Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Brønsted activity of two-dimensional zeolites compared to bulk materials
Joachim
Sauer
*
Humboldt-Universität, Institut für Chemie, Berlin, Germany. E-mail: js@chemie.hu-berlin.de
First published on 11th April 2016
Abstract
Different reactivity parameters yield different results for the relative acidity of zeolitic Brønsted sites in thin films and in bulk materials. Whereas the adsorption energies of ammonia and pyridine are about the same, the energy of deprotonation is much lower for two-dimensional systems than for three-dimensional systems. It is shown that this is due to the smaller effective dielectric constant of two-dimensional systems, which leads to much lower deprotonation energies, but also to much lower interaction energies between the protonated molecule and the negatively charged surface site. In the total adsorption energies, both effects nearly compensate each other.
1. Introduction
The catalytic activity1,2 of the proton forms of zeolites originates from bridging Al–O(H)–Si groups which represent Brønsted acid sites.3,4 They are located at the internal surface of the channels and cavities of the crystalline zeolite structures. In recent years two-dimensional zeolites, i.e. nanosheets as thin as single unit cells of, e.g. MFI,5,6 have found much interest.7 To understand if “being two-dimensional” affects the catalytic activity, we compare the protonation ability of bridging Al–O(H)–Si groups of three-dimensional (3d, bulk) and two-dimensional (2d) zeolites. As a model for the latter, we consider an aluminosilicate bilayer with a two-dimensional arrangement of hexagonal prisms (H-2dH) and compare it with the protonated form of H-chabazite (H-CHA)8,9 and other zeolites. Such a protonated aluminosilicate bilayer has indeed been grown as an ultra-thin film under UHV conditions by deposition of O2, Si and Al on the Ru(0001) surface and subsequent adsorption and desorption of water.8,10 It has also been speculated that low-density large-pore zeolites can be assembled from such double-layer aluminosilicate sheets.11A flat surface may be considered as the limiting case of a pore with an infinitely large curvature. The surface curvature effect of Derouane12 (Fig. 1) has been invoked to explain the smaller adsorption energies for CO, C2H4, and pyridine on the two-dimensional system with a flat surface compared to the microporous bulk systems.8 If this effect (largely due to dispersion) is approximately eliminated from the adsorption system, the remaining “specific” interaction is larger for Brønsted sites on the 2d system as compared to the 3d system.8
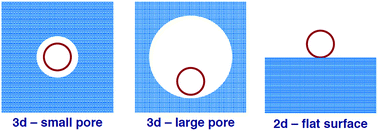 | ||
| Fig. 1 Worsening of the fit of a molecule (red circle) to the surface from a small pore to a large pore 3d system and to a flat surface. | ||
IR spectroscopy has shown that the OH frequency-shifts on adsorption of carbon monoxide and ethene are indeed somewhat larger for the proton forms of aluminosilicate bilayers than for acidic zeolites (H-CHA and H-ZSM-5).8 Both the 2d and 3d systems are able to protonate ammonia and pyridine.8 In contrast, much lower deprotonation energies have been obtained for the two-dimensional systems (H-2dH) compared to the bulk system (H-CHA).9 Hence, the deprotonation energy points to a special effect of being two-dimensional, different from the surface curvature effect, and the question arises: Why is this not seen in the energies of adsorption of ammonia or pyridine that involve proton transfer? Answering this question is the aim of this study.
2. Methods
There are different measures of acidity strength,13–15i.e. parameters that are related to the (free) energy for the protonation of a substrate molecule S by a zeolitic hydroxyl group ZO–H,| Z–OH + S → Z–O−⋯HS+. | (1) |
They may be categorized according to different stages of reaction (1), ranging from little mutual perturbation of the reactants (“early” transition state) to completed proton transfer (“late” transition state). Parameters that describe the intrinsic acidity without any interaction with a substrate molecule are the OH frequency13,16 (lower frequency – weaker OH bond – stronger acid) and the 1H-NMR shift17,18 (larger shift – more positive charge on proton site – stronger acid) for the initial state, as well as the deprotonation energy (or the proton affinity of the deprotonated Brønsted site) for the final state.4
Although the deprotonation energy neglects interactions between the substrate and the catalyst surface, often it predicts reactivity trends correctly. An example is the H-SSZ-13/H-SAPO-34 comparison: two catalysts with the same active site and framework structure (CHA), but with different framework compositions, SiO2/AlPO4. The calculations yielded deprotonation energies that were, depending on the specific method, 30–40 kJ mol−1 higher for H-SAPO-34 than for H-SSZ-13, indicating a lower acidity of the former. This fits the statement “Another key feature of the SAPO-34 molecular sieve is its optimized acidity relative to aluminosilicate based zeolitic materials” in a publication on the UOP/hydro methanol-to-olefin process.19
The OH frequency shift on adsorption of weak bases such as CO and C2H2 is among the parameters that take the interaction with a probe molecule (model substrate) into account and represent an “early” transition state. In contrast, adsorption of strong bases like ammonia or pyridine implies a late transition state with the proton transferred to the reaction partner.
The advantage of a computational approach is that all these parameters can be calculated and their relation can be analysed, see ref. 18, 20 and 21 for comparisons between different parameters. The calculations use density functional theory (DFT) and apply periodic boundary conditions.
The hybrid QM/MM calculations are performed with the B3LYP hybrid functional (QM) and shell-model ion-pair potential for the periodic structure (MM).22 Harmonic frequencies are calculated for the QM cluster models at the QM:MM structures. They are scaled with a factor of 0.9716, obtained by comparison of the B3LYP frequencies for water and methanol with experimental values.22 When calculating deprotonation energies, a constant is added to eliminate the effect of different systematic errors in different computational models. Values of −46 and 0 kJ mol−1 for Hartree–Fock and DFT (B3LYP) results, respectively, are obtained from calibration calculations for the related molecules methanol and silanol for which very accurate deprotonation energies are known.9,23 For further details on QM/MM calculations see ref. 9.
Details of the full DFT (PBE functional + dispersion) slab calculations of the interaction of CO, C2H4, NH3, and NC5H5 with acidic sites are given in ref. 8. The OH vibrational wavenumbers for the 2dH system have been calculated from the DFT bond distances making use of the ω/r correlation with anharmonicity corrections proposed by Nachtigall.24
3. Results and discussion
Table 1 shows the OH stretching frequency of the H-2dH system and compares it with those of other zeolite frameworks. In the CHA and FAU frameworks there is only one crystallographic position for substitution of Si by Al, but there are two different oxygen positions at which the protons can sit. This gives rise to a high-frequency and a low-frequency band for H-CHA and H-FAU. Table 1 shows that the result for H-2dH is bracketed by the high- and low-frequencies of H-CHA, which themselves are embraced by the corresponding frequencies for H-FAU. The calculations reproduce the sequence of the experimental frequencies and, on both scales, the H-2dH result is very close to the result for H-ferrierite (H-FER).| Zeolite | Sitea | ν OH, obsdb | ν OH, calcd | E DP | E DP |
|---|---|---|---|---|---|
| a Crystallographic position. b See computational articles for original references to experimental results. c Ref. 22. d Ref. 25. e Ref. 9 – the Ru(0001) surface was included in the model. f Ref. 26 and 27 – corrected by −46 kJ mol−1, cf.ref. 28. g The correction term is zero. h Ref. 23 – Al/Si = 1/11 for CHA. i Ref. 9 – Al/Si = 1/7 for 2dH, the Ru(0001) surface was not included in the 2dH model. j Ref. 25. | |||||
| FAU | O1H | 3623 | 3626c | 1206 | 1198h |
| MFI | O7–H | 3610 | 3608c | 1235 | |
| CHA | O1H | 3603 | 3606c | 1225 | 1233i |
| FER | O7–H | 3609 | 3598d | 1218j | |
| 2dH | O2–H | 3594 | 3600e | 1069i | |
| CHA | O3–H | 3579 | 3588c | ||
| FAU | O3–H | 3550 | 3563c | 1203 | |
Whereas the unperturbed OH group of H-2dH does not show any peculiarity among the zeolitic OH groups, this changes when probe molecules are used. The OH frequency shift measured on adsorption of CO on H-2dH is 379 cm−1, 63 cm−1 larger than the shifts for H-CHA.8 The calculated shift is also larger, but only 23 cm−1.8 If one uses the relation between frequency shift and deprotonation enthalpy suggested by Paukshtis and Yurchenko,29,30 these frequency shifts translate into deprotonation enthalpies for H-2dH that are 35 and 12 kJ mol−1, respectively, lower than the values for H-CHA, indicating a higher acidity of the two-dimensional system.9
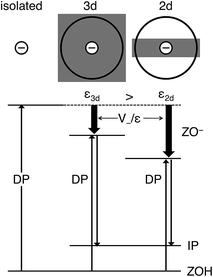
The surprise comes when the deprotonation energy is explicitly calculated. The value for H-2dH is as much as 164 kJ mol−1 lower than that for H-CHA (Table 2). Obviously, the correlation between an initial state parameter (OH frequency shift on complex formation with the weak base CO) and a final state parameter (deprotonation energy) breaks down. The exceptionally low value is a true consequence of the low dimensionality of the thin film that results in a much lower effective dielectric constant for the thin film (1.8) than for the bulk material of the same chemical composition (2.9). As a result, in the thin film, the charge created on deprotonation is less screened and better stabilized by electrostatic interactions with the surrounding ions, as illustrated in Fig. 2. The acidity increase will be less strong if the 2d-system is not a free-standing film in vacuum, but a film on a metal substrate,9 or if the 2d zeolite sheet is not used for gas phase catalysis, but is surrounded by solvent molecules.
| Zeolite | H-CHA | H-2dH | Difference | |||
|---|---|---|---|---|---|---|
| ΔEDP(ZOH)a | 1234 | 1069 | −165 | |||
| a Ref. 9. b Ref. 31. c Ref. 8. d E Ads(S) – dispersion. | ||||||
| S | NH3 | NC5H5 | NH3 | NC5H5 | NH3 | NC5H5 |
| −ΔEPA(S)b | 854 | 929 | 854 | 929 | 854 | 929 |
| +ΔEIP | −539 | −487 | −375 | −285 | 163 | 202 |
| ΔEAds(S)c | −159 | −182 | −160 | −145 | −2 | 37 |
| Dispersionc | −27 | −66 | −29 | −47 | +2 | 19 |
| “Active site”d | −131 | −116 | −131 | −98 | 0 | 18 |
Such a high acidity seems to be in contradiction with the protonation ability of Brønsted sites in the H-2dH films. As other zeolites, they are found to protonate NH3 and NC5H5, whereas on adsorption of CO and C2H4 the proton is not transferred.8 Moreover, when looking at the adsorption energy differences for the molecules that undergo protonation, we find almost no change for NH3, whereas for NC5H5 we find a 37 kJ mol−1 less favourable reaction energy on the H-2dH film. We can use the dispersion contribution to the binding energy to estimate the surface curvature effect12 which yields larger energies for adsorption in a micropore as compared to a flat surface (Table 2). If we remove the dispersion contribution, we will get an interaction energy that relates to the “active site”. For NH3 this parameter does not indicate any difference between the Brønsted site reactivity in a 3d bulk material and a 2d material, while for NC5H5 it indicates an even higher activity for the 3d bulk material.
How can this be understood in view of the much lower deprotonation energy of the H-2dH system? The energy of adsorption, ΔEAds, for the formation of an interacting ion pair (eqn (1)) can be decomposed27,32 into the energy of the interaction, ΔEIP, between the protonated species and the deprotonated surface site,
| Z–O− + HS+ → Z–O−⋯HS+ | (2) |
| Z–OH → Z–O− + H+ | (3) |
| S + H+ → HS+. | (4) |
Hence,
| ΔEAds = ΔEIP + ΔEDP + ΔEPA. | (5) |
Table 2 shows the different contributions to ΔEAds. The ion-pair stabilization ΔEIP is much smaller for the 2d system than for the 3d bulk system. For NH4+ formation, the difference of the ion-pair stabilization energies outweighs the difference of the deprotonation energies, whereas for HNC5H5 formation, the decrease in ion-pair stabilization (202 kJ mol−1) is even larger than the decrease of the deprotonation energy (165 kJ mol−1). The lower (in absolute terms) ion-pair stabilization energy for 2d systems has the same origin as the lower deprotonation energy: the negative charge in the deprotonated system is more stabilized by surrounding charges if it is less screened (small dielectric constant), and it costs less energy to create it. In turn, less energy is gained when annihilating this charge by ion-pair formation.
For different zeolite structures, it has been shown before that the sequence of deprotonation energies and the sequence of NH3 adsorption energies is not the same (Table 3).27 For the four different framework structures, the deprotonation energies vary over 29 kJ mol−1 and the ion-pair formation energies over 23 kJ mol−1, whereas the energies of NH3 adsorption vary over 10 kJ mol−1 only. The differences of the ion-pair formation energies partially outweigh differences of the deprotonation energies. This general compensation effect is connected with the properties of conjugated acid–base pairs. The anion Z–O− formed on deprotonation of a stronger acid Z–OH is a weaker base and, hence, binds the “acid” NH4+ less strongly. In addition, there are effects connected with the specific local surface structure around the active site.
| Zeolite | H-FAU | H-CHA | H-MOR | H-MFI |
|---|---|---|---|---|
| ΔEDP | 1206 | 1225 | 1230 | 1235 |
| ΔEIP | −457 | −476 | −484 | −480 |
| ΔEAds | −109 | −109 | −116 | −106 |
For the isomorphous substitution of metal ions into microporous aluminium phosphates (AlPO-34) and chabazite, the trend has been confirmed that NH3 adsorbs more strongly if the deprotonation energy becomes smaller, but the correlation is not quantitative.21 Although a good correlation between the heat of NH3 adsorption and the enthalpy barrier for the methylation of propene could be established,21 this may not always be the case, for example different reactivity sequences of H-MFI and H-MOR have been predicted if substituted amines and pyridines are used as probe molecules.33
4. Conclusions
Calculated deprotonation energies indicate a much higher intrinsic acidity for Brønsted sites in two-dimensional aluminosilicates than in bulk zeolites, whereas the energy of ammonia adsorption shows hardly any difference. The difference between the two reactivity parameters is due to the interaction of the NH4+ cation formed on protonation of NH3 with the negatively charged surface site. The differences of this interaction between the two- and three-dimensional case outweigh the differences in the deprotonation energies.Whereas the deprotonation energy is a perfect measure of the intrinsic acidity for gas phase reactions, it is not a suitable reactivity parameter for the design of solid acids.
Acknowledgements
This work has been supported by the German Research Foundation (DFG) within the Collaborative Research Center (CRC 1109) and by the “Fonds der Chemischen Industrie”. I thank Dr Marcin Rybicki for comments on the manuscript and discussions.References
- A. Corma, Chem. Rev., 1995, 95, 559 CrossRef CAS.
- A. Corma, J. Catal., 2003, 216, 298 CrossRef CAS.
- W. O. Haag, R. M. Lago and P. B. Weisz, Nature, 1984, 309, 589 CrossRef CAS.
- W. J. Mortier, J. Sauer, J. A. Lercher and H. Noller, J. Phys. Chem., 1984, 88, 905 CrossRef CAS.
- M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246 CrossRef CAS PubMed.
- Y. Seo, K. Cho, Y. Jung and R. Ryoo, ACS Catal., 2013, 3, 713 CrossRef CAS.
- W. J. Roth, P. Nachtigall, R. E. Morris and J. Čejka, Chem. Rev., 2014, 114, 4807 CrossRef CAS PubMed.
- J. A. Boscoboinik, X. Yu, E. Emmez, B. Yang, S. Shaikhutdinov, F. D. Fischer, J. Sauer and H.-J. Freund, J. Phys. Chem. C, 2013, 117, 13547 CAS.
- M. Rybicki and J. Sauer, Phys. Chem. Chem. Phys., 2015, 17, 27873 RSC.
- J. A. Boscoboinik, X. Yu, B. Yang, F. D. Fischer, R. Włodarczyk, M. Sierka, S. Shaikhutdinov, J. Sauer and H.-J. Freund, Angew. Chem., Int. Ed., 2012, 51, 6005 CrossRef CAS PubMed.
- C. J. Dawson, M. A. B. Pope, M. O'Keeffe and M. M. J. Treacy, Chem. Mater., 2013, 25, 3816 CrossRef CAS.
- E. G. Derouane, J.-M. Andre and A. A. Lucas, J. Catal., 1988, 110, 58 CrossRef CAS.
- J. Sauer, J. Mol. Catal., 1989, 54, 312 CrossRef CAS.
- W. E. Farneth and R. J. Gorte, Chem. Rev., 1995, 95, 615 CrossRef CAS.
- E. G. Derouane, J. C. Védrine, R. R. Pinto, P. M. Borges, L. Costa, M. A. N. D. A. Lemos, F. Lemos and F. R. Ribeiro, Catal. Rev. Sci. Eng., 2013, 55, 454 CAS.
- H. Kriegsmann, Z. Phys. Chem. (Leipzig), 1988, 269, 178 CAS.
- H. Pfeifer, D. Freude and M. Hunger, Zeolites, 1985, 5, 274 CrossRef CAS.
- U. Fleischer, W. Kutzelnigg, A. Bleiber and J. Sauer, J. Am. Chem. Soc., 1993, 115, 7833 CrossRef CAS.
- J. Q. Chen, A. Bozzano, B. Glover, T. Fuglerud and S. Kvisle, Catal. Today, 2005, 106, 103 CrossRef CAS.
- M. Sierka, U. Eichler, J. Datka and J. Sauer, J. Phys. Chem. B, 1998, 102, 6397 CrossRef CAS.
- C.-M. Wang, R. Y. Brogaard, B. M. Weckhuysen, J. K. Nørskov and F. Studt, J. Phys. Chem. Lett., 2014, 5, 1516 CrossRef CAS PubMed.
- M. Sierka and J. Sauer, J. Phys. Chem. B, 2001, 105, 1603 CrossRef CAS.
- M. Sierka and J. Sauer, Faraday Discuss., 1997, 106, 41 RSC.
- P. Nachtigall, O. Bludsky, L. Grajciar, D. Nachtigallova, M. R. Delgado and C. O. Arean, Phys. Chem. Chem. Phys., 2009, 11, 791 RSC.
- V. Nieminen, M. Sierka, D. Y. Murzin and J. Sauer, J. Catal., 2005, 231, 393 CrossRef CAS.
- U. Eichler, M. Brändle and J. Sauer, J. Phys. Chem. B, 1997, 101, 10035 CrossRef CAS.
- M. Brändle and J. Sauer, J. Am. Chem. Soc., 1998, 120, 1556 CrossRef.
- J. Sauer, in Modelling of Structure and Reactivity in Zeolites, ed. C. R. A. Catlow, Academic Press, London, 1992, p. 183 Search PubMed.
- E. A. Paukshtis, R. I. Soltanov and E. N. Yurchenko, React. Kinet. Catal. Lett., 1981, 16, 93 CrossRef.
- E. A. Paukshtis and E. N. Yurchenko, Russ. Chem. Rev., 1983, 52, 242 CrossRef.
- E. P. Hunter and S. G. Lias, in NIST Chemistry WebBook, NIST Standard Reference Database Number 69, ed. P. J. Linstrom and W. G. Mallard, National Institute of Standards and Technology, Gaithersburg MD, 2005, vol. 20899 Search PubMed.
- M. Brändle and J. Sauer, J. Mol. Catal. A: Chem., 1997, 119, 19 CrossRef.
- C. Lee, D. J. Parrillo, R. J. Gorte and W. E. Farneth, J. Am. Chem. Soc., 1996, 118, 3262 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |