheng, J. G. Lyons, T. K. Tan, Y. Wang, T. T. Hsu, D. Min, L. Succar, G. K. Rangan, M. Hu, B. R. Henderson, S. I. Alexander and D. C. Harris, Am. J. Pathol., 2009, 175, 580–591 CrossRef CAS PubMed.
- W. C. Prozialeck and R. J. Niewenhuis, Toxicol. Appl. Pharmacol., 1991, 107, 81–97 CrossRef CAS.
- G. Jacquillet, O. Barbier, M. Cougnon, M. Tauc, M. C. Namorado, D. Martin, J. L. Reyes and P. Poujeol, Am. J. Physiol. Renal Physiol., 2006, 290, F127–F137 CrossRef CAS PubMed.
- L. Arreola-Mendoza, L. M. Del Razo, M. E. Mendoza-Garrido, D. Martin, M. C. Namorado, J. V. Calderon-Salinas and J. L. Reyes, Toxicol. Lett., 2009, 191, 279–288 CrossRef CAS PubMed.
- T. N. Meyer, C. Schwesinger, J. Ye, B. M. Denker and S. K. Nigam, J. Biol. Chem., 2001, 276, 22048–22055 CrossRef CAS PubMed.
- E. Molina-Jijón, R. Rodriguez-Munoz, C. Namorado Mdel, J. Pedraza-Chaverri and J. L. Reyes, Free Radical Biol. Med., 2014, 72, 162–175 CrossRef PubMed.
- T. Esatbeyoglu, P. Huebbe, I. M. Ernst, D. Chin, A. E. Wagner and G. Rimbach, Angew. Chem., 2012, 51, 5308–5332 CrossRef CAS PubMed.
- A. Goel, A. B. Kunnumakkara and B. B. Aggarwal, Biochem. Pharmacol., 2008, 75, 787–809 CrossRef CAS PubMed.
- T. Osawa, Adv. Exp. Med. Biol., 2007, 595, 407–423 CrossRef.
- A. Gonzalez-Salazar, E. Molina-Jijon, F. Correa, G. Zarco-Marquez, M. Calderon-Oliver, E. Tapia, C. Zazueta and J. Pedraza-Chaverri, Cardiovasc. Toxicol., 2011, 11, 357–364 CrossRef CAS PubMed.
- F. Correa, M. Buelna-Chontal, S. Hernandez-Resendiz, W. R. García-Niño, F. J. Roldán, V. Soto, A. Silva-Palacios, A. Amador, J. Pedraza-Chaverri, E. Tapia and C. Zazueta, Free Radical Biol. Med., 2013, 61, 119–129 CrossRef CAS PubMed.
- I. Carmona-Ramirez, A. Santamaria, J. C. Tobon-Velasco, M. Orozco-Ibarra, I. G. Gonzalez-Herrera, J. Pedraza-Chaverri and P. D. Maldonado, J. Nutr. Biochem., 2013, 24, 14–24 CrossRef CAS PubMed.
- L. M. Reyes-Fermin, S. Gonzalez-Reyes, N. G. Tarco-Alvarez, M. Hernandez-Nava, M. Orozco-Ibarra and J. Pedraza-Chaverri, Nutr. Neurosci., 2012, 15, 34–41 CrossRef CAS PubMed.
- M. Ueki, M. Ueno, J. Morishita and N. Maekawa, J. Biosci. Bioeng., 2013, 115, 547–551 CrossRef CAS PubMed.
- E. Tapia, V. Soto, K. M. Ortiz-Vega, G. Zarco-Marquez, E. Molina-Jijon, M. Cristobal-Garcia, J. Santamaria, W. R. Garcia-Nino, F. Correa, C. Zazueta and J. Pedraza-Chaverri, Oxid. Med. Cell. Longevity, 2012, 2012, 269039 Search PubMed.
- E. Tapia, Z. L. Zatarain-Barron, R. Hernandez-Pando, G. Zarco-Marquez, E. Molina-Jijon, M. Cristobal-Garcia, J. Santamaria and J. Pedraza-Chaverri, Phytomedicine, 2013, 20, 359–366 CrossRef CAS PubMed.
- E. Molina-Jijon, E. Tapia, C. Zazueta, M. El Hafidi, Z. L. Zatarain-Barron, R. Hernandez-Pando, O. N. Medina-Campos, G. Zarco-Marquez, I. Torres and J. Pedraza-Chaverri, Free Radical Biol. Med., 2011, 51, 1543–1557 CrossRef CAS PubMed.
- A. T. Dinkova-Kostova and P. Talalay, Mol. Nutr. Food Res., 2008, 52(Suppl. 1), S128–S138 Search PubMed.
- V. Calabrese, T. E. Bates, C. Mancuso, C. Cornelius, B. Ventimiglia, M. T. Cambria, L. Di Renzo, A. De Lorenzo and A. T. Dinkova-Kostova, Mol. Nutr. Food Res., 2008, 52, 1062–1073 CAS.
- J. M. Perez-Rojas, C. Cruz, P. Garcia-Lopez, D. J. Sanchez-Gonzalez, C. M. Martinez-Martinez, G. Ceballos, M. Espinosa, J. Melendez-Zajgla and J. Pedraza-Chaverri, Free Radical Res., 2009, 43, 1122–1132 CrossRef CAS PubMed.
- J. M. Perez-Rojas, C. E. Guerrero-Beltran, C. Cruz, D. J. Sanchez-Gonzalez, C. M. Martinez-Martinez and J. Pedraza-Chaverri, Food Chem. Toxicol., 2011, 49, 2631–2637 CrossRef CAS PubMed.
- C. E. Guerrero-Beltran, P. Mukhopadhyay, B. Horvath, M. Rajesh, E. Tapia, I. Garcia-Torres, J. Pedraza-Chaverri and P. Pacher, J. Nutr. Biochem., 2012, 23, 494–500 CrossRef CAS PubMed.
- Y. I. Chirino and J. Pedraza-Chaverri, Exp. Toxicol. Pathol., 2009, 61, 223–242 CrossRef CAS PubMed.
- J. Trujillo, E. Molina-Jijon, O. N. Medina-Campos, R. Rodriguez-Munoz, J. L. Reyes, M. L. Loredo, E. Tapia, L. G. Sanchez-Lozada, D. Barrera-Oviedo and J. Pedraza-Chaverri, Toxicol. Mech. Methods, 2014, 24, 520–528 CrossRef CAS PubMed.
- A. Kuhad, S. Pilkhwal, S. Sharma, N. Tirkey and K. Chopra, J. Agric. Food Chem., 2007, 55, 10150–10155 CrossRef CAS PubMed.
- S. Ugur, R. Ulu, A. Dogukan, A. Gurel, I. P. Yigit, N. Gozel, B. Aygen and N. Ilhan, Renal Failure, 2015, 37, 332–336 CrossRef CAS PubMed.
- K. Sahin, M. Tuzcu, H. Gencoglu, A. Dogukan, M. Timurkan, N. Sahin, A. Aslan and O. Kucuk, Life Sci., 2010, 87, 240–245 CrossRef CAS PubMed.
- K. Sahin, M. Tuzcu, N. Sahin, S. Ali and O. Kucuk, Food Chem. Toxicol., 2010, 48, 2670–2674 CrossRef CAS PubMed.
- L. M. Aleksunes, M. J. Goedken, C. E. Rockwell, J. Thomale, J. E. Manautou and C. D. Klaassen, J. Pharmacol. Exp. Ther., 2010, 335, 2–12 CrossRef CAS PubMed.
- U. Kilic, E. Kilic, Z. Tuzcu, M. Tuzcu, I. H. Ozercan, O. Yilmaz, F. Sahin and K. Sahin, Nutr. Metab., 2013, 10, 7 CAS.
- G. Briassoulis, E. Briassouli, D. M. Fitrolaki, I. Plati, K. Apostolou, T. Tavladaki and A. M. Spanaki, BioMed Res. Int., 2014, 2014, 101023 Search PubMed.
- J. Trujillo, E. Molina-Jijon, O. N. Medina-Campos, R. Rodriguez-Munoz, J. L. Reyes, D. Barrera and J. Pedraza-Chaverri, J. Biochem. Mol. Toxicol., 2015, 29, 149–156 CrossRef CAS PubMed.
- I. F. Benzie and J. J. Strain, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS PubMed.
- S. Yanagisawa, A. Koarai, H. Sugiura, T. Ichikawa, M. Kanda, R. Tanaka, K. Akamatsu, T. Hirano, K. Matsunaga, Y. Minakata and M. Ichinose, Respir. Res., 2009, 10, 50 CrossRef PubMed.
- M. I. Cervantes, P. M. de Oca Balderas, J. de Jesus Gutierrez-Banos, M. Orozco-Ibarra, B. Fernandez-Rojas, O. N. Medina-Campos, M. Espinoza-Rojo, M. Ruiz-Tachiquin, A. Ortiz-Plata, M. I. Salazar, M. Rubio-Osornio, E. Castaneda-Saucedo, J. Pedraza-Chaverri, F. Calzada and P. Aguilera, Food Chem., 2013, 140, 343–352 CrossRef CAS PubMed.
- L. Gaona-Gaona, E. Molina-Jijon, E. Tapia, C. Zazueta, R. Hernandez-Pando, M. Calderon-Oliver, G. Zarco-Marquez, E. Pinzon and J. Pedraza-Chaverri, Toxicology, 2011, 286, 20–27 CrossRef CAS PubMed.
- P. Van Antwerpen and J. Neve, Eur. J. Pharmacol., 2004, 496, 55–61 CrossRef CAS PubMed.
- D. Huang, B. Ou, M. Hampsch-Woodill, J. A. Flanagan and R. L. Prior, J. Agric. Food Chem., 2002, 50, 4437–4444 CrossRef CAS PubMed.
- M. Waseem, P. Kaushik and S. Parvez, Cell Biochem. Funct., 2013, 31, 678–684 CrossRef CAS PubMed.
- N. Srisawat, R. Murugan and J. A. Kellum, Nephron. Clin. Pract., 2014, 127, 185–189 CrossRef CAS PubMed.
- C. E. Guerrero-Beltran, M. Calderon-Oliver, E. Tapia, O. N. Medina-Campos, D. J. Sanchez-Gonzalez, C. M. Martinez-Martinez, K. M. Ortiz-Vega, M. Franco and J. Pedraza-Chaverri, Toxicol. Lett., 2010, 192, 278–285 CrossRef CAS PubMed.
- Y. I. Chirino, R. Hernandez-Pando and J. Pedraza-Chaverri, BMC Pharmacol., 2004, 4, 20 CrossRef PubMed.
- A. S. Jimenez-Osorio, A. Picazo, S. Gonzalez-Reyes, D. Barrera-Oviedo, M. E. Rodriguez-Arellano and J. Pedraza-Chaverri, Int. J. Mol. Sci., 2014, 15, 20290–20305 CrossRef CAS PubMed.
- R. P. Miller, R. K. Tadagavadi, G. Ramesh and W. B. Reeves, Toxins, 2010, 2, 2490–2518 CrossRef CAS PubMed.
- R. Imamdi, M. de Graauw and B. van de Water, J. Pharmacol. Exp. Ther., 2004, 311, 892–903 CrossRef CAS PubMed.
- A. B. Kramer, M. M. van Timmeren, T. A. Schuurs, V. S. Vaidya, J. V. Bonventre, H. van Goor and G. Navis, Am. J. Physiol. Renal Physiol., 2009, 296, F1136–F1145 CrossRef CAS PubMed.
- J. V. Bonventre, Nephrol., Dial., Transplant., 2009, 24, 3265–3268 CrossRef CAS PubMed.
- I. N. Namboothiri, D. Panda, I. Deb, A. Mehta and V. Wadhawan, Bombay Pat., 254150, 2008 Search PubMed; US Pat., SN, 2012 Search PubMed.
- A. Atessahin, A. O. Ceribasi, A. Yuce, O. Bulmus and G. Cikim, Basic Clin. Pharmacol. Toxicol., 2007, 100, 121–126 CAS.
- B. Rasoulian, M. Jafari, M. Mahbod, M. E. Dehaj, M. Nowrozi, H. Wahhabaghai, M. Mofid, A. Ghasemi, M. R. Bigdeli and A. Khoshbaten, Renal Failure, 2010, 32, 234–242 CrossRef CAS PubMed.
- A. J. Danford, D. Wang, Q. Wang, T. D. Tullius and S. J. Lippard, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 12311–12316 CrossRef CAS PubMed.
- N. M. Yunos, P. Beale, J. Q. Yu and F. Huq, Anticancer Res., 2011, 31, 4283–4289 CAS.
- E. Tapia, L. G. Sanchez-Lozada, W. R. Garcia-Nino, E. Garcia, A. Cerecedo, F. E. Garcia-Arroyo, H. Osorio, A. Arellano, M. Cristobal-Garcia, M. L. Loredo, E. Molina-Jijon, J. Hernandez-Damian, M. Negrette-Guzman, C. Zazueta, S. Huerta-Yepez, J. L. Reyes, M. Madero and J. Pedraza-Chaverri, Free Radical Res., 2014, 48, 1342–1354 CrossRef CAS PubMed.
- A. S. Awad and A. A. El-Sharif, Int. Immunopharmacol., 2011, 11, 992–996 CrossRef CAS PubMed.
- A. M. El-Mahalaway, Int. J. Clin. Exp. Pathol., 2015, 8, 6019–6030 Search PubMed.
- Y. O. Ilbey, E. Ozbek, M. Cekmen, A. Simsek, A. Otunctemur and A. Somay, Hum. Reprod., 2009, 24, 1717–1725 CrossRef CAS PubMed.
- Y. Kawai, T. Satoh, D. Hibi, Y. Ohno, Y. Kohda, K. Miura and M. Gemba, J. Pharmacol. Sci., 2009, 111, 433–439 CrossRef CAS.
- N. Wang, G. Wang, J. Hao, J. Ma, Y. Wang, X. Jiang and H. Jiang, Dig. Dis. Sci., 2012, 57, 1792–1801 CrossRef CAS PubMed.
- K. Sahin, C. Orhan, Z. Tuzcu, M. Tuzcu and N. Sahin, Food Chem. Toxicol., 2012, 50, 4035–4041 CrossRef CAS PubMed.
- R. Radi, Acc. Chem. Res., 2013, 46, 550–559 CrossRef CAS PubMed.
- S. Ikeda, A. Fukuzaki, H. Kaneto, S. Ishidoya and S. Orikasa, Int. J. Urol., 1999, 6, 245–250 CrossRef CAS.
- T. Gomez-Sierra, E. Molina-Jijon, E. Tapia, R. Hernandez-Pando, W. R. Garcia-Nino, P. D. Maldonado, J. L. Reyes, D. Barrera-Oviedo, I. Torres and J. Pedraza-Chaverri, J. Pharm. Pharmacol., 2014, 66, 1271–1281 CrossRef CAS PubMed.
- L. Gonzalez-Mariscal, M. C. Namorado, D. Martin, J. Luna, L. Alarcon, S. Islas, L. Valencia, P. Muriel, L. Ponce and J. L. Reyes, Kidney Int., 2000, 57, 2386–2402 CrossRef CAS PubMed.
- L. Gonzalez-Mariscal, R. Tapia and D. Chamorro, Biochim. Biophys. Acta, 2008, 1778, 729–756 CrossRef CAS PubMed.
- E. Molina-Jijon, R. Rodriguez-Munoz, C. Namorado Mdel, P. Bautista-Garcia, O. N. Medina-Campos, J. Pedraza-Chaverri and J. L. Reyes, J. Nutr. Biochem., 2015, 26, 441–454 CrossRef CAS PubMed.
- T. A. Wynn, J. Pathol., 2008, 214, 199–210 CrossRef CAS PubMed.
- D. Pohlers, J. Brenmoehl, I. Loffler, C. K. Muller, C. Leipner, S. Schultze-Mosgau, A. Stallmach, R. W. Kinne and G. Wolf, Biochim. Biophys. Acta, 2009, 1792, 746–756 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5fo00624d |
| ‡ Both authors contributed equally to this work and should be considered as first authors. |
| This journal is © The Royal Society of Chemistry 2016 |