Open Access Article
Open Access ArticleCharacterising and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systems†
Katherine E.
Fish
 *a,
A. Mark
Osborn
b and
Joby
Boxall
a
*a,
A. Mark
Osborn
b and
Joby
Boxall
a
aPennine Water Group, Department of Civil and Structural Engineering, The University of Sheffield, Sir Frederick Mappin Building, Mappin Street, Sheffield, S1 3JD, UK. E-mail: K.fish@sheffield.ac.uk; Tel: +44 (0)114 22 25767
bSchool of Science, RMIT University, PO Box 71 Bundoora, Melbourne, VIC3083, Australia
First published on 4th April 2016
Abstract
Drinking water quality deteriorates during transportation through drinking water distribution systems (DWDS). Microbial activity and ecology, particularly within biofilms that occur on the inner-pipe surface of DWDS, are emerging as important drivers in the degradation process. Yet, we have little real-world applicable understanding of the DWDS biofilms. This paper provides a critical discussion of current drinking water biofilm research, highlighting the importance of biofilms, including the extracellular polymeric substances (EPS) and their interactions with the physico-chemical environment. Evidence is presented that the tools for biofilm analysis are becoming more accessible and there is now the opportunity to translate microbial research from idealised bench-top settings to practical real-world applications. It is essential that we understand biofilms and manage them within ageing, deteriorating DWDS infrastructure to protect public health and wellbeing.
Water impactDrinking water distribution systems (DWDS) contain multi-species microbial biofilms, which influence water quality during transportation. Evidence is drawn from experimental and full-scale systems to explore the complex-interactions between biofilms (community and physical structure) and the physical-chemical DWDS environment. Particular emphasis is placed upon the need and new directions for DWDS biofilm research, to safeguard water quality and improve DWDS management. |
1 Introduction
Treated drinking water is a perishable resource and quality deterioration during distribution is an important issue for suppliers, consumers and regulatory bodies, alike. Drinking water distribution systems (DWDS) are networks of pipe infrastructure that transport potable water from treatment works to consumers. DWDS are central to supplying safe drinking water but microbial interactions between DWDS and water quality are often overlooked due to various engineering/environmental complexities and (commonly) a greater emphasis given to the chemistry of DWDS than the biology. DWDS are typically buried, with an evolving piecemeal design and construction, which experience ever-changing demands with variation in pipeline conditions and water quality.1,2 New pressures are emerging that are driving the future development and use of our DWDS. In particular, with climate change and population increases reported to be causing water stress,3,4 the water industry is faced with providing continually higher volumes of drinking water at a maintained or improved quality, all with diverse, ageing and deteriorating infrastructure.5Drinking water quality is affected by a multitude of interacting chemical, physical and (micro)-biological factors. Within DWDS, regulatory requirements and historic research have focused on planktonic cells (i.e. cells in the water column). However, microorganisms are more commonly found in a sessile-state termed biofilms; highly hydrated, heterogenic microbial assemblages consisting of cells embedded within a self-produced matrix of extracellular polymeric substances (EPS), where organics and inorganics (including metals) also accumulate.6,7 Biofilms form upon the inner pipe walls which provide a vast surface area in contact with drinking water (for example, approximately 169 km2 in U.K. systems). Compared to the planktonic microbiota, biofilms have a distinct community composition7–10 and substantially greater cell concentrations: 103 to 105 cells ml−1 have been reported (post-treatment) in the water column,11–13 compared to 106 to 1011 cells cm−2 at the pipe wall.7,14 However, a direct comparison between planktonic and sessile cell counts is not feasible due to the difference in the units of measurement, which is reviewed in detail in Liu et al.15 Nevertheless, it is accepted that the majority of the microbial load within a DWDS is found at the pipe wall and the water–pipe interface is where other interactions that influence water quality occur (e.g. discolouration and corrosion); therefore an understanding of biofilm ecology at this interface is essential.
Various abiotic and biotic properties influence the presence, architecture and composition of biofilms, which subsequently affect various characteristics of the DWDS. Biofilms mediate processes that contribute to aesthetic degradation, possess the potential to inoculate the pipeline downstream if mobilised and, in chlorinated DWDS, place a chlorine demand upon the system. Thus the presence and activity of microorganisms within the DWDS, particularly as biofilms, substantially affect the infrastructure, network management and, arguably more importantly, water quality.
In this review, we highlight the importance of understanding biofilms at the pipe–water interface, with particular reference to EPS and the interactions with the DWDS physico-chemical environment. We discuss the current understanding of DWDS biofilms with respect to water quality. The merits and limitations of the methodological approaches and the model systems used to investigate DWDS biofilms are considered. We build upon previous research and reviews to define the state of the art and the need for research on biofilms in DWDS. Emphasized are the importance of the role that biofilms play in safeguarding drinking water quality as it is transported, and the two way feedback between biofilms and the abiotic/biotic aspects of the DWDS environment. This is particularly relevant with the increasing concern for water availability and quality, and a greater appreciation that DWDS are ageing, deteriorating infrastructures that we need to further understand in order to better manage to safeguard the future of high-quality drinking water. Concluding remarks are made identifying key knowledge gaps and directions for future research regarding DWDS biofilm systems.
2 Microbial drinking water quality
2.1 Microbial water quality guidelines
Drinking water contains low concentrations of soluble and particulate material including inorganics/organics, disinfectant residuals and microbial cells. Legislation regarding the acceptable concentrations of these (e.g. Table S1†) has been established by governing bodies to control water quality.6–18 However, these guidelines have limitations; there is no international consensus on the standards to be met or the location and frequency of sample collection. Internationally, planktonic bacteria are the only microorganisms monitored with respect to water quality (Table S1†), apart from Swedish regulations which include fungi (≤100 CFU per 100 ml (ref. 19)). Monitoring remains heavily reliant on culture-based enumeration (which greatly underestimates microbial concentrations11,20) of coliforms (a group of Gram-negative bacteria, including Escherichia coli), which are used to indicate potential faecal contamination,16,17 a major source of pathogens. Crucially, these regulations are for the bulk water, which provides assurance for the planktonic microbial quality. However, there are currently no guidelines available regarding biofilms within DWDS but research has demonstrated that biofilm communities differ considerably from the planktonic community9 in cell counts and composition. Therefore, biofilms present an unknown (and unmonitored) risk to water quality.2.2 Public health impacts
In developed countries drinking water quality is generally high. Nevertheless, occasional microbial water quality failures occur such as an Escherichia coli O517 outbreak during 2000, in Walkerton, Canada, which led to seven deaths21 or a Cryptosporidium contamination in Yorkshire, U.K. in 2014, which affected ∼575![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people.22 Large-scale outbreaks are commonly attributed to treatment work failures, i.e. “internal” contamination due to microorganisms evading treatment23 (facilitated by their size and physiology24). However, contaminants may also originate from “external” sources. For example, certain fungi have been detected exclusively in recently replaced regions of DWDS,25 likely resulting from poor practices (non-sterilised construction materials) and negative pressures (which reverse flows facilitating the intrusion of particles). Regardless of their origin, planktonic particles (including microorganisms) may be incorporated into the (unmonitored) DWDS biofilms, masking a contamination and causing delayed issues with water quality. Indeed, incidents occur where “finished” water complies with regulations but “endpoint” water does not, indicating the (often overlooked) impact of DWDS as bio-chemical reactors affecting water quality during transportation.
000 people.22 Large-scale outbreaks are commonly attributed to treatment work failures, i.e. “internal” contamination due to microorganisms evading treatment23 (facilitated by their size and physiology24). However, contaminants may also originate from “external” sources. For example, certain fungi have been detected exclusively in recently replaced regions of DWDS,25 likely resulting from poor practices (non-sterilised construction materials) and negative pressures (which reverse flows facilitating the intrusion of particles). Regardless of their origin, planktonic particles (including microorganisms) may be incorporated into the (unmonitored) DWDS biofilms, masking a contamination and causing delayed issues with water quality. Indeed, incidents occur where “finished” water complies with regulations but “endpoint” water does not, indicating the (often overlooked) impact of DWDS as bio-chemical reactors affecting water quality during transportation.
It is not only large scale pathogen outbreaks that can affect water quality. Potentially undocumented or undetected small-scale outbreaks, which do not violate standards (or are not sampled under regulatory regimes), occur during distribution. The resulting continuous low-level microbial concentrations may seed DWDS biofilms downstream and influence the distribution of systemic infections,26 which could have substantial socio-economic consequences. Roberts et al.27 stated that diarrheal disease costs the U.K. ∼£743 million per annum, due to absence from work, although it is unclear what proportion of this value is attributed to a drinking water cause.
2.3 Aesthetic impacts
The activity of non-pathogenic microorganisms either within or released from a biofilm may affect water quality by degrading aesthetics and impact DWDS operation. In countries with long-established DWDS, the water supply is often seen as a “service industry”, where customer satisfaction is paramount. In countries such as Australia,28 Holland1 or the U.K.,29 the majority of water quality-related consumer contacts with water suppliers are due to aesthetic degradation, which is a worldwide issue, of which discolouration is the leading example. Discolouration events (indicated by increased turbidity) often occur following changes in DWDS hydraulics and are primarily considered as an aesthetic problem but have also been positively correlated with gastro-illness.30 Hunter et al.31 discovered an unexpected positive relationship between the occurrence of cryptosporidiosis and a disrupted water supply (p < 0.001), which was stronger than the correlation with interactions with an infected individual (p = 0.001). Although correlative results do not prove causation, the observed trends demonstrated that hydraulic changes caused discolouration events, which could mask health issues.The processes driving the accumulation and release of “discolouration material” within pipelines have yet to be fully proven but the material is thought to originate from biological interactions, corrosion and chemical reactions.32 Various studies modelling discolouration have assumed this process is governed by sedimentation of particles (controlled by gravitational settling) but even low hydraulic forces within the DWDS would be sufficient to keep the particles suspended.33 Alternatively, the Prediction of Discolouration in Distribution Systems (PODDS) model is based upon the “cohesive layer” theory, which suggests that interactions at the pipe–water interface lead to particles actively concentrating into attached “layers” at the pipe-wall at different adhesive strengths, determined by the hydraulic regime within the pipeline.33 Mobilization of the attached material then occurs when hydraulic forces exceed those experienced during conditioning. PODDS has been validated as an empirical tool by various field and laboratory studies34–36 but provides limited understanding of what the interactions that cause material accumulation are. It is plausible that these interactions are (micro)biological; PODDS is in line with the concept of biofilms (i.e. attached material) occurring at the pipe wall and playing a significant role in water quality events (such as discolouration) during transportation through the DWDS.
Ultimately, reducing the incidence of water quality failures (aesthetic and pathogenic) is of paramount importance; to do so requires further understanding of the processes and interactions occurring at the pipe wall during distribution, in which microbial ecology is emerging as key driver. Consequently, a continued increase in research evaluating both the planktonic and biofilm communities is needed, which, combined with molecular analysis or fluorescence microscopy, is generating a more accurate evaluation of the microbial life in our pipelines.9,37–39
3 Investigating DWDS biofilms
3.1 DWDS simulation, biofilm development and sampling
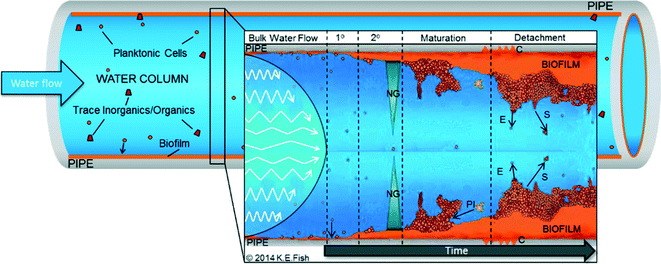
Fig. 1 highlights the generalised stages of the “biofilm cycle” set within a DWDS context. Understanding of DWDS biofilm colonisation and dynamics (attachment/detachment) is commonly informed from other environments, laboratory experiments or inferred from planktonic samples because of the challenges in investigating biofilms within buried, operational systems. DWDS biofilm samples are generally obtained following network refurbishment or routine maintenance,40–42 which provides valuable real world data but poses challenges with regard to replication, representative sampling, aseptic removal and control, and/or determination of environmental variables. Therefore, biofilms are often developed within flow cells, bioreactors (reviewed in ref. 43) or in small-scale pilot systems (Table 1). Such laboratory-scale research provides valuable insights into DWDS microbial ecology and allows environmental control while providing the possibility to design systems to facilitate the removal of biofilm samples.9,44,45 However, full-scale DWDS present a unique heterogeneous environment in which microorganisms (planktonic and biofilm) are exposed to many diverse (and interacting) physico-chemical and biotic variables. These are not necessarily replicated by bench-top systems such as bioreactors, which may not replicate the DWDS microbiota accurately46 and are often designed to investigate a single variable (Table 1).| Research focus | Experimental system | |
|---|---|---|
| Bench-top scale | Simulation pipe rig | |
| Material effect on biofilm formation/growth | Stainless steel and PVC; seven biofilm reactors connected in series, fed with municipal drinking water at a flow of 10 cm s−1 (ref. 79) | 2 m long stainless steel pipes (different grades), both 20 mm in diameter, connected with brass compression joints83 |
| Coupons (3 cm diameter) of six materials in glass reactor39 | — | |
| Hydraulics, shear stress and biofilm stability/cohesion | PVC annular reactor, cell responses to shear stress102 | — |
| Chemostat bioreactor53,103,104 | — | |
| Glass coupons, rotating reactor (0.01–3500 RPM), 24 hour residence time, tap water inoculum54 | — | |
| Cultured Pseudomonas aeruginosa inoculation of glass flow cells50 | — | |
| Water composition (nutrients/inorganics) | Drinking water annular reactor, assessing bacterial water quality71 | — |
| Iron and manganese within biofilms, glass reactor, 60 cm long, 19.5 mm diameter, flow 0.28 l min−1 (ref. 28) | — | |
| Disinfection/water treatment effect on biofilms | PVC coupons within a reactor, fed with monochloraminated ground water132 | Reclaimed pipe length from DWDS, 9 m MDPE and 9 m cast iron151 |
| Six cement, iron and PVC pipes 65 inches (1.65 m) long131 | — | |
| Biofilm community analysis/cellular quantification | — | 12.2 m looped reactor, 2 hours retention time, flow 0.07 ms−1, fed with non-disinfected groundwater55 |
| — | 90 m coiled HPPE loop with removable coupons that fit to the curvature of the inner pipe surface44 | |
| — | Two stainless steel loops with removable plugs82 | |
| Biofilm adhesion mechanisms | Fermenter and test cell, Pseudomonas fluorescens culture93 | — |
| Protozoan activity | — | Three pipe loops (31 m long, 100 mm internal diameter), PVC or polyurethane foam coupons71 |
| Characterising the EPS and community structure of biofilms | — | Three coiled HDPE pipes 200 m long, with removable HDPE coupons, fed with water from the local DWDS45 |
The use of DWDS-relevant materials has increased the relevance of findings from experimental systems (Table 1) to the real world, but many studies use small surface areas which differ considerably from the pipe surface characteristics and surface-area-to-volume ratio comprising full-scale DWDS. Also, typically, a steady state flow rate scenario is considered but varied flow regimes occur in operational DWDS. Consequently, boundary layer hydraulics are not accurately reproduced in most model systems which, in combination with the surface-area-to-volume ratio, are integral to replicating the shear forces imposed upon a biofilm, as well as the disinfectant exposure, nutrient supply and exchange of microorganisms between the biofilm and the water column. Equally important is the inoculum used to seed the biofilms; commonly wastewater,47,48 liquid medium containing a single species49,50 or an artificial mix of species51,52 are used. None of these reflect the lower cell numbers or greater species richness that a DWDS is ordinarily exposed to via drinking water. Ultimately, the replication of the complex DWDS internal environment must be improved to generate real-world relevant knowledge.
Biofilm investigations have ranged from days50,53 to months or years.28,39,54 Short timescales may not be sufficient to observe a change in the microbiota, particularly as a response to environmental variation and Martiny et al.55 rightly argue that they may not reflect the effects of the longer developmental time seen in live DWDS. However, the biofilms of DWDS are products of an old system (past working practices, prior microbial contamination and previous pipelines) and can be the result of decades of growth, which is ongoing; therefore laboratory based tests will never fully converge with the real system. Although younger biofilms may have a different structure and diversity to mature assemblages, in-depth research over a shorter time scale offers a critical insight into the initial biofilm colonisation of “new” pipes and environmental impacts upon this, which provides information crucial to managing the longer-term sustainability of DWDS.
3.2 Biofilm analysis
Regardless of how a DWDS biofilm sample is obtained there exist many potential methods for analysis of the DWDS microbial communities (see ref. 56). However, many have been designed to test planktonic samples or biofilms from other environments and their accuracy has yet to be established, particularly as DWDS biofilms often have limited quantities of biomass available for analysis compared to biofilms from non-oligotrophic environments. Additionally, most studies are concerned with variations in microbial community (particularly bacterial) structure and diversity38,39 with little or no integration of analysis of the EPS molecules (primarily carbohydrates and proteins). Fish et al.45 previously demonstrated that DWDS biofilms from a full-scale system have an extensive EPS matrix, with a greater volume than the microorganisms embedded within; indeed EPS synthesis (Fig. 1) is crucial to any biofilm as without it, cells would remain planktonic. EPS has many roles (reviewed in ref. 57 and 58) including promoting biofilm stability (mechanical and chemical), the accumulation of inorganic/organic concentration and protection against disinfection. Given the integral role of EPS, research should seek to assess the combined impact that DWDS environmental conditions and microbiota have upon the characteristics of EPS (e.g. quantity, composition) and the resultant properties that EPS conveys (e.g. stability, structure).3.3 Biofilm detachment
Although one of the least studied biofilm processes, detachment (Fig. 1) is arguably one of the most important, particularly with respect to water quality management. Consistent low-level detachment of small aggregates is referred to as erosion (Fig. 1), which, within DWDS, is unlikely to violate microbiological quality guidelines.24,59 Instead, detachment provides a persistent slow-release of unknown microbial quantities into the water supply. Detachment of larger fractions of the biomass is commonly termed sloughing (often defined differently between studies). The available research indicates that sloughing occurs less frequently than erosion but presents a greater risk of water quality failures (due to the release of higher cell numbers and other particles from the EPS). For example, large aggregates (cell clusters exceeding 1000 μm2) represented only 10% of detachment events from biofilms within a chemostat but accounted for >60% of the material detached.60 Mobilisation of pathogenic cells in this way could explain the previously observed correlation between turbidity and gastro-illnesses.31 While various studies have investigated or modelled detachment,61–63 few consider this with respect to DWDS biofilms and it has yet to be established if the patterns from other environments are transferable to pipelines.The limitations discussed throughout this section apply to all of the literature considered throughout this review and as the choice of experimental design and sample analysis influences data collection, comparison between studies should be undertaken with care. Although the insights from these studies may not accurately reflect real-world DWDS biofilm characteristics entirely, they can nonetheless be used to inform and target future research.
4 The microbial diversity within DWDS pipelines
DWDS microbiomes are taxonomically diverse but bacteria are the most commonly studied and identified microorganisms therein (Table 2). Members of the phylum Proteobacteria are particularly predominant in planktonic64,65 and biofilm samples,9,66 regardless of pipe material,39 disinfection technique67 or time of sampling.38 Environmental variables do, however, influence the microbial community composition; various taxa have been identified in the course of DWDS-associated microbial studies worldwide (Table 2) and community structure and species composition differs both within and between networks.38,65 Interestingly, the application of molecular techniques has revealed that many drinking water isolates (>57% in some instances) are “difficult to classify” but closely match other unclassified sequences originating from drinking water,37,38,64 possibly indicating the existence of novel bacteria adapted to the DWDS.| Kingdom | Phylum | Class/order | Example genus/species | Sample type | Ref.c |
|---|---|---|---|---|---|
| a Biofilms generally taken from discontinued pipes obtained via routine maintenance/dismantling of a system, in Sibille et al.71 coupons were suspended within reservoirs. b Laboratory set ups described in Table 1. c All references have identified the corresponding class/order of microorganisms but the example species are not necessarily found in all the referenced studies. | |||||
| Bacteria | Proteobacteria | α-Proteobacteria | Agrobacteria | Biofilm – fielda | 8, 152 |
| Sphingomonas | Biofilm – laboratoryb | 39, 55, 131 | |||
| Methylobacterium | Planktonic | 38, 64, 76, 87, 153 | |||
| β-Proteobacteria | Burkholderia | Biofilm – fielda | 8, 152 | ||
| Thiobacillus | Biofilm – laboratoryb | 39, 110, 131 | |||
| Nitrosomonas | Planktonic | 8, 64, 65, 76, 110, 153 | |||
| γ-Proteobacteria | Pseudomonas aeruginosa | Biofilm – fielda | 8, 42, 152 | ||
| Escherichia coli | Planktonic | 8, 38, 64, 65, 76, 85, 87, 153 | |||
| Legionella pneumophila | |||||
| ε-Proteobacteria | Helicobacter pylori | Biofilm – fielda | 41 | ||
| Actinobacteria | Actinomycetales | Arthrobacter | Biofilm – fielda | 40, 42, 152 | |
| Mycobacterium avium, M. gordonae, M. intracellulare | Biofilm – laboratoryb | 39, 83, 131 | |||
| Planktonic | 8, 38, 40, 64, 65, 87, 153 | ||||
| Bacteroidetes | — | — | Biofilm – laboratoryb | 39, 131 | |
| Planktonic | 8, 38, 153 | ||||
| Acidobacteria | — | — | Biofilm – laboratoryb | 55 | |
| Nitrospirae | Nitrospira | — | |||
| Planktonic | 64, 87 | ||||
| Cyanobacteria | — | — | Planktonic | 38, 64 | |
| Planctomycetes | — | — | Biofilm – fielda | 152 | |
| Biofilm – laboratoryb | 55 | ||||
| Planktonic | 38, 64, 153 | ||||
| Archaea | Euryarchaeota | — | — | Biofilm – laboratoryb | 132 |
| Crenarchaeota | — | — | |||
| Fungi | Basidiomycota | Sporidiales | Cryptococcus | Biofilm – fielda | 7 |
| Rhodotorula | |||||
| Ascomycota | Saccharomycetes | Candida | |||
| Eurotiales | Penicillium spinulosum, Aspergillus calidoustus | Planktonic | 25, 135 | ||
| Hypocreales | Trichoderma viride | ||||
| Acremonium butyri | Biofilm – fielda | 7, 96 | |||
| Planktonic | 7, 25, 96, 135 | ||||
| Chaetothyriales | Phialophora reptans | Biofilm – fielda | 96 | ||
| Exophiala lecanii-corni, E. castellani | Planktonic | 7, 25, 96 | |||
| Dothideomycetes | Cladosporium malorum, C. cladosporioides | Biofilm – fielda | 7, 96 | ||
| Planktonic | 37, 96, 135 | ||||
| Protists | Apicomplexa | Eucoccidiorida | Cryptosporidium paryum | Biofilm – fielda | 154 |
| Ciliophora | — | Acanthamoeba | Biofilm – laboratoryb | 71 | |
| Sarcomastigophora | — | Giardia | Planktonic | 37, 71, 154 | |
| Amoebozoa | Tubulinida | Hartmannella | |||
Various fungi, particularly filamentous fungi, are known to be autochthonous to DWDS (Table 2) and are becoming accepted as a diverse component of the DWDS microbiota. In some instances, relatively little difference has been found between the fungal communities of raw and treated water.7,37,68,69 Fungi within DWDS biofilms have not been well explored and their contribution to the planktonic community varies between studies,25,37 possibly due to different DWDS characteristics and water sources, or varying limits of detection between methods.
Protozoa (eukaryotes) have been recovered from drinking water (Table 2), especially when disinfection use is limited.68 van Lieverloo et al.70 found that 78% of water samples taken from DWDS without disinfection residuals contained protozoa, with Rotifera and Nematoda also being particularly abundant. Protozoa have been found actively predating upon other microorganisms and feed on organic compounds within drinking water biofilms.71 Despite the detection of protozoa in DWDS and their known role in biofilm ecology, little is known about their impact upon water quality. Currently the U.S. Environmental Protection Agency Contaminants Candidate Lists includes Naegleria fowleri72 as a potential health risk in drinking water systems, although quality control legislation does not yet incorporate protozoa.
Planktonic archaea have been detected, although only a few species have been identified (Table 2) as researchers rarely seek to detect or isolate these organisms post-water treatment. We have recently reported the presence of archaea during the early stages of biofilm formation in DWDS.45 Similarly, viruses within DWDS remain relatively unexplored, although rotavirus was detected in a non-disinfected drinking water sample.73 Laboratory-based studies have established that viruses can survive within the water column24 and be incorporated into the biofilm74 but contamination by human viruses may be less likely in full-scale networks. Small invertebrates have been found in drinking water storage tanks and pipelines (iron and plastic), particularly those with a ground water source and no chlorination;70,75 microbial biofilms may serve as a nutrient supply for these larger organisms.
The co-existence and interactions between these different taxa are not well understood but their presence within a biofilm could degrade water quality and potentially cause systematic infections. Critically, microorganisms within operational DWDS have generally been identified in the planktonic phase via isolation from end-point drinking water (household taps or outlet fittings). Pinto et al.76 provide an exception to this as planktonic samples were taken from various points along a DWDS. However, such samples are not necessarily representative of the microbiome within the biofilms of operational systems, for which taxonomic identification remains limited to the analysis of biofilms obtained from pipelines removed from service (ref. 77 and 78; Table 2), rather than sampled from a live DWDS.
5 Ecological/engineering effects and biofilm response
DWDS vary greatly with respect to infrastructure, system management (e.g. hydraulic conditions, disinfection) and water composition (e.g. organic/inorganic concentrations, microbial content and physico-chemistry). Essentially, biofilm development and structure (both community and physical) are mediated by multiple influences; externally, by the complex interactions of the DWDS environmental parameters, and intrinsically, by the microorganisms which modify the surrounding environment via their metabolic activity and community processes, acting as “ecosystem engineers”. As biofilms are ubiquitous and their complete eradication is an impossible demand, we assert that improving our understanding of these two-way interactions between biofilms and the environment is paramount to safeguarding high quality drinking water.5.1 The pipeline surface
DWDS infrastructure presents a vast surface area-to-volume ratio (in U.K. systems this ratio has been calculated as 11 m2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 m3) so a substantial area of pipe is in contact with drinking water during transportation. DWDS are composed of pipes of diverse age, material, diameter (from centimetres to metres), length and condition. Metals, plastics and cement have all been and still are used in DWDS construction (Fig. S1†). Internationally, many pipelines are iron dominated, however, polyvinyl chloride (PVC) and high or medium density polyethylene (HDPE/MDPE) are now steadily replacing older pipelines5,35 and current practice (where cost effective) is to re-line with epoxy based resins resulting in a surface with plastic-like properties (although other linings such as cement have been used historically). Pipe materials and surface condition have been found to influence biofilm growth, particularly density and community composition7,39,79 with adhesion occurring more quickly on rougher surfaces. Increased biofilm growth can accelerate infrastructure deterioration via microbially influenced corrosion of the pipe surface, which can cause pitting, simultaneously releasing nutrients from the pipe material and forming by-products that affect surface roughness or porosity.80 For example, iron oxidising bacteria (e.g. Shewanella, some Pseudomonas spp. and filamentous bacteria such as Gallionella) corrode iron and the by-products accumulate forming tubercles of Fe3+ which increases surface roughness and promotes biofilm development.81 This may in turn cause unaccounted energy losses, influencing DWDS hydrodynamics. Bio-corrosion can also cause water quality degradation; “red”, “black” or “blue” water problems can occur due to the activity of iron-, sulphate- or copper-reducing bacteria, respectively.82
1 m3) so a substantial area of pipe is in contact with drinking water during transportation. DWDS are composed of pipes of diverse age, material, diameter (from centimetres to metres), length and condition. Metals, plastics and cement have all been and still are used in DWDS construction (Fig. S1†). Internationally, many pipelines are iron dominated, however, polyvinyl chloride (PVC) and high or medium density polyethylene (HDPE/MDPE) are now steadily replacing older pipelines5,35 and current practice (where cost effective) is to re-line with epoxy based resins resulting in a surface with plastic-like properties (although other linings such as cement have been used historically). Pipe materials and surface condition have been found to influence biofilm growth, particularly density and community composition7,39,79 with adhesion occurring more quickly on rougher surfaces. Increased biofilm growth can accelerate infrastructure deterioration via microbially influenced corrosion of the pipe surface, which can cause pitting, simultaneously releasing nutrients from the pipe material and forming by-products that affect surface roughness or porosity.80 For example, iron oxidising bacteria (e.g. Shewanella, some Pseudomonas spp. and filamentous bacteria such as Gallionella) corrode iron and the by-products accumulate forming tubercles of Fe3+ which increases surface roughness and promotes biofilm development.81 This may in turn cause unaccounted energy losses, influencing DWDS hydrodynamics. Bio-corrosion can also cause water quality degradation; “red”, “black” or “blue” water problems can occur due to the activity of iron-, sulphate- or copper-reducing bacteria, respectively.82
Pedersen79 established that matt steel accumulated 1.44 times more biofilm than electro-polished steel, when used in a drinking water fed bioreactor. Rougher surfaces likely promote biofilm growth because they provided a greater area for adhesion,66 more niches for colonisation and decreased detachment (pits offer protection from shear forces and disinfection79). Percival et al.83 also found a positive correlation between surface roughness and microbial density, but established that the quantity of EPS (carbohydrates) was unaffected by steel grade, though it did increase linearly with time. After 12 months, microbial density no longer differed between the steel surfaces, suggesting that surface roughness governs the rate of primary colonisation but has little influence once a mature biofilm is established. These findings are relevant to biofilm formation within real DWDS as most have been exposed to microbial colonisation for >12 months; in the U.K., the majority (69%) of the DWDS (for which age is known) has actually been in place for at least 35 years (Fig. S1†). However, steel is rarely used in DWDS and as various materials are known to influence biofilm development differently, the results cannot directly be applied to operational DWDS.
Worldwide, biofilms are found upon various materials associated with DWDS, including lead service lines in Illinois,84 stainless steel taps in Romania,14 unplasticised PVC (uPVC) pipeline in the Netherlands85 and asbestos cement pipes in Australia.69 Standards exist to maintain high quality of these diverse materials and minimise their biofilm forming potential (BFP).86 However, BFP variation exists between materials due to differences in surface characteristics and the likelihood of leaching (water-soluble) organics or releasing nutrients when exposed to biological activity, and this has caused debate in the literature as to which material has the lowest BFP. For instance, Schwartz et al.66 reported reduced bacterial cell densities upon steel compared to plastics (HDPE and PVC), with significant differences in community composition between the metal and plastics. Similarly, Douterelo et al.87 established that biofilm communities mobilised from a cast iron pipe had lower bacterial richness and diversity than equivalent communities from a cast iron pipe. Copper pipes also support a less diverse bacterial community39 and have a lower BFP than plastics,66,88 likely because copper corrosion produces inhibitory substances that most microorganisms find toxic. However, enumeration was generally limited to (culture-based) analysis of bacteria, which does not accurately evaluate the total microbiota. Conversely, a growing body of literature indicates that plastics (PVC, uPVC, HDPE/MDPE, polybutylene and polypropylene) support a reduced abundance and diversity of bacteria when compared to metals (steel,7,39 iron80,86) or cement.89 Doubling times (exponential growth phase) for a heterotrophic bacterial community grown on cast iron and plastic (uPVC, MDPE) were determined to be 13.2 hours and 15.6 hours, respectively.86 This lag in biofilm development rate upon plastic compared to iron, resulted in lower cell abundance upon plastic in both the short (21 days) and longer (7 months) term.86 Considering the aforementioned link that is proposed between biofilms and discolouration in DWDS, further evidence for slower accumulation upon plastics than metals is provided from a discolouration based study: discolouration material accumulation (i.e. biofilm growth) was reported to take 4 years in plastic pipes but just 1.5 years in iron pipes.35 Lehtola et al.88 showed that the initial lag in biofilm growth rate between PE and copper was less evident with prolonged development time, which reaffirms the results from Percival et al.83 regarding surface roughness – initial biofilm development rate depends upon characteristics of the substratum but overtime a plateau phase is achieved. The concept of a plateau again mirrors practical discolouration research, where material is conceptualised as reaching an upper limit dictated by the normal hydraulic conditions within a given pipeline.35
There is currently no definitive consensus as to which plastic has the lowest BFP, some studies have found no difference between the types tested,7,66 others state that PVC39,89 or polypropylene90 accumulate the lowest cell densities. Rożej et al.91 found that, within a model system fed with chlorinated water, HDPE pipelines supported a thicker biofilm than either cross-linked polyethylene (PEX) or PVC but that total cell counts were greatest in PEX. This implies that the HDPE biofilms had a more extensive EPS than the PEX biofilms but as no EPS analysis was applied this remains conjecture, indeed the impact of pipe material upon the EPS in general has yet to be explored. Hallam et al.92 found that the effect of pipe material upon microbial occurrence was less significant than the impact of chlorine; hence material may be most influential upon biofilms formed within non-chlorinated systems or in areas of DWDS that experience reduced residuals. However, differences in biofilm formation rate between materials is particularly evident in studies using chlorinated water as an inoculum.80,92
The relationship between pipe material and biofilms within DWDS is complex. Pipes and their microorganisms seed each other in operational systems hence location within the network may be more influential than the pipe material. Henne et al.8 established that initial bacterial colonisation within DWDS may be surface-specific but, over time, biofilms demonstrate increased similarity to their neighbouring biofilms; community fingerprints from a looped fire-main grouped together in a single cluster, despite growing on different materials. Again, this suggests that pipe material influences initial biofilm development but is less influential as a mature biofilm coats that surface.
5.2 Hydraulic conditions
DWDS hydraulics (flow rate, velocity, turbulence and shear stress) vary temporally (diurnally and seasonally) with changes in water demand, and spatially with distant sections of the network, dead ends or looped pipelines commonly experiencing lower flows than other DWDS areas. Spatial variation also occurs at the small scale. For example, compared to the middle of the pipeline, a more turbulent water flow is observed adjacent to the pipe surface (rapidly reaching zero at the pipe wall; Fig. 1). This region is termed the boundary layer and is the region of water to which biofilms are exposed. Hydraulics (in combination with pipe diameter) influence the exchange of trace nutrients, disinfectants, oxygen, heat and microorganisms at this pipe–water interface,48,49,51,93 which all interact to affect biofilm development. For instance, Beyenal and Lewandowski49 found high velocities promote biofilm density and inhibit nutrient diffusion, possibly indicating that biofilm communities prioritise mechanical pliability over nutrient transport.49 Contrastingly, an alternative study demonstrated that the penetration of substrates into Pseudomonas fluorescens cultures increased with increasing velocity, however, density was still greater at the higher velocities.94 Although the velocities investigated (0.28 and 1.00 ms−1) are atypical of DWDS pipelines (in the U.K. pipes of 75–100 mm in diameter have an average flow of 0.4 ls−1,95 which is ∼0.08 ms−1) this study highlights the importance of considering the interaction between parameters such as hydraulics and nutrients. Hydraulics also govern residence time (i.e. the time taken for water to reach the consumer, which can vary from minutes to days) influencing water age and quality2,26 and potentially biofilm colonisation. Extended residence times increase the longevity of contact with the network infrastructure, a decline in disinfectant residuals2 and hence an increased propensity for cell transfer between the planktonic and biofilm states,93 which may facilitate the growth of species less able to form biofilms. For instance, the fungi Exophiala lecanii-corni and Ochroconis mirabilis can colonise and dominate biofilms during static periods.96Within bioreactors, hydrodynamics have been reported to shape not only biofilm density, composition and structure but also cohesion and erosion.49,50,54 However, the biofilm–hydraulic interactions reported do not always converge and cannot always be attributed conclusively to hydraulic impacts. For instance, biofilms within bioreactors subjected to turbulent flows were described as patchy, “ripple” structures.50 However, turbulent flows are extremely chaotic and, consequently, the consistent ripple pattern observed is more likely a consequence of the scaffold surface characteristics than the hydraulics. More commonly, turbulent flows (with a greater shear stress) have been observed to increase biofilm density and cohesion, and reduce thickness, possibly due to compression;97 characteristics which seem to promote detachment resistance.98 Interestingly, Abe et al.54 found the reverse to be true; eight-week-old biofilms (up to 107 cells cm2) had a greater cohesive strength (measured via atomic force microscopy – AFM) when formed under a lower shear stress. Biofilms developed under 0.230 Pa were removed with 20 kPa, a quarter of the force required to detach the lower shear stress (0.120 Pa) conditioned biofilms.54 However, if these AFM-applied forces are comparable to hydraulic forces, then the conditioning shear stresses were below that experienced in an average DWDS (the aforementioned average flow of 0.4 ls−1 corresponds to ∼0.30 Pa). Moreover, the detachment forces were far greater than those occurring under normal operational conditions; typical maximum flows (achieved if fully opening a hydrant due to an extreme event or planned cleaning) in U.K. DWDS correspond to ∼10 Pa (dependent upon pipeline surface roughness). Abe et al.54 also observed that the force required to detach biofilm decreased with increasing biofilm volume; this is in contrast to Lehtola et al.99 who found large biofilm clumps (>25 μm) required more energy to be detached. The contrasting trends observed in the literature (likely due to the use of different reactors and operating conditions) make it difficult to predict biofilm behaviour as a response to shear stress variation in operational DWDS. Additionally, biofilms are generally cultured under steady-state flows (albeit at different rates) at a bench-top scale whereas real networks experience varied flow patterns. Hence such results are limited in their ability to inform the dynamics of DWDS biofilms.
Biofilm detachment occurs within pilot-scale pipelines when shear stress increases at the pipe wall, simultaneously increasing the turbidity, iron, copper and phosphorus concentrations in the water-column.99 Within DWDS, correlations have been found between daily and weekly hydraulic patterns and planktonic cells counts,100 which supports the occurrence of interactions between shear stress and release of material under operational conditions. Furthermore, a burst or seasonal increase in demand could cause the shear stress to exceed historic levels, overcoming the EPS cohesive strength which was in equilibrium with the previous external shear forces,49,50 resulting in biofilm mobilisation, which could, in turn, affect water quality. However, detachment events do not remove all biofilm material. Both Abe et al.54 and Lehtola et al.99 state that biofilms persisted after exposure to detachment forces, the depth of this strongly adhered base biofilm layer may be affected by carbon concentrations.101 Various studies have provided evidence of biofilm stratification with areas that possess different adhesive/cohesive strengths and thus areas that detach at different rates.54,97 Detachment has been hypothesised to initiate an “energy spill”, causing proton translocation across cell membranes, which alters cell surface characteristics (e.g. a decrease in the net-negative charge of the cell surface) such that cell–cell interactions are promoted and the formation of a stronger biofilm is more likely.102 Variations in biofilm stability may be due to the alignment of polysaccharides, proteins, ionic concentrations and hydration of the EPS. This theory has yet to be fully explored but it is logical as the adhesive forces of the EPS molecules provide mechanical stability to the biofilm.57 Simoes et al.103,104 provide a rare insight into the interaction between hydrodynamics and EPS, with respect to Pseudomonas fluorescens and Bacillus cereus biofilms within a reactor. In brief, protein mass was positively correlated with shear stress but polysaccharide mass was negatively correlated103 and B. cereus (a Gram-positive bacterium) produced smaller quantities of EPS than P. fluorescens (Gram-negative bacterium), but experienced lower biofilm loss,104 potentially indicating increased stability. These speculative links regarding specific EPS profiles and biofilm stability require further investigation before clear conclusions can be made. Nonetheless, it is feasible that a higher shear stress during biofilm development may condition the EPS and/or cells to be more resistant to detachment in the future; a theory which mirrors the “cohesive layer” theory of discolouration36,95 although this requires further investigation.
5.3 Biodegradable matter and inorganic nutrients
Nutrients (e.g. ammonium, nitrates, phosphates) and energy (i.e. a carbon source) are crucial for biological growth and are found at oligotrophic levels in drinking water. Carbon and nutrients follow a gradient (Fig. 1) towards the pipe wall, driven by the turbulence of the water, further highlighting the need to accurately replicate DWDS hydraulics and volume-to-surface ratio which will affect nutrient availability/transfer within the biofilm. Compared to the water column, the biofilm presents a habitat that is rich in nutrients and carbon, where non-oligotrophic microorganisms are able to thrive.105 Trace substrates become trapped in the EPS, and biofilm-bound microorganisms corrode pipe surfaces, releasing diverse substrates which are then bioavailable88,92 (leaching may also occur as soluble-organics are released from the pipe material into the biofilm/water column).Biofilms comprise both autotrophic and heterotrophic microorganisms. Of direct relevance to the heterotrophs are the microbially accessible organics, collectively termed biodegradable organic matter (BOM) and generally represented by the assimilable organic carbon (AOC) and bio-available dissolved organic carbon (BDOC). The specific organics (or inorganics) and their concentration in DWDS vary with source water, treatment processes (removal and/or addition of organisms or particles), residence times, the microbial load of the network (cells contribute organic carbon) and disinfectant by-products (DBPs), which may themselves be a source of AOC.106,107 BOM, particularly the AOC fraction (reported at concentrations of 3–500 AOC μg l−1), has a considerable influence on microbial diversity, especially of heterotrophic bacteria105,106 and affects microbial growth.40 Therefore, carbon is often considered the limiting factor of microbial growth in DWDS. Growth limiting concentrations of ≤10.9 AOC μg l−1 have been reported,108 although this will vary between DWDS and with varying water quality parameters such as disinfection (standards for AOC differ between systems with and without disinfectant residuals) or temperature. Microscopy-based studies have indicated that carbon (and nitrogen) also affect biofilm physical structure with carbon increases altering a thin, filamentous biofilm to a thicker structure supporting “mushroom” cell clusters,59 although the direct impact upon EPS composition has yet to be explored.
The autotrophs of the DWDS include the nitrifiers, ammonia oxidising archaea (AOA)109 or bacteria (AOB), such as the betaproteobacteria Nitrosomonas which have been identified in biofilm and water samples.110,111 Disinfection with chloramines can promote the growth of AOB (or potentially AOA) because ammonia is introduced as a residual from the synthesis of the chloramines and as a by-product from their decay.110 The by-products produced by AOB (namely nitrite) can cause water quality issues and potentially sustain nitrite-oxidising-bacteria (e.g. Nitrobacter spp.) as well as heterotrophs110,111 because metabolites are cycled between cells within a biofilm providing substrates that would otherwise be unobtainable. Hence, unsurprisingly, increasing ammonia concentrations increases total biofilm biomass and growth rate.112 Such cooperation of (primarily) bacteria with diverse metabolisms is an important biofilm-specific function that aids microbial growth in DWDS. These findings demonstrate that biofilms represent a reservoir of not only microorganisms but also substrates, which can degrade water quality in situ and if mobilised would be available for use by biofilms downstream.
Phosphorus, rather than carbon, may be the limiting substrate within certain DWDS as microbially available phosphorus (MAP) is essential for bio-molecule synthesis (including phospholipids and nucleic acids) and many cell functions. MAP is generally present in DWDS at ≤10 μg l−1, although upstream detachment events may increase the concentration.99 The effect of increased MAP upon the microbial community is debated; some studies find a positive correlation with biofilm growth,113 others find a negative correlation.114 This is perhaps because test waters are not phosphorus-limited113 or because many studies do not account for sources of phosphorus other than the water, for example corroded iron115 or bio-corrosion of plastic pipes which can contribute phosphorous (and nitrogen) to the DWDS.116
Metals, especially iron and manganese, have been found to facilitate biofilm accumulation and activity within reactors inoculated with biomass from a surface water source.28 Bacteria such as Pseudomonas spp. and Escherichia coli, are capable of oxidising iron as part of their metabolic processes,81,117 whilst manganese is often released following the biocorrosion of PVC pipes118 by manganese-oxidising bacteria such as Leptothrix spp.119 Heavy metal resistant bacteria have also been found in DWDS biofilms, including species which are able to release nutrients from copper pipes, resulting in increased copper concentrations, causing “blue water” issues.120 Metal oxides may be used as an energy source or may offer protection by reacting with chlorine residuals and forming deposits that accumulate on the pipe surface, as has been reported with respect to manganese.118 The potential for metals to convey protection from disinfection could have a significant impact upon biofilm management, although the occurrence of this process within DWDS has yet to be established.
Variations in iron and manganese concentrations have been found to influence microbial community diversity;7 with metal concentrations negatively correlated with AOA abundance, potentially because AOA were outcompeted by other autotrophs that were able to utilise the abundant metal particles.12 Torvinen et al.121 established that the abundance of mycobacteria was positively correlated with iron concentrations. However, as this was observed at distal areas of a DWDS, which also experienced dramatically decreased chlorine concentrations, it is possible that the iron oxides reacted with chlorine in place of cells. These interactions between nutrients and bacterial community composition are speculative and have yet to be thoroughly investigated within the DWDS and with respect to other microbial taxa. As iron and manganese, in particular, are linked to discolouration,95,122 their presence within a biofilm presents a water quality risk if detachment occurs; therefore it is essential to better understand their role in DWDS biofilm ecology.
5.4 Disinfection
The efficiency of current planktonic microbial control strategies in managing DWDS biofilms is uncertain. Commonly chemical disinfection is applied at the treatment works to “inactivate” microbial cells and then as a residual during distribution, to limit microbial regrowth and contamination. In the USA, Japan, the U.K. and various other European countries chlorine (Cl) or chloramines (NH2Cl or NHCl2) are generally provided in finished water.123 The efficiency of chemical disinfection is dependent on hydrodynamics, water chemistry, biofilm biomass and biocide action.48 Ultimately, disinfectant residuals may injure or kill planktonic cells but they do not prevent biofilm development,124,125 even inactivated/injured cells will form or attach to existing biofilms, within which they can recover.126 At best, disinfectant residuals slow biological activity.92 Ginige et al.28 found that previously non-chlorinated biofilms decreased in activity from 55.12 ng adenosine triphosphate (ATP) cm−2 to 4.10 ng ATP cm−2 within two days of chlorine application. Simultaneously the turbidity of the water increased by 8.5 nephelometric turbidity units (NTU), indicating that discolouration may be a biofilm driven response to any substantial change in the DWDS environment from historic conditions, not just the hydraulics. This study developed biofilms within a glass reactor inoculated with biomass from surface waters, so the inoculant is relevant to real DWDS but the material and hydraulics are not, which may alter interactions which are significant in governing the efficiency and action of chlorine in full-scale systems.Chloramines are generally accepted as being less reactive than chlorine, thus they degrade less quickly and produce fewer (regulated) DBPs, which can be an energy source for microorganisms within the DWDS and have been associated with public health issues.127 Consequently, chloramines have been suggested to be safer and more effective128 than chlorine. However, some non-regulated chloraminated DBPs have been shown to be very toxic and several are on contaminant lists. Therefore while fewer DBPs may be produced under disinfection with chloramines compared to chlorine, the DBPs that are produced may actually present a greater risk to water quality and public health. Chloramines have been stated to have a greater penetration potential and thus a greater disinfection effect upon the biofilm communities.81,124 However, Wang et al.129 established that bacteria and protozoa were more abundant within a chloraminated-simulated distribution system than in a chlorinated system. Hallam et al.92 also found chlorine to have the strongest disinfection action across all investigated experimental permutations involving different disinfectants, temperatures and pipe materials. Research has shown that fungi are tolerant of bacterial disinfection regimes commonly used in DWDS and that ozonation is the most effective treatment to inactivate fungi.130 Clearly, disinfection research needs to be undertaken in more representative systems and with a greater consideration of the overall diversity of microorganisms within DWDS, which are interacting with the disinfectants.
Disinfectant decay during distribution promotes microbial activity, which can adversely impact taste and odour, increase the microbial load of the DWDS biofilms (and finished water) and thus place a higher disinfection demand on the system. As a result, disinfection application may increase causing a subsequent rise in DBPs, elevated operating costs124 and potentially selecting for chlorine-resistant microorganisms. This could lead to biofilms which are very difficult to eradicate and better able to shelter potential pathogens. Although this theory has yet to be investigated thoroughly, particularly with respect to DWDS biofilms, disinfection variation within model systems has been shown to cause bacterial community shifts. During chlorination, AOB have been observed to decrease; upon switching to chloramination the reverse was true,67 this is likely due to the different organics supplied to the system. Wang et al.131 established that the same major microbial species were present regardless of the disinfection agent (chlorine or chloramines) employed but that relative abundance of those bacterial and eukaryotic species differed such that the communities developed under each disinfectant were distinct from each other.131 In the short term, these interactions could affect the microorganisms (quantity and species) and material that could be detached; in the longer term, the environmental pressures may encourage the development of a highly resistant biofilm that is more difficult to manage.
Disinfection application may also alter the biofilm physical structure; Ling and Liu132 found that with increased chloramine concentration and contact time biofilms persisted but became thinner and more compact. However, this study only stained the cells of the biofilm so, while providing some insight into the distribution of cells post-chloramination, no conclusions can be made regarding the EPS, which, as argued here, is a key component of the biofilm. Wang et al.133 clearly showed that the EPS of Pseudomonas aeruginosa or P. putida cultures interacts with chlorine as there was a positive correlation between EPS volume and DBP formation. Moreover, the chemical composition of the EPS also influenced the type of DBPs: P. putida EPS is predominantly protein-based and produced double the amount of nitrogenous DBPs compared to P. aeruginosa cultures, where EPS was primarily carbohydrate-based. While this study was based upon single-species biofilms rather than multi-species biofilms, it is plausible that similar disinfection and EPS interactions occur within DWDS.
A number of utilities in countries such as Norway (∼25%), Germany (∼50%) and the Netherlands (approaching 100%) do not use a disinfection residual during distribution but some do use UV radiation at the treatment stage.19,134 Hageskal et al.130 demonstrated that the most common fungi in Norwegian DWDS135 are those with an ability to survive the UV disinfection (potentially due to their pigmentation), primarily Penicillium spinulosum, and Trichoderma viride. The Netherlands and Switzerland, also aim towards producing high-quality drinking water via implementation of alternative methods to chemical disinfection11 such as ultra-filtration or reverse osmosis,136 which primarily control growth-limiting substrates. This is in response to customers' preference for drinking water without a chlorine residual, due to the taste and potentially harmful DBPs that can develop.134 The arguments for alternative treatments that enhance the chemical quality of drinking water are compelling, particularly as, irrespective of the disinfection process(es) applied, microorganisms prevail in DWDS. However, the influence of these methods (or perhaps better thought of as the influence of a lack of disinfection residual) upon the biofilm composition, structure and stability and thus their potential risk to water quality degradation is unknown.
Internationally, disinfection regimes, regardless of the specific approach, focus upon the management of planktonic microorganisms. Biofilms potentially present a bigger threat to water quality than the planktonic community, not only due to a greater microbial abundance but because biofilm-bound bacteria and fungi tend to be more resistant to residuals than their planktonic counterparts.130,137 The mechanisms behind increased disinfectant resistance are debated in the literature. It has been suggested that biofilm disinfection resistance is due to an abundance of “persistor” cells which do not automatically lyse when injured or stressed or, alternatively, that biofilm cells are less susceptible due to biochemical changes (e.g. alterations in membrane composition), slow growth or phenotypic differences from the free-living cells.138,139 As it has been established that many biocide agents are more effective at lysing or injuring fast growing cells, this theory is feasible. However, generally it is accepted that the EPS provides physical protection in the form of a barrier which the disinfectant agents cannot penetrate,57 either because they bind to the EPS rather than reacting with the cells140 or because enzymes in the matrix degrade the residuals.139 It is possible that EPS cohesive forces may be weakened by this disinfection action, as has also been reported to occur with biofilm ageing and decreased nutrient concentration,28,141 which increases the likelihood of the biofilm detaching under shear stresses which it could previously withstand and causing discolouration events as previously mentioned.
Wingender et al.137 and Xue et al.142 showed that the EPS of Pseudomonas aeruginosa cultures limited the action of many disinfection agents but not hydrogen peroxide, demonstrating that this resistance mechanism is not universal for all biocides. Interestingly, a study of cultured P. fluorescens found that EPS was associated with planktonic cells but the composition differed from that within biofilm, although the authors acknowledged that improved polysaccharide extraction is necessary to more robustly differentiate between the two EPS types.143 Nevertheless, there is the potential for detached cells to retain an EPS coating, which affords a degree of protection against disinfectant residuals that previously unbound cells do not have. Despite the apparent interactions between EPS and disinfectants, these reactions and their effects upon biofilms within DWDS are not well explored.
5.5 Other environmental parameters
Environmental parameters such as pH, oxygen and temperature vary temporally and spatially throughout and between DWDS. Due to the complex interactions between the various parameters, a change in one can substantially affect another. For instance, temperature or pH can impact the efficiency of chlorine; Keevil et al.144 report that biocide activity rapidly decreases in alkaline conditions such that, at pH 8, a threefold increase in chlorine concentration is necessary to retain the disinfection activity seen at pH 7. Very few studies address pH variation in DWDS with respect to microbiology, probably because treated water is managed to be close to neutral with minimal variation during distribution. However, pH remains an important issue with respect to water chemistry, disinfection and corrosion, which will subsequently affect the microbial ecology of DWDS. Meckes et al.145 developed biofilms under pH 5 to pH 10, within identical test loops and monitored growth by heterotrophic plate counts (HPC). Biofilm growth was greatest in the alkaline conditions but HPC does not provide a comprehensive assessment of microbial presence and correlation does not imply causation – the pH change may have affected the pipe material or nutrient cycling rather than directly limiting growth. Additionally, compared to pH variation in DWDS, the pH ranges tested are extreme; further research is necessary to determine the influence of smaller pH changes upon DWDS biofilms.The influence of oxygen availability upon DWDS biofilms also remains somewhat unexplored. In biofilms from other environments, the basal layer is generally anaerobic but the influences of this upon biofilm community and architecture have not been thoroughly explored. Paul et al.97 provide a rare insight into these interactions, showing that anaerobic conditions resulted in denser, thicker biofilms than were seen under aerobic conditions. Fluorescent beads have been tracked through biofilms within a reactor and illustrated that channels within the EPS were filled with flowing liquid,146 facilitating the circulation of oxygen48 and so it is possible that DWDS biofilms are not anaerobic at the basal level. Microelectrodes for the detection of pH, nitrification substrates, ammonia, sulphate-reduction and sulphate-oxidation have demonstrated that these are also circulated around wastewater-47 and nutrient rich stream-147 biofilms. These environments are a stark contrast to DWDS but it is feasible that drinking water biofilms also express this physical structure.
As in all biological systems, temperature regulates reaction rates within DWDS, particularly in those supplying water with no disinfectant residual.92,148 Temperature has been thought to be most influential in above ground water storage units, as buried pipes experience lower thermal variation, Husband et al.95 recorded a range of 4–14 °C in U.K. DWDS and ranges of 5–22 °C were found in 90 US systems.126 Bacteriological issues are more common in warmer months, likely because microbial growth is accelerated.90 Various studies have shown a significant increase in bacteria (including coliforms) at temperatures ≥15 °C (ref. 92, 126) and it has been established that naturally cooler waters experience a coliform increase at 10 °C.126 Bagh et al.148 demonstrated that warm drinking waters contain a greater bacterial diversity than cool waters, but Revetta et al.38 failed to confirm these findings, potentially due to differences in diversity assessment and species identification methods. Seasonal variation in temperature has been suggested to influence the microbial community in source water and subsequently affect the abundance and diversity of microbes inoculating the DWDS pipelines.90 Pinto et al.76 clearly established that planktonic bacterial communities within a DWDS experienced seasonal variation; lower richness was observed over winter/spring (December–May) than in summer/autumn (June–November), with alphaproteobacteria and betaproteobacteria dominating the communities, respectively. The planktonic communities sampled at the exit of the treatment works were (on average) 20–40% different from the communities sampled from the DWDS.76 This observation indicates a substantial interaction between the biofilm-microbiome found upon the pipe walls of DWDS and the microbiome of the water, during distribution. Similarly, El-Chakhtoura et al.149 found that the planktonic microbiome at the treatment works and within the DWDS differed, with 35–42% of taxa being specific to one of the sites. The DWDS bacterial community had a greater total cell concentration (evaluated via flow-cytometry) and some taxa from the treatment works were absent from DWDS samples, possibly due to their incorporation into biofilms.149 As these differences occur under normal operating conditions (during a water quality event the difference between the bacterial communities at the treatment works and in the DWDS is likely to be greater), seasonal effects upon DWDS biofilms may be possible due to the differential seeding from the bulk-water cells. However, as biofilm communities are shaped by the historic environments of the DWDS and are distinct from the planktonic communities, any seasonal variation may not be as noticeable. Research by Sharpe150 confirmed that temperature impacted the accumulation of cells at the pipe wall in a full-scale DWDS test facility, with greater cell coverage occurring at 16 °C than at 8 °C. Furthermore, this study showed that influence of temperature upon the accumulation (and subsequent mobilization) of cells was greater under steady-state flows than varied flow conditions. Temperature effects are seemingly restricted to the assessment of pathogens or whole communities (via fingerprinting or more recently, high throughput sequencing), with limited study of the impacts upon EPS, understanding of which is critical to predict biofilm behavior.
6 Summary and concluding comments
Understanding the interactions at the pipe–water interface is critical to managing and protecting water quality, and, subsequently, public wellbeing. This interface is where biofilms occur, which may affect water quality during distribution via the processes they mediate or due to detachment/mobilisation of biofilm material, which contributes to aesthetic degradation (e.g. discolouration) or microbiological quality failures. Therefore, it is essential to gain an understanding of biofilm community and physical composition within DWDS.Previous research has focused predominantly upon environmental effects upon the bacterial community, the only taxa for which water quality legislation exists world-wide. However, the microbiomes of DWDS are extremely diverse and even with advances in molecular analysis, the majority of the microbial world within DWDS remains unidentified, illustrating the under-representation of drinking water-derived sequences in databases. Consequently it is essential that future research considers a wider range of taxa than just bacteria; fungi, archaea, protozoa and viruses are also found within DWDS but the co-existence and ecological interactions of these different taxa and their resulting impacts upon water quality are relatively unexplored.
Critical to improving DWDS biofilm management is further understanding the environmental/engineering effects upon biofilm stability and detachment. From an engineering perspective, material (organic and inorganic) has been described to develop at the pipe wall in cohesive layers conditioned by the network hydraulics. Biofilms are known to exhibit cohesive properties via their EPS matrix, which is critical in both the formation and detachment of the assemblages and is the characteristic difference between planktonic and biofilm communities. The EPS matrix governs mechanical stability of the biofilm, an attribute which is prioritised by biofilm communities and influenced by DWDS hydraulics. However, based upon the currently available literature, it is difficult to predict the response of biofilms to hydraulic (or other environmental/engineering) changes within full-scale pipe lines due to the use of bench-top scale experimental systems. It is essential that research seeks to address this and improve simulation of the full-scale DWDS environment with respect to engineering and microbial parameters. Additionally, the impact of environmental conditions upon biofilms needs to be addressed in an integrated manner as interactions are complex. For instance, hydraulics affect the exchange of nutrients, microorganisms, and disinfectants at the pipe–water interface. It is also essential that future investigations combine the study of the physical structure (i.e. EPS) of biofilms with microbial community analysis. Achieving such an integrated approach will allow the essential transition and implementation of research generated knowledge to the operation and biofilm management in full-scale DWDS, ideally also improving the prediction of water quality failures (likelihood and risk level), such as discolouration events.
A common public and industry perception is that biofilms should be completely eradicated, an impossible and, arguably, unnecessary demand. To inform and change this mind-set from biofilm eradication to better biofilm management, it is necessary to better appreciate the complex abiotic and biotic interactions occurring between the pipe-wall and the water column. Invaluable initial insights into DWDS biofilm ecology have been provided in the literature to date. With advances in technology there is an opportunity (and need) for future research to build upon this knowledge and bridge the critical gap between bench-top based systems and the field. To achieve this requires an increased applicability of laboratory growth conditions to the engineering and physico-chemical environment of operational DWDS. The opportunities and protocols for biofilm sampling from within real DWDS also require improvement, which will require cooperation and coordination with water suppliers. Of particular relevance may be further understanding and evaluating the impacts of hydraulics and disinfection (especially chlorination) upon the biofilm microbiota, and crucially also incorporating characterization of the EPS and associated inorganics. Future research agendas need to address drinking water biofilm research via multidisciplinary approaches, in order to fully appreciate and more effectively model both the microbial and engineering details of these complex but crucial systems. Only by doing this, can effective biofilm management strategies be developed, which will sustain both the distribution infrastructure and a high quality water supply into the future.
Acknowledgements
KEF was funded by a Natural Environment Research Council PhD studentship (NE/H52489X/1). The research was conducted within the Pipe Dreams project, supported by the U.K. Engineering and Physical Sciences Research Council (Challenging Engineering: EP/G029946/1) and the Pennine Water Group EPSRC Platform Grant (EP/1029346/1).References
- I. J. H. G. Vreeburg and D. J. B. Boxall, Water Res., 2007, 41, 519–529 CrossRef CAS PubMed.
- J. Machell, J. Boxall, A. Saul and D. Bramley, J. Water Resour. Plan. Manag., 2009, 135, 382–391 CrossRef.
- D. Karanja, S. J. Elliott and S. Gabizon, Curr. Opin. Environ. Sustain., 2011, 3, 467–470 CrossRef.
- WHO and UNICEF, WHO, 2012.
- UKWIR, National Database of Mains Failures 2003, 2003 Search PubMed.
- J. Costerton, K. Cheng, G. Geesey, T. Ladd, J. Nickel, M. Dasgupta and T. Marrie, Annu. Rev. Microbiol., 1987, 41, 435–464 CrossRef CAS PubMed.
- O. M. Zacheus, M. J. Lehtola, L. K. Korhonen and P. J. Martikainen, Water Res., 2001, 35, 1757–1765 CrossRef CAS PubMed.
- K. Henne, L. Kahlisch, I. Brettar and M. G. Höfle, Appl. Environ. Microbiol., 2012, 78, 3530–3538 CrossRef CAS PubMed.
- I. Douterelo, R. L. Sharpe and J. B. Boxall, Water Res., 2013, 47, 503–516 CrossRef CAS PubMed.
- G. Roeselers, J. Coolen, P. W. J. J. van der Wielen, M. C. Jaspers, A. Atsma, B. de Graaf and F. Schuren, Environ. Microbiol., 2015, 17, 2505–2514 CrossRef PubMed.
- F. Hammes, M. Berney, Y. Wang, M. Vital, O. Köster and T. Egli, Water Res., 2008, 42, 269–277 CrossRef CAS PubMed.
- D. Hoefel, W. Grooby, P. Monis, S. Andrews and C. Saint, J. Microbiol. Methods, 2003, 55, 585–597 CrossRef PubMed.
- M. Vital, M. Dignum, A. Magic-Knezev, P. Ross, L. Rietveld and F. Hammes, Water Res., 2012, 46, 4665–4676 CrossRef CAS PubMed.
- A. A. Morvay, M. Decun, M. Scurtu, C. Sala, A. Morar and M. Sarandan, Water Sci. Technol.: Water Supply, 2011, 11, 252 CrossRef CAS.
- G. Liu, J. Q. J. C. Verberk and J. C. Van Dijk, Appl. Microbiol. Biotechnol., 2013, 97, 9265–9276 CrossRef CAS PubMed.
- D. W. I. DWI, 2008.
- E. U. EU, Council of 3rd November 1998 on the quality of water intended for human consumption, 1998 Search PubMed.
- D. D. Ratnayaka, M. J. Brandt and K. M. Johnson, Twort's Water Supply, Butterworth-Heinemann, Great Britain, 6th edn, 2009 Search PubMed.
- G. Hageskal, P. Gaustad, B. Heier and I. Skaar, J. Appl. Microbiol., 2007, 102, 774–780 CrossRef CAS PubMed.
- J. Staley and A. Konopka, Annu. Rev. Microbiol., 1985, 39, 321–346 CrossRef CAS PubMed.
- M. T. Osterholm, Clin. Infect. Dis., 2000, 31, i–iii CAS.
- Drinking Water Inspectorate, 2014.
- M. Polychronopolous, K. Dudley, G. Ryan and J. Hearn, Water Sci. Technol.: Water Supply, 2003, 3, 295–306 Search PubMed.
- F. Quignon, M. Sardin, L. Kiene and L. Schwartzbrod, Water Sci. Technol., 1997, 311–318 CrossRef CAS.
- E. Göttlich, W. van der Lubbe, B. Lange, S. Fiedler, I. Melchert, M. Reifenrath, H.-C. Flemming and S. de Hoog, Int. J. Hyg. Environ. Health, 2002, 205, 269–279 CrossRef PubMed.
- S. Tinker, C. Moe, M. Klein, W. Flanders, J. Uber, A. Amirtharajah, P. Singer and P. Tolbert, J. Water Health, 2009, 7, 332–343 CrossRef PubMed.
- J. A. Roberts, P. Cumberland, P. N. Sockett, J. Wheeler, L. C. Rodrigues, D. Sethi and P. J. Roderick, Infectious Intestinal Disease Study Executive, Epidemiol. Infect., 2003, 130, 1–11 CrossRef CAS PubMed.
- M. P. Ginige, J. Wylie and J. Plumb, Biofouling, 2011, 27, 151–163 CrossRef CAS PubMed.
- D. W. I. DWI, 2001.
- J. Schwartz, R. Levin and R. Goldstein, J. Epidemiol. Community Health, 2000, 54, 45–51 CrossRef CAS PubMed.
- P. R. Hunter, R. M. Chalmers, S. Hughes and Q. Syed, Clin. Infect. Dis., 2005, 40, e32–34 CrossRef PubMed.
- G. J. Kirmeyer, Guidance manual for maintaining distribution system water quality, American Water Works Association, 2000 Search PubMed.
- J. B. Boxall, P. J. Skipworth and A. J. Saul, in Proceedings of the Computing and Control in the Water Industry Conference, DeMonfort University, UK, 2001 Search PubMed.
- J. Boxall and A. Saul, J. Environ. Eng., 2005, 131, 716–725 CrossRef CAS.
- S. Husband and J. B. Boxall, J. Environ. Eng., 2010, 136, 86–94 CrossRef CAS.
- P. S. Husband and J. B. Boxall, Water Res., 2011, 45, 113–124 CrossRef CAS PubMed.
- J.-B. Poitelon, M. Joyeux, B. Welté, J.-P. Duguet, J. Peplies and M. S. DuBow, Lett. Appl. Microbiol., 2009, 49, 589–595 CrossRef CAS PubMed.
- R. Revetta, A. Pemberton, R. Lamendella, B. Iker and J. Domingo, Water Res., 2010, 44, 1353–1360 CrossRef CAS PubMed.
- J. Yu, D. Kim and T. Lee, Water Sci. Technol., 2010, 61, 163–171 CrossRef CAS PubMed.
- J. O. I. Falkinham, C. D. Norton and M. W. LeChevallier, Appl. Environ. Microbiol., 2001, 67, 1225–1231 CrossRef CAS PubMed.
- S. R. Park, W. G. Mackay and D. C. Reid, Water Res., 2001, 35, 1624–1626 CrossRef CAS PubMed.
- J. J. Kelly, N. Minalt, A. Culotti, M. Pryor and A. Packman, PLoS One, 2014, 9(5), e98542 Search PubMed.
- I. B. Gomes, M. Simões and L. C. Simões, Water Res., 2014, 62, 63–87 CrossRef CAS PubMed.
- P. Deines, R. Sekar, P. Husband, J. Boxall, A. Osborn and C. Biggs, Appl. Microbiol. Biotechnol., 2010, 87, 749–756 CrossRef CAS PubMed.
- K. E. Fish, R. Collins, N. H. Green, R. L. Sharpe, I. Douterelo, A. M. Osborn and J. B. Boxall, PLoS One, 2015, 10, e0115824 Search PubMed.
- X. Luo, K. L. Jellison, K. Huynh and G. Widmer, PLoS One, 2015, 10, e0133427 Search PubMed.
- M. Kűhl and B. B. Jørgensen, Appl. Environ. Microbiol., 1992, 58, 1164–1174 Search PubMed.
- D. de Beer, R. Srinivasan and P. S. Stewart, Appl. Environ. Microbiol., 1994, 60, 4339–4344 CAS.
- H. Beyenal and Z. Lewandowski, Biotechnol. Prog., 2002, 18, 55–61 CrossRef CAS PubMed.
- B. Purevdorj, J. Costerton and P. Stoodley, Appl. Environ. Microbiol., 2002, 68, 4457–4464 CrossRef CAS PubMed.
- D. de Beer, P. Stoodley and Z. Lewandowski, Water Res., 1996, 30, 2761–2765 CrossRef CAS.
- P. Stoodley, R. Cargo, C. J. Rupp, S. Wilson and I. Klapper, J. Ind. Microbiol. Biotechnol., 2002, 29, 361–367 CrossRef CAS PubMed.
- M. Simoes, M. Pereira and M. Vieira, Water Res., 2005, 39, 5142–5152 CrossRef CAS PubMed.
- Y. Abe, S. Skali-Lami, J.-C. Block and G. Francius, Water Res., 2012, 46, 1155–1166 CrossRef CAS PubMed.
- A. C. Martiny, T. M. Jørgensen, H.-J. Albrechtsen, E. Arvin and S. Molin, Appl. Environ. Microbiol., 2003, 69, 6899–6907 CrossRef CAS PubMed.
- I. Douterelo, J. B. Boxall, P. Deines, R. Sekar, K. E. Fish and C. A. Biggs, Water Res., 2014, 65C, 134–156 CrossRef PubMed.
- T. Neu and J. Lawrence, in Microbial Glycobiology: Structures, Relevance and Applications, Elsevier, San Diego, 2009, pp. 735–758 Search PubMed.
- H. Flemming and J. Wingender, Nat. Rev. Microbiol., 2010, 8, 623–633 CAS.
- P. Stoodley, L. Hall-Stoodley and H. Lappin-Scott, Microb. Growth Biofilms Pt B, 2001, vol. 337, pp. 306–318 Search PubMed.
- P. Stoodley, S. Wilson, L. Hall-Stoodley, J. Boyle, H. Lappin-Scott and J. Costerton, Appl. Environ. Microbiol., 2001, 67, 5608–5613 CrossRef CAS PubMed.
- P. Stoodley, I. Dodds, J. Boyle and H. Lappin-Scott, J. Appl. Microbiol., 1999, 85, 19S–28S CrossRef PubMed.
- C. Picioreanu, M. van Loosdrecht and J. Heijnen, Biotechnol. Bioeng., 2000, 72, 205–218 CrossRef.
- E. Morgenroth and P. A. Wilderer, Water Res., 2000, 34, 417–426 CrossRef CAS.
- M. Williams, J. Domingo, M. Meckes, C. Kelty and H. Rochon, J. Appl. Microbiol., 2004, 96, 954–964 CrossRef CAS PubMed.
- K. A. Kormas, C. Neofitou, M. Pachiadaki and E. Koufostathi, Environ. Monit. Assess., 2010, 165, 27–38 CrossRef CAS PubMed.
- T. Schwartz, S. Hoffmann and U. Obst, Water Res., 1998, 32, 2787–2797 CrossRef CAS.
- J. W. Santo Domingo, M. C. Meckes, J. M. Simpson, B. Sloss and D. J. Reasoner, Water Sci. Technol., 2003, 47, 149–154 CAS.
- E. Otterholt and C. Charnock, Water Res., 2011, 45, 2527–2538 CrossRef CAS PubMed.
- N. B. Sammon, K. M. Harrower, L. D. Fabbro and R. H. Reed, Int. J. Environ. Res. Public Health, 2011, 8, 713–732 CrossRef PubMed.
- J. H. M. van Lieverloo, W. Hoogenboezem, G. Veenendaal and D. van der Kooij, Water Res., 2012, 46, 4918–4932 CrossRef CAS PubMed.
- I. Sibille, T. Sime-Ngando, L. Mathieu and J. Block, Appl. Environ. Microbiol., 1998, 64, 197–202 CAS.
- F. Codony, L. M. Pérez, B. Adrados, G. Agustí, M. Fittipaldi and J. Morató, Future Microbiol., 2012, 7, 25–31 CrossRef CAS PubMed.
- E. Lambertini, M. A. Borchardt, B. A. Kieke, S. K. Spencer and F. J. Loge, Environ. Sci. Technol., 2012, 46, 9299–9307 CrossRef CAS PubMed.
- K. Helmi, S. Skraber, C. Gantzer, R. Willame, L. Hoffmann and H.-M. Cauchie, Appl. Environ. Microbiol., 2008, 74, 2079–2088 CrossRef CAS PubMed.
- S. C. B. Christensen, E. Nissen, E. Arvin and H.-J. Albrechtsen, Water Res., 2011, 45, 3215–3224 CrossRef CAS PubMed.
- A. J. Pinto, J. Schroeder, M. Lunn, W. Sloan and L. Raskin, mBio, 2014, 5(3), e01135–14 CrossRef PubMed.
- H. Sun, B. Shi, Y. Bai and D. Wang, Sci. Total Environ., 2014, 472, 99–107 CrossRef CAS PubMed.
- H. Ren, W. Wang, Y. Liu, S. Liu, L. Lou, D. Cheng, X. He, X. Zhou, S. Qiu, L. Fu, J. Liu and B. Hu, Appl. Microbiol. Biotechnol., 2015, 99, 10713–10724 CrossRef CAS PubMed.
- K. Pedersen, Water Res., 1990, 24, 239–243 CrossRef.
- P. Niquette, P. Servais and R. Savoir, Water Res., 2000, 34, 1952–1956 CrossRef CAS.
- M. LeChevallier, C. Lowry, R. Less and D. Gibbon, J. - Am. Water Works Assoc., 1993, 85, 111–123 CAS.
- R. Boe-Hansen, H.-J. Albrechtsen, E. Arvin and C. Jørgensen, Water Res., 2002, 36, 4477–4486 CrossRef CAS PubMed.
- S. Percival, J. Knapp, R. Edyvean and D. Wales, Water Res., 1998, 32, 243–253 CrossRef CAS.
- C. White, M. Tancos and D. A. Lytle, Appl. Environ. Microbiol., 2011, 77, 5557–5561 CrossRef CAS PubMed.
- B. A. Wullings, G. Bakker and D. Van Der Kooij, Appl. Environ. Microbiol., 2011, 77, 634–641 CrossRef CAS PubMed.
- C. Kerr, K. Osborn, G. Roboson and P. Handley, J. Appl. Microbiol., 1999, 85, 29S–38S CrossRef PubMed.
- I. Douterelo, S. Husband and J. B. Boxall, Water Res., 2014, 54, 100–114 CrossRef CAS PubMed.
- M. Lehtola, K. Miettinen, M. Keinanen, T. Kekki, O. Laine, A. Hirvonen, T. Vartiainen and P. Martikainen, Water Res., 2004, 38, 3769–3779 CrossRef CAS PubMed.
- A. K. Camper, K. Brastrup, A. Sandvig, J. Clement, C. Spencer and A. J. Capuzzi, J. - Am. Water Works Assoc., 2003, 95, 107–121 CAS.
- Z. G. Tsvetanova and D. N. Dimitrov, Water Sci. Technol.: Water Supply, 2012, 12, 720 CrossRef CAS.
- A. Rożej, A. Cydzik-Kwiatkowska, B. Kowalska and D. Kowalski, World J. Microbiol. Biotechnol., 2015, 31, 37–47 CrossRef PubMed.
- N. Hallam, J. West, C. Forster and J. Simms, Water Res., 2001, 35, 4063–4071 CrossRef CAS PubMed.
- M. Vieira, R. Oliveira, L. Melo, M. Pinheiro and V. Martins, Colloids Surf., B, 1993, 1, 119–124 CrossRef CAS.
- M. Vieira and L. Melo, Bioprocess Eng., 1999, 20, 369–375 CrossRef CAS.
- P. Husband, J. Boxall and A. Saul, Water Res., 2008, 42, 4309–4318 CrossRef CAS PubMed.
- G. Heinrichs, I. Hübner, C. K. Schmidt, G. S. Hoog and G. Haase, Mycopathologia, 2013, 175, 387–397 CrossRef CAS PubMed.
- E. Paul, J. C. Ochoa, Y. Pechaud, Y. Liu and A. Liné, Water Res., 2012, 46, 5499–5508 CrossRef CAS PubMed.
- C. M. Manuel, O. C. Nunes and L. F. Melo, Water Res., 2007, 41, 551–562 CrossRef CAS PubMed.
- M. Lehtola, I. Miettinen, A. Hirvonen, T. Vartiainen and P. Martikainen, in IWA Biofilm Systems IV, Amsterdam, 2006 Search PubMed.
- R. Sekar, P. Deines, J. Machell, A. M. Osborn, C. A. Biggs and J. B. Boxall, J. Appl. Microbiol., 2012, 112, 1220–1234 CrossRef CAS PubMed.
- S.-K. Park, J.-H. Choi and J. Y. Hu, Desalination, 2012, 296, 7–15 CrossRef CAS.
- Y. Liu and J. Tay, J. Appl. Microbiol., 2001, 90, 337–342 CrossRef CAS PubMed.
- M. Simoes, M. Pereira and M. Vieira, Water Sci. Technol., 2003, 47, 217–223 CAS.
- M. Simoes, S. Cleto, M. Pereira and M. Vieira, Water Sci. Technol.: Water Supply, 2007, 55, 473–480 CrossRef CAS.
- C. J. Volk and M. W. LeChevallier, Appl. Environ. Microbiol., 1999, 65, 4957–4966 CAS.
- D. van der Kooij, J. - Am. Water Works Assoc., 1992, 84, 57–65 CAS.
- I. Escobar, A. Randall and J. Taylor, Environ. Sci. Technol., 2001, 35, 3442–3447 CrossRef CAS PubMed.
- Y. Ohkouchi, B. T. Ly, S. Ishikawa, Y. Kawano and S. Itoh, Environ. Monit. Assess., 2013, 185, 1427–1436 CrossRef CAS PubMed.
- P. W. J. J. V. D. Wielen, S. Voost and D. Van Der Kooij, Appl. Environ. Microbiol., 2009, 75, 4687–4695 CrossRef PubMed.
- J. Regan, G. Harrington and D. Noguera, Appl. Environ. Microbiol., 2002, 68, 73–81 CrossRef CAS PubMed.
- J. M. Regan, G. W. Harrington, H. Baribeau, R. D. Leon and D. R. Noguera, Water Res., 2003, 37, 197–205 CrossRef CAS PubMed.
- S. Okabe, H. Satoh and Y. Watanabe, Appl. Environ. Microbiol., 1999, 65, 3182–3191 CAS.
- B. M. R. Appenzeller, Y. B. Duval, F. Thomas and J.-C. Block, Environ. Sci. Technol., 2002, 36, 646–652 CrossRef CAS PubMed.
- M. M. Keinanen, L. K. Korhonen, M. J. Lehtola, I. T. Miettinen, P. J. Martikainen, T. Vartiainen and M. H. Suutari, Appl. Environ. Microbiol., 2002, 68, 434–439 CrossRef CAS PubMed.
- S. Morton, Y. Zhang and M. Edwards, Water Res., 2005, 39, 2883–2892 CrossRef CAS PubMed.
- D. Brocca, E. Arvin and H. Mosbaek, Water Res., 2002, 36, 3675–3680 CrossRef CAS PubMed.
- L. E. Hersman, J. H. Forsythe, L. O. Ticknor and P. A. Maurice, Appl. Environ. Microbiol., 2001, 67, 4448–4453 CrossRef CAS PubMed.
- J. M. Cerrato, L. P. Reyes, C. N. Alvarado and A. M. Dietrich, Water Res., 2006, 40, 2720–2726 CrossRef CAS PubMed.
- J. Kielemoes, I. Bultinck, H. Storms, N. Boon and W. Verstraete, FEMS Microbiol. Ecol., 2002, 39, 41–55 CrossRef CAS PubMed.
- M. Critchley, R. Pasetto and R. J. O'Halloran, J. Appl. Microbiol., 2004, 97, 590–597 CrossRef CAS PubMed.
- E. Torvinen, S. Suomalainen, M. J. Lehtola, I. T. Miettinen, O. Zacheus, L. Paulin, M.-L. Katila and P. J. Martikainen, Appl. Environ. Microbiol., 2004, 70, 1973–1981 CrossRef CAS PubMed.
- (Environmental Protection Agency) US EPA, Potential Contamination Due to Cross-Connection and Backflow and the Associated Health Risks, 2004 Search PubMed.
- Euro Chlor, 2006.
- J. Chandy and M. Angles, Water Res., 2001, 35, 2677–2682 CrossRef CAS PubMed.
- M. Williams and E. Braun-Howland, Appl. Environ. Microbiol., 2003, 69, 5463–5471 CrossRef CAS PubMed.
- M. LeChevallier, N. Welch and D. Smith, Appl. Environ. Microbiol., 1996, 62, 2201–2211 CAS.
- J. Wei, B. Ye, W. Wang, L. Yang, J. Tao and Z. Hang, Sci. Total Environ., 2010, 408, 4600–4606 CrossRef CAS PubMed.
- P. S. Stewart, F. Roe, J. Rayner, J. G. Elkins, Z. Lewandowski, U. A. Ochsner and D. J. Hassett, Appl. Environ. Microbiol., 2000, 66, 836–838 CrossRef CAS PubMed.
- H. Wang, S. Masters, Y. Hong, J. Stallings, J. O. Falkinham 3rd, M. A. Edwards and A. Pruden, Environ. Sci. Technol., 2012, 46, 11566–11574 CrossRef CAS PubMed.
- G. Hageskal, I. Tryland, H. Liltved and I. Skaar, Water Sci. Technol.: Water Supply, 2012, 12, 220 CrossRef CAS.
- H. Wang, S. Masters, M. A. Edwards, J. O. Falkinham and A. Pruden, Environ. Sci. Technol., 2014, 48, 1426–1435 CrossRef CAS PubMed.
- F. Ling and W.-T. Liu, Microbes Environ., 2013, 28, 50–57 CrossRef PubMed.
- Z. Wang, J. Kim and Y. Seo, Environ. Sci. Technol., 2012, 46, 11361–11369 CrossRef CAS PubMed.
- W. Uhl, G. Schaule and R. Gimbel, Water Sci. Technol.: Water Supply, 2001, 2, 259–266 Search PubMed.
- G. Hageskal, A. K. Knutsen, P. Gaustad, G. S. de Hoog and I. Skaar, Appl. Environ. Microbiol., 2006, 72, 7586–7593 CrossRef CAS PubMed.
- J. Kruithof, P. Kamp, H. Folmer, M. Nederlof and S. van Hoof, Membranes in Drinking and Industrial Water Production II, 2001, vol. 1, pp. 261–271 Search PubMed.
- J. Wingender, S. Grobe, S. Fiedler and H. Flemming, Biofilms Aquat. Environ., 1999, pp. 93–100 Search PubMed.
- K. Lewis, Antimicrob. Agents Chemother., 2001, 45, 999–1007 CrossRef CAS PubMed.
- T. F. C. Mah and G. A. O'Toole, Trends Microbiol., 2001, 9, 34–39 CrossRef CAS PubMed.
- X. Chen and P. S. Stewart, Environ. Sci. Technol., 1996, 30, 2078–2083 CrossRef CAS.
- B. M. Peyton and W. G. Characklis, Biotechnol. Bioeng., 1993, 41, 728–735 CrossRef CAS PubMed.
- Z. Xue, C. M. Hessler, W. Panmanee, D. J. Hassett and Y. Seo, FEMS Microbiol. Ecol., 2013, 83, 101–111 CrossRef CAS PubMed.
- J. Kives, B. Orgaz and C. SanJose, Colloids Surf., B, 2006, 52, 123–127 CrossRef CAS PubMed.
- C. Keevil, C. W. Mackerness and J. S. Colbourne, Int. Biodeterior., 1990, 169–179 CrossRef CAS.
- M. C. Meckes, R. Haught, M. Dosani, R. Clark and M. Sivaganesan, in Proceedings of the American Water Works Association Water Quality Technology Conference, AWWA, Denver, CO, 1999 Search PubMed.
- P. Stoodley, D. de Beer and Z. Lewandowski, Appl. Environ. Microbiol., 1994, 60, 2711–2716 CAS.
- L. P. Nielsen, P. B. Christensen, N. P. Revsbech and J. Sørensen, Microb. Ecol., 1990, 19, 63–72 CrossRef CAS PubMed.
- L. Bagh, H. Albrechtsen, E. Arvin and K. Ovesen, Water Sci. Technol., 2002, 46, 95–101 CAS.
- J. El-Chakhtoura, E. Prest, P. Saikaly, M. van Loosdrecht, F. Hammes and H. Vrouwenvelder, Water Res., 2015, 74, 180–190 CrossRef CAS PubMed.
- R. Sharpe, Ph.D Thesis, The University of Sheffield, 2012 Search PubMed.
- B. Holden, M. Greetham, B. T. Croll and J. Scutt, Water Sci. Technol., 1995, 32, 213–220 CrossRef CAS.
- R. Liu, Z. Yu, H. Zhang, M. Yang, B. Shi and X. Liu, Can. J. Microbiol., 2012, 58, 261–270 CrossRef CAS PubMed.
- K. Lautenschlager, C. Hwang, W.-T. Liu, N. Boon, O. Köster, H. Vrouwenvelder, T. Egli and F. Hammes, Water Res., 2013, 47, 3015–3025 CrossRef CAS PubMed.
- R. M. Valster, B. A. Wullings, G. Bakker, H. Smidt and D. van der Kooij, Appl. Environ. Microbiol., 2009, 75, 4736–4746 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ew00039h |
| This journal is © The Royal Society of Chemistry 2016 |