Impact of microbial activities and hydraulic retention time on the production and profile of long chain fatty acids in grease interceptors: a laboratory study†
Xia
He
and
Tao
Yan
*
Department of Civil and Environmental Engineering, University of Hawaii at Manoa, 2540 Dole Street, 383 Holmes Hall, Honolulu, HI 96822, USA. E-mail: taoyan@hawaii.edu; Fax: +1 808 956 5014; Tel: +1 808 956 6024
First published on 17th March 2016
Abstract
Fat, oil and grease (FOG) deposits are a major cause of sanitary sewer overflows (SSOs), and calcium salts of long chain fatty acids (LCFAs) have recently been identified as key components of FOG deposits in sewer systems. Since grease interceptors (GIs) are commonly used to prevent sewer FOG deposits, this study aims to investigate how microbial activities and hydraulic retention time (HRT) of laboratory GI reactors affect the production and profile of LCFAs. The GI reactors with microbial activities exhibited five times higher total LCFAs than the control reactors where microbial activities were inhibited, and a significant positive correlation was observed between microbial activities and total LCFA concentration in the reactor effluent. However, HRT did not exhibit a consistent impact on microbial activities, COD, and total LCFA concentrations in the GI reactor effluent, which was likely caused by the stratified operational mode of the GI reactors. A significantly higher concentration of LCFAs was detected within the GI reactors than in the effluent, corresponding to the stratification of LCFAs within the GI reactors. High similarity of LCFA profile was observed between the GI effluent and real FOG deposits, while the LCFAs retained within the GI reactors, which contained more unsaturated LCFAs, showed different profiles than that in the GI effluent. Together, our data indicate that microbial activities in GIs can significantly impact the quantity and profile of LCFAs, which should be taken into consideration in the GI design and operation in order to reduce the formation of FOG deposits in sewer systems.
Water impactLong chain fatty acids (LCFAs) are important components of sewer FOG deposits, which threaten the proper operation of sewer systems and cause environmental damages. This study showed that microbial activities can significantly impact the quantity and profile of LCFAs in grease interceptors (GIs) and hence should be considered in the GI design and operation to reduce FOG deposit formation in sewer systems. |
Introduction
The formation of hardened fat, oil and grease (FOG) deposits often causes severe operational and maintenance problems in sewer systems, with the worst scenario being sanitary sewer overflows (SSOs). It was estimated that about 50–75% of SSOs in the United States1 and about 21% of sewer blockages in Australia2 were caused primarily by the formation of FOG deposits in sewer pipes. Although FOG deposits were traditionally perceived as direct FOG accumulation in sewer pipelines, recent studies have demonstrated a strong compositional similarity between sewer FOG deposits and laboratory-prepared calcium-based fatty acid salts, indicating that a significant portion of FOG deposits are actually saponified calcium fatty acid salts.3 Four major components, including calcium, free fatty acids, FOG, and water, are important to the formation of FOG deposits on sewer pipe walls.4 Since the majority of free fatty acids in FOG deposits are long-chain fatty acids (LCFAs; C14 to C22),4–6 understanding the sources of LCFAs in sewer systems is important in controlling the formation of FOG deposits in sewer pipelines.Currently, the most commonly used approach to prevent the formation of FOG deposits is to install grease interceptors (GIs) between commercial kitchen wastewater discharge points and sewer pipelines in order to trap and remove FOG from the wastewater stream. Given the newly discovered role of LCFAs in the formation of sewer FOG deposits, it is important to understand how GIs affect LCFAs entering the sewer systems and specifically whether GIs are potential sources of LCFAs. High concentrations of LCFAs were previously reported in samples collected from within sewer GIs (e.g. up to 8–12%7). Although some of the LCFAs might originate from the upstream cooking processes,8 additional LCFAs may also be produced by microbial activities within the GIs. Many bacteria are capable of producing extracellular lipases that hydrolyze the ester bonds of FOG,9 and studies on wastewater treatment processes and contaminated soils have reported microbial production of LCFAs as intermediates of FOG biodegradation.10,11
Several aspects of GI installation and operation may affect the microbial production and biotransformation of LCFAs within the GIs as well as their eventual discharge into sewer systems. First of all, the sizing of GI for a given wastewater stream could result in different hydraulic retention times (HRTs), which in the field typically ranges from 0.3 to 4.4 days.12 Different HRTs may affect the quantity of LCFAs produced by microbial activities within GIs, as the incubation time often controls the production of bacterial lipases that catalyze oil hydrolysis.7,13 Different HRTs may also affect the LCFA composition in GIs, as faster biodegradation of unsaturated LCFAs than that of saturated LCFAs was previously observed in an activated sludge process.14 Similar observations were also made in sewer systems where a higher ratio of oleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to palmitic acid (C16
1) to palmitic acid (C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) was observed in the upstream than in the downstream.5 Furthermore, GIs are normally operated in a multi-layer form, including a FOG top layer, an aqueous middle layer, and a bottom sediment layer. Since effluent from the GIs is usually discharged from the middle aqueous layer, this stratified operational mode could help retain LCFAs within the GIs and affect the types of LCFAs exiting the GIs.
0) was observed in the upstream than in the downstream.5 Furthermore, GIs are normally operated in a multi-layer form, including a FOG top layer, an aqueous middle layer, and a bottom sediment layer. Since effluent from the GIs is usually discharged from the middle aqueous layer, this stratified operational mode could help retain LCFAs within the GIs and affect the types of LCFAs exiting the GIs.
Therefore, the aim of the study is to determine how microbial activities and hydraulic retention time (HRT) of GIs affect the production and profile of LCFAs. Laboratory GI reactors with and without microbial activities were operated in parallel and compared to determine the concentration and profile of LCFAs in the GI effluent. Different HRTs were used to determine the impact of HRT on LCFAs in the reactor effluent. The concentration and compositional profile of LCFAs retained within the GI reactors were also determined and compared with that in the effluent.
Materials and methods
Reactor setup
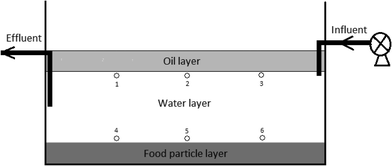
Bench scale GI reactors were constructed using high density polypropylene bottles and consisted of a top FOG layer, a middle water layer, a bottom sediment layer, an inlet pipe positioned right below the top oil layer, and an outlet pipe positioned in the middle of the water layer (Fig. 1). Influent artificial wastewater was delivered from an external reservoir using a peristaltic pump, while effluent from the reactor was pumped at the same flow rate as the influent to a glass bottle stored at 4 °C in the dark. The FOG, water, and sediment layers were 20%, 60% and 20% by volume, respectively. Canola oil was used to simulate wastewater FOG, cooked rice was used to simulate carbohydrate food residuals as the sediment, and the water layer was filled with artificial wastewater. The artificial wastewater contains, per liter of deionized water, 0.20 g of NH4Cl, 0.33 g of KCl, 0.32 g of MgCl2·6H2O, 0.30 g of NaCl, 0.15 g of CaCl2·2H2O, 0.04 M potassium phosphate buffer (pH = 7), 0.40 g of yeast extract, and 1 ml of trace element stock solution.13 All laboratory GI reactors were inoculated with one ml aliquots of a fully mixed FOG wastewater sample that was collected from a local wastewater treatment plant.For the experiment investigating LCFA production by microbial activities, three laboratory GI reactors with microbial activities (experiment) and three without microbial activities (control) were established. The three control GI reactors received the artificial wastewater amended with 2 g L−1 sodium azide in order to inhibit microbial activities. The total reactor volume for this experiment was 2 liters, and the flow rate was 0.5 L d−1, which resulted in a HRT of four days. The water layer within the reactors was maintained at a pH in the range of 6 to 7 using either sodium hydroxide or hydrochloric acid. For the experiment investigating the impact of HRT on LCFA production, a total of nine bench-scale GI reactors (one-liter volume) were established, and three different HRTs (0.5 day, 1.0 day, and 2.0 days) were tested. The pH in the water layer of these reactors was not adjusted in order to mimic the operational conditions in real sewer GIs.
Reactor operation and sample collection
All laboratory GI reactors in this study were operated with an intermittent flow pattern that involved pumping artificial wastewater through the reactors twice a day to simulate the two diurnal peak flows of real GIs.12 The two pumping events lasted for one hour each and were separated from each other by five hours. All reactors were operated for 60 days in the dark and at room temperature (23–25 °C). All experiments were conducted using triplicate reactors.Effluent samples (50 ml) were collected twice a week from completely mixed effluent collection bottles. For the experiment using different HRTs, in addition to the effluent samples, aqueous samples were also collected from multiple locations within the GI reactors after 60 days of operation, which was used to determine the concentration and compositional profiles of LCFAs within the reactors. This was done by positioning the inlet of the glass pipettes at sampling points 1 to 6 inside each GI reactor (Fig. 1) and withdrawing 10 ml of sample with minimum disturbance to the reactors. The samples were analyzed immediately for pH, COD, and microbial activities. Aliquots of the samples were stored at −20 °C for later LCFA analyses.
Chemical analyses
pH was monitored for all effluent samples using an UltraBASIC pH meter (Denver Instrument, Bohemia, New York). Chemical oxygen demand (COD) of the GI effluent samples was measured using the HACH COD test kit (HACH, Loveland, Colorado) following the reactor digestion method (HACH method 8000). The LCFAs in the GI samples were extracted using a mixture of chloroform and methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v),15 and the extracts were analyzed to determine the LCFA concentration and composition using a procedure previously described.16 Briefly, 8 ml of sample, 8 μl of 5 mg ml−1 tridecanoic acid as internal standard, 10 ml of methanol, and 10 ml of chloroform were added sequentially to a centrifuge tube, which was shaken vigorously for 2 min. Another 10 ml of chloroform was subsequently added, and the tube was shaken vigorously for 2 min. After mixing, the tube was centrifuged at 3000 × g for 30 min before 5 ml of the bottom chloroform layer was transferred into a screw capped glass tube and dried under a nitrogen stream. 1.5 ml of methanol and 0.3 ml of 8.0% HCl solution in methanol were added sequentially to the glass tube and then incubated at 45 °C for 16 hours. After cooling to room temperature, 1 ml of hexane and 1 ml of water were added and vortexed for 1 min to extract fatty acid methyl esters (FAMEs). After settling for 10 min, the top, hexane layer was transferred to a new tube containing anhydrous sodium sulfate to remove any residual water. Analysis for FAMEs was performed using a Hewlett Packard 5890 SERIES II Gas Chromatograph with a flame ionization detector (FID) using split injection. A capillary RT-2560 column (Restek, Bellefonte, PA) was used, and the temperature gradient was 100 °C with a 4 min hold time, which was increased at 3 °C per min to a final temperature of 240 °C.
1, v/v),15 and the extracts were analyzed to determine the LCFA concentration and composition using a procedure previously described.16 Briefly, 8 ml of sample, 8 μl of 5 mg ml−1 tridecanoic acid as internal standard, 10 ml of methanol, and 10 ml of chloroform were added sequentially to a centrifuge tube, which was shaken vigorously for 2 min. Another 10 ml of chloroform was subsequently added, and the tube was shaken vigorously for 2 min. After mixing, the tube was centrifuged at 3000 × g for 30 min before 5 ml of the bottom chloroform layer was transferred into a screw capped glass tube and dried under a nitrogen stream. 1.5 ml of methanol and 0.3 ml of 8.0% HCl solution in methanol were added sequentially to the glass tube and then incubated at 45 °C for 16 hours. After cooling to room temperature, 1 ml of hexane and 1 ml of water were added and vortexed for 1 min to extract fatty acid methyl esters (FAMEs). After settling for 10 min, the top, hexane layer was transferred to a new tube containing anhydrous sodium sulfate to remove any residual water. Analysis for FAMEs was performed using a Hewlett Packard 5890 SERIES II Gas Chromatograph with a flame ionization detector (FID) using split injection. A capillary RT-2560 column (Restek, Bellefonte, PA) was used, and the temperature gradient was 100 °C with a 4 min hold time, which was increased at 3 °C per min to a final temperature of 240 °C.
Microbial activity quantification
Microbial activities in the samples were quantified using a modified dehydrogenase activity (DHA) assay.17,18 Briefly, 15 ml of liquid sample was centrifuged to pellet the microbial biomass. The microbial biomass pellet was subsequently resuspended in 1 ml of TTC reagent (2 g L−1 2,3,5-triphenyltetrazolium chloride), 1 ml of glucose solution (0.18 g of glucose in 20 ml of (40 mM) phosphate buffer), and 3 ml of lysozyme solution (0.15 g of lysozyme in 1 liter (40 mM) of phosphate buffer). The mixture was vigorously vortexed and then incubated at 37 °C in the dark for one hour, during which the mixture was agitated by inverting the tubes every 10 min. After incubation, the mixture was centrifuged at 4000 × g for 12 min, and the supernatant was discarded, and then 5 ml of ethanol was added and vortexed to extract triphenylformazan. After being kept in the dark for 10 min, the mixture in the tube was centrifuged at 4000 × g for 3 min. Then, the supernatant was used for active biomass analysis by measuring the TF spectrophotometrically at 485 nm.Statistical analysis
Linear models were considered for each of the various physical and chemical parameters measured in the experiment (denoted generally by Yij). These models all take the form Yij = μ + τi + Eij, where μ is the overall mean value of the parameter, τi denotes the effects of the ith treatment (HRT), j is an index for the days in which the measurements were made, and Eij denotes the experimental error or variability not explained by the treatment effect. These errors were assumed to be normally distributed with constant variance. The models were fitted using the GLM procedure of the SAS statistical package (SAS, Cary, NC).Results
Microbial production of LCFAs
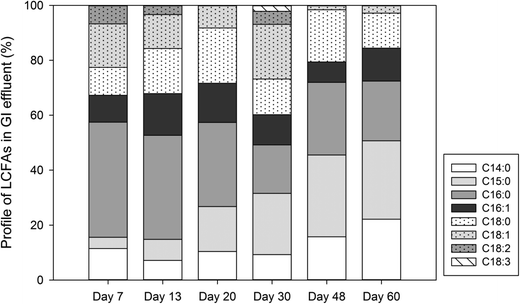
To determine the microbial production of LCFAs, the laboratory GI reactors with microbial activities (experiment) were operated in parallel with the reactors where microbial activities were inhibited (control). The experimental reactors exhibited a significantly higher microbial biomass than the control reactors (Fig. 2a). The experimental reactors showed a rapid increase in microbial activities within the first week of incubation, reaching a maximum DHA value of 12.9 mg TF per L on day 7. The DHA then subsided and fluctuated in the range of 1.2–4.7 mg TF per L from day 10 to day 60. In contrast, the control GI reactors consistently exhibited a DHA activity near zero (range: 0–1 mg TF per L). Corresponding to the microbial dynamics, the experimental GI reactors also discharged a significantly higher concentration of COD in the effluent than the control reactors did, and the COD concentration dynamics also followed that of microbial activities (Fig. 2b).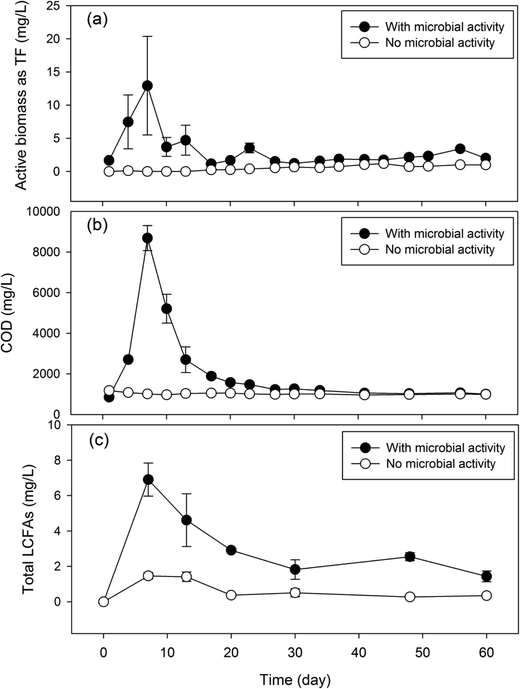 | ||
| Fig. 2 Concentration dynamics of microbial activities (a), COD (b) and total LCFAs (c) in laboratory GI reactors with or without microbial activities. | ||
The presence of microbial activities in the experimental GI reactors led to a significantly higher concentration of total LCFAs (Fig. 2c) than in the control reactors. The average total concentration of LCFAs in the effluent of the experimental GI reactors was 3.38 mg L−1, which is approximately 5 times higher than that in the control reactors. Corresponding to the peaking of microbial activities and COD on day 7, maximum total LCFAs (6.91 mg L−1) was also reached on day 7 in the experimental GI reactors, indicating microbial production of LCFAs. This is contrasted by the significantly lower total LCFAs (range: 0.27–1.41 mg L−1) in the control reactors, which remained near zero over the entire experimental course. The total LCFA concentration in the GI effluent showed a significant and positive correlation with microbial activities (Pearson's r = 0.81, P = 0.05) and a marginal and positive correlation with COD (Pearson's r = 0.79, P = 0.06). Although microbial cell membranes also contain LCFAs, their contributions to the total amount of LCFAs were deemed to be limited. The average concentration of total lipids from microbial cells was estimated to be 0.91 mg L−1 based on the average microbial activities estimated by the DHA assay,18 which accounts for up to 27% of total LCFAs detected in the GI effluent with microbial activities.
Impact of HRT on LCFA production
To determine the impact of HRT on microbial production of LCFAs, laboratory GI reactors were operated under three different HRTs (0.5 d, 1 d, and 2 d) for 60 days, and the concentration dynamics of microbial activities, COD, and total LCFAs in the reactor effluent were quantified over time (Fig. 3). No statistically significant difference (P ≥ 0.06) in microbial activities was observed in the reactors with different HRTs. Similarly, the difference in COD between the GI reactors with 1 d HRT and 2 d HRT (P = 0.13) as well as that between the GIs with 0.5 d HRT and 2 d HRT (P = 0.06) were not significant either, albeit a significant difference in effluent COD concentration was observed between reactors with 0.5 d HRT and 1 d HRT (P = 0.001). The average concentrations of total LCFAs in the GI effluent over the 60-day operational period were 1.71 mg L−1, 2.08 mg L−1 and 2.45 mg L−1 for the reactors with a HRT of 0.5 d, 1 d and 2 d, respectively (Table 1), indicating that a longer HRT resulted in higher concentration of total LCFAs in the reactor effluent. Although the difference in total concentration of LCFAs between 0.5 d HRT and 1 d HRT was not statistically significant (P = 0.18), a significant difference was found between 0.5 d HRT and 2 d HRT (P = 0.003) as well as between 1 d HRT and 2 d HRT (P = 0.03).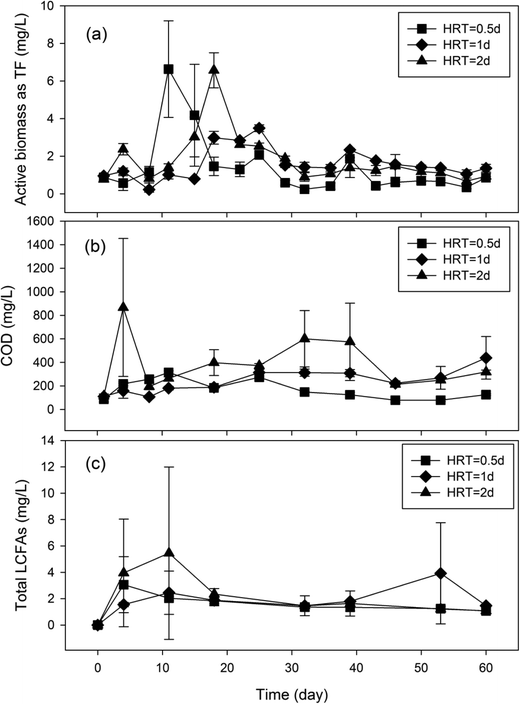 | ||
| Fig. 3 Concentration dynamics of microbial activities (a), COD (b) and total LCFAs (c) in laboratory GI reactors with different HRTs. | ||
| HRT | In GI effluent (mg L−1) | Within GI reactors (mg L−1) | |
|---|---|---|---|
| Top water layer | Bottom water layer | ||
| 0.5 d | 1.71 ± 0.69 | 79.5 ± 42.1 | 106.9 ± 12.5 |
| 1 d | 2.08 ± 0.88 | 180.3 ± 115.0 | 410.6 ± 113.6 |
| 2 d | 2.45 ± 1.65 | 227.2 ± 97.3 | 225.7 ± 29.5 |
The different impacts of HRT on microbial activities, COD and total LCFAs in the reactor effluent were likely caused by the stratified operational conditions and the lack of mixing within the GI reactors. To overcome this limitation of effluent sampling, additional samples were also collected from within the GI reactors to quantify the total LCFAs retained within the reactors on day 60. Compared to the average concentration of LCFAs in GI effluent discharged from the middle of the water layer, a much higher concentration of LCFAs was detected at the top and bottom of the water layer within all GI reactors (Table 1). For the top of the water layer, the average concentrations of total retained LCFAs are around 46, 87 and 93 times higher than the average concentrations of total LCFAs in the GI effluent under HRTs of 0.5 d, 1 d and 2 d, respectively. Similarly, the concentrations of the retained LCFAs at the bottom of the water layer were 63, 198 and 93 times higher than the average concentrations of LCFAs in the GI effluent under HRTs of 0.5 d, 1 d and 2 d, respectively. This indicates that the majority of the microbiologically produced LCFAs were retained within the GI reactors.
LCFA profile
Since different LCFAs were detected in real sewer FOG deposits5 and different LCFAs can exhibit different biodegradation patterns,13 the LCFA profiles in the laboratory GI reactor effluent and in the aqueous layer within the GI reactors were analyzed and compared. The effluent samples from the GI reactors exhibited a total of eight different LCFAs (C14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C15
0, C15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C16
0, C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C16
0, C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C18
1, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C18
0, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C18
1, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and C18
2 and C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (Fig. 4), six of which, including C14
3) (Fig. 4), six of which, including C14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C16
0, C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C18
0, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C18
0, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C18
1, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and C18
2 and C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, were previously reported in real sewer FOG deposit samples.5 The primary saturated and poly-unsaturated LCFAs in the GI reactor effluent were C16
3, were previously reported in real sewer FOG deposit samples.5 The primary saturated and poly-unsaturated LCFAs in the GI reactor effluent were C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (29.4 ± 9.3%) and C18
0 (29.4 ± 9.3%) and C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (2.5 ± 2.9%), respectively, which were also the primary saturated and poly-unsaturated LCFAs detected in real sewer FOG deposits.6 A relatively high percentage of C18
2 (2.5 ± 2.9%), respectively, which were also the primary saturated and poly-unsaturated LCFAs detected in real sewer FOG deposits.6 A relatively high percentage of C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was the primary mono-unsaturated LCFA detected in real sewer FOG deposits, was also observed in the GI effluent (10.1 ± 7.3%), which was only slightly lower than that of the primary mono-unsaturated LCFA (C16
1, which was the primary mono-unsaturated LCFA detected in real sewer FOG deposits, was also observed in the GI effluent (10.1 ± 7.3%), which was only slightly lower than that of the primary mono-unsaturated LCFA (C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with 11.6 ± 2.9%) in the GI effluent.
1 with 11.6 ± 2.9%) in the GI effluent.
Similar LCFA composition profiles were also observed in the effluent samples of GI reactors under different HRTs (Fig. S1†). No significant difference in LCFA composition was observed amongst the different HRTs, which corresponds well to the similar observation in the concentration of total LCFAs in the effluent samples (Fig. 3). All the samples collected from within the GI reactors showed similar LCFA profiles to each other regardless of HRT; however, the LCFA profiles in the samples collected from within the GI reactors showed a considerable difference than the corresponding effluent samples (Fig. 5). For example, in the GI reactors with a HRT of 2 days (Fig. 5, Eff-2d), the three most dominant LCFAs in the effluent samples were C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C18
0, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, and C16
0, and C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and their average percentages were 27.4 ± 7.8%, 20.8 ± 11.9%, and 16.6 ± 8.8%, respectively. In contrast, the three most dominant LCFAs at the top of the water layer within the reactors were C18
1, and their average percentages were 27.4 ± 7.8%, 20.8 ± 11.9%, and 16.6 ± 8.8%, respectively. In contrast, the three most dominant LCFAs at the top of the water layer within the reactors were C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C18
1, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and C18
2, and C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 5, T-2d), accounting for 63.2 ± 0.2%, 18.8 ± 0.6%, and 6.9 ± 0.3% of the total LCFAs, respectively. Similarly, the total amount of these three LCFAs at the bottom of the water layer (Fig. 5, B-2d) was as high as 80.7%. Additionally, five LCFAs, including C12
3 (Fig. 5, T-2d), accounting for 63.2 ± 0.2%, 18.8 ± 0.6%, and 6.9 ± 0.3% of the total LCFAs, respectively. Similarly, the total amount of these three LCFAs at the bottom of the water layer (Fig. 5, B-2d) was as high as 80.7%. Additionally, five LCFAs, including C12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C17
0, C17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C20
0, C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C20
0, C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and C22
1 and C22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, were detected only in the samples collected from within the GI reactors but absent in the GI effluent samples. Similar differences were also observed in the GI reactors with either a 0.5 d HRT or a 1 d HRT (Fig. 5).
0, were detected only in the samples collected from within the GI reactors but absent in the GI effluent samples. Similar differences were also observed in the GI reactors with either a 0.5 d HRT or a 1 d HRT (Fig. 5).
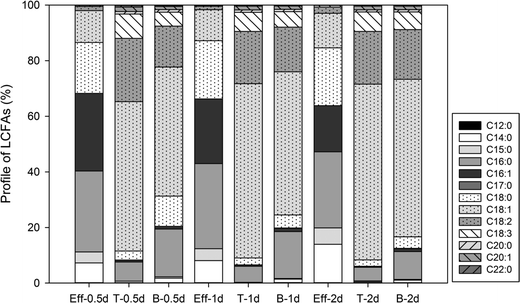 | ||
| Fig. 5 Profiles of LCFAs in samples in the GI effluent (Eff) and samples from the top (T) and bottom (B) of the water layers within GIs under different HRTs (0.5 d, 1 d, and 2 d). | ||
Discussion
Microbial production of LCFAs in the laboratory GI reactors was clearly demonstrated in this study by comparing experimental GI reactors with microbial activities to the control reactors without microbial activities (Fig. 2). The experimental GI reactors showed four times higher amount of LCFAs in the reactor effluent than the control reactors, supporting microbial activities as the primary cause of LCFA production inside the reactors. The association between microbial activities and LCFA production was also supported by the significantly positive correlation between microbial activities and total LCFAs in the GI reactor effluent. The microbial activities peaked on day 7, corresponding to the peaking of total LCFA concentration on the same day, while a subsequent decrease in microbial activities also concurred with the decrease in the total LCFA concentration in the reactor effluent, which further supports microbial production of LCFAs within the GI reactors.The decrease in microbial activities after day 7 was probably caused by the inhibitory effects of LCFAs on microorganisms. LCFAs can adhere to the outer surface of microbial cells, creating a physical barrier that disrupts various cellular functions, including nutrient transport and certain enzyme activities.19–22 It is possible that the microbiologically produced LCFAs accumulated within the GI reactors and exerted inhibitory effects on the microorganisms, leading to the decrease in microbial activities, which was manifested in the reactor effluent. In the control GI reactors, the small spike of LCFAs detected in the GI effluent at the beginning of the experiment may be attributed to limited FOG hydrolysis catalyzed by the residual lipase from the carbohydrate food residuals. A similar phenomenon was found in real sewer GIs where FOG underwent hydrolysis in the presence of high moisture content and lipase from food particles to form glycerol and LCFAs.7
The majority of LCFAs detected in the laboratory GI reactor effluent (Fig. 4) are the same as the LCFAs commonly detected in real sewer FOG deposits, indicating that GIs can be a source of LCFAs contributing to the formation of FOG deposits in sewer systems. Since unsaturated LCFAs was barely detected in the effluent from control GIs (data not shown), the unsaturated LCFAs in the experimental GI effluent should be attributed primarily to microbial activities in the GIs. Unsaturated LCFAs may be more detrimental to downstream sewer pipelines because the calcium salts of unsaturated LCFAs (such as palmitic acid) are more adhesive FOG deposits than those formed by saturated fatty acids.4 For the unsaturated fatty acids (such as C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and C18
1 and C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with increasing number of double bonds, stickier FOG deposits may be formed leading to more corrosion on concrete pipes.4 Therefore, microbial activities in the GIs not only can produce more LCFAs but also contribute to the higher proportion of unsaturated fatty acids in the GI effluent that tend to form more adhesive and corrosive FOG deposits in downstream sewer lines.
2) with increasing number of double bonds, stickier FOG deposits may be formed leading to more corrosion on concrete pipes.4 Therefore, microbial activities in the GIs not only can produce more LCFAs but also contribute to the higher proportion of unsaturated fatty acids in the GI effluent that tend to form more adhesive and corrosive FOG deposits in downstream sewer lines.
Since HRT is an important controlling parameter in completely-mixed bioreactors, it was interesting to observe that HRT did not exert a consistent impact on microbial activities, COD, and LCFAs (Fig. 3) or the composition profile of total LCFAs (Fig. S1†) in the GI reactor effluent. This could be attributed primarily to the fact that the GI reactors were stratified into three layers from top to bottom (FOG, water and solids), while the microbial activities responsible for LCFA production are expected to be concentrated near the interface between FOG and water, which consequently alleviated the impact of HRT. Although the concentration of the retained LCFAs at the top of the water layer increased with longer HRT, the concentration of LCFAs at the bottom within GIs with 2 d HRT was much lower than that in GIs with 1 d HRT. Since the inhibitory effects of LCFA on microorganisms are believed to be concentration dependent,13,23,24 the production of LCFAs in the GI reactors with 2 d HRT may be inhibited due to the higher concentration of LCFAs at the top of the water layer, leading to the lower amount of produced LCFAs than that in GI with 1 d HRT to form less calcium salts of LCFAs retained at the bottom of the water layer.
The detection of drastically higher concentrations of total LCFAs in the samples collected from within the GI reactors than in the effluent samples (Table 1) corresponds well to the stratified operation mode of the GI reactors and suggests possible stratified distribution of microbiologically produced LCFAs within the GI reactors. The solubility of LCFAs in water is very low,25 which is in agreement with the observation that a low concentration of LCFAs was observed in the GI effluent while a much higher concentration of LCFAs was retained within the GI reactors (Table 1). LCFAs are expected to accumulate in two areas within the GI reactors, including the top of the water layer (as LCFAs are less dense than water) and the bottom of the water layer (due to the precipitation of calcium salts of LCFAs). Albeit only a very small portion of the LCFAs was discharged in the effluent in this study, the large amount of LCFAs retained within the GI reactors represent a significant LCFA reservoir that could be released into the sewer systems under different hydraulic conditions (such as disturbances). During the operation of these laboratory GI reactors, two highly viscous samples that were collected on day 53 in a GI reactor with a 1 d HRT and on day 39 in a GI reactor with a 2 d HRT exhibited a drastic increase in total LCFA concentration and different LCFA composition profiles than the other samples (Fig. 3c and S1†), which was probably caused by disturbance during sampling. Other environmental conditions encountered in real sewer GIs, such as the presence of kitchen surfactants, may also allow the discharge of the LCFAs from GIs into the sewer system.
The significantly different LCFA profiles between the GI effluent samples and the samples collected from within the GI reactors (Fig. 5) can be explained by the different physicochemical properties and biodegradability of the LCFAs. Typically, the water solubility of LCFAs decreases with increasing carbon chain length and degree of branching,25 which is in agreement with the observation that the majority of LCFAs in the GI effluent were fatty acids with a carbon length equal to or less than 18. In contrast, LCFAs with a carbon chain length greater than 18 (such as C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C20
0, C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and C22
1 and C22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) were only detected in samples collected from within the GI reactors. The other potential cause could be the biodegradation of unsaturated LCFAs (such as C18
0) were only detected in samples collected from within the GI reactors. The other potential cause could be the biodegradation of unsaturated LCFAs (such as C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to short chain saturated fatty acids. Previous studies found that palmitic acid (C16
1) to short chain saturated fatty acids. Previous studies found that palmitic acid (C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) was the main intermediate and the key inhibitory species during the anaerobic degradation of C18
0) was the main intermediate and the key inhibitory species during the anaerobic degradation of C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via β-oxidation,26,27 which is consistent with our observation that palmitic acid was the primary LCFA in the GI effluent (Fig. 4 and 5).
1 via β-oxidation,26,27 which is consistent with our observation that palmitic acid was the primary LCFA in the GI effluent (Fig. 4 and 5).
The LCFA composition profile in the samples collected from the top/bottom of the water layer within the GI reactors (Fig. 5) is highly similar to the LCFA composition of the FOG used in the reactors, indicating that the retained LCFAs were derived from FOG within the GI reactors. For example, the samples collected at the top of the GI reactors with a 2 d HRT contained 4.9% of C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 2.3% of C18
0, 2.3% of C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 63.2% of C18
0, 63.2% of C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 18.9% of C18
1, 18.9% of C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 7.0% of C18
2 and 7.0% of C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, while canola oil consists of 3.49% of C16
3, while canola oil consists of 3.49% of C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 0.85% of C18
0, 0.85% of C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 64.4% of C18
0, 64.4% of C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 22.3% of C18
1, 22.3% of C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 8.23% of C18
2 and 8.23% of C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.7 Besides, the composition profile of the retained LCFAs also indicates that LCFAs from microbial cell membranes are negligible because some of the dominant LCFAs in the cell membrane (such as C16
3.7 Besides, the composition profile of the retained LCFAs also indicates that LCFAs from microbial cell membranes are negligible because some of the dominant LCFAs in the cell membrane (such as C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and C18
0 and C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 028) were observed to be few inside GIs, while the dominant polyunsaturated LCFAs in GIs (such as C18
028) were observed to be few inside GIs, while the dominant polyunsaturated LCFAs in GIs (such as C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and C18
2 and C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) are barely observed in the cell membrane.28,29
3) are barely observed in the cell membrane.28,29
Regarding the formation of FOG deposits in the sewer systems, the disturbance of the LCFA layer within GI can be more detrimental to the downstream sewer pipeline as a higher concentration of unsaturated fatty acids (such as C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C18
1, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and C18
2 and C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) would be discharged. Since the depth of FOG and the food particle layer increased significantly during the operation of real GIs,30 the water layer in the middle of GI would be gradually reduced in volume. Thus, the layers of LCFAs and calcium salts of LCFAs are more likely to be disturbed by influent flow at the end of the operational days, especially for GIs with longer HRT where a higher concentration of unsaturated fatty acids was observed in this study. Therefore, the pump out frequency for GIs with longer HRT may be increased to avoid the occurrence of this scenario. Nevertheless, as a spike of LCFAs discharged from GI was observed at the beginning of the operational period (within the first 20 days) for all GIs under all HRTs, an increasing frequency of pump out service would result in increasing spikes of LCFAs discharged. Thus, a further study on the optimization of the pump out frequency for GIs may be helpful.
3) would be discharged. Since the depth of FOG and the food particle layer increased significantly during the operation of real GIs,30 the water layer in the middle of GI would be gradually reduced in volume. Thus, the layers of LCFAs and calcium salts of LCFAs are more likely to be disturbed by influent flow at the end of the operational days, especially for GIs with longer HRT where a higher concentration of unsaturated fatty acids was observed in this study. Therefore, the pump out frequency for GIs with longer HRT may be increased to avoid the occurrence of this scenario. Nevertheless, as a spike of LCFAs discharged from GI was observed at the beginning of the operational period (within the first 20 days) for all GIs under all HRTs, an increasing frequency of pump out service would result in increasing spikes of LCFAs discharged. Thus, a further study on the optimization of the pump out frequency for GIs may be helpful.
Conclusions
Microbial activities in GIs can lead to the production of LCFAs, as illustrated by the significantly higher concentration of LCFAs in the effluent of the experimental GI reactors than the control reactors, which was also supported by the significantly positive correlation between microbial activities and the concentration of LCFAs in the GI effluent. HRT did not exhibit a consistent impact on microbial activities, COD, and total LCFA concentrations in the GI effluent, which was likely caused by the stratified operational mode of the GI reactors. High similarity of LCFA profile was observed between the effluent from the laboratory GI reactors and real FOG deposits. Significantly higher levels of LCFAs were accumulated at the top and bottom of the water layer within the GI reactors than in the effluent. The LCFAs retained within the GI reactors showed a different profile than that in the effluent, containing more unsaturated LCFAs. In summary, GIs can impact the quantity and profile of LCFAs, and this should be taken into consideration in the GI design and operation in order to reduce the formation of FOG deposits in sewer systems.Acknowledgements
We would like to thank the United States Environmental Protection Agency (USEPA grant R834871) for funding this research. The contents of this research are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.References
- J. J. Ducoste, K. M. Keener, J. W. Groninger and L. M. Holt, Fats, Roots, Oils and Grease (FROG) in Centralized and Decentralized Systems, Water Environment Research Foundation, London, 2008 Search PubMed.
- D. R. Marlow, F. Boulaire, D. J. Beale, C. Grundy and M. Moglia, J. Infrastruct. Syst., 2011, 17, 42–51 CrossRef.
- X. He, M. Iasmin, L. O. Dean, S. E. Lappi, J. J. Ducoste and F. L. de los Reyes, 3rd, Environ. Sci. Technol., 2011, 45, 4385–4391 CrossRef CAS PubMed.
- X. He, F. L. De los Reyes, M. L. Leming, L. O. Dean, S. E. Lappi and J. J. Ducoste, Water Res., 2013, 47, 4451–4459 CrossRef CAS PubMed.
- J. B. Williams, C. Clarkson, C. Mant, A. Drinkwater and E. May, Water Res., 2012, 46, 6319–6328 CrossRef CAS PubMed.
- K. M. Keener, J. J. Ducoste and L. M. Holt, Water Environ. Res., 2008, 80, 2241–2246 CrossRef CAS PubMed.
- M. J. Montefrio, X. W. Tai and J. P. Obbard, Appl. Energy, 2010, 87, 3155–3161 CrossRef CAS.
- M. Canakci, Bioresour. Technol., 2007, 98, 183–190 CrossRef CAS PubMed.
- R. Gupta, N. Gupta and P. Rathi, Appl. Microbiol. Biotechnol., 2004, 64, 763–781 CrossRef CAS PubMed.
- M. H. El-Masry, E. El-Bestawy and N. I. El-Adl, World J. Microbiol. Biotechnol., 2004, 20, 551–557 CrossRef CAS.
- H. K. Shon, D. Tian, D.-Y. Kwon, C.-S. Jin, T.-J. Lee and W.-J. Chung, J. Microbiol. Biotechnol., 2002, 12, 583–591 CAS.
- T. N. Aziz, L. M. Holt, K. M. Keener, J. W. Groninger and J. J. Ducoste, Water Environ. Res., 2012, 84, 237–246 CrossRef CAS PubMed.
- X. He, Q. Zhang, M. J. Cooney and T. Yan, Appl. Microbiol. Biotechnol., 2015, 99, 6059–6068 CrossRef CAS PubMed.
- J. T. Novak and D. L. Kraus, Water Res., 1973, 7, 843–851 CrossRef CAS.
- T. Seppanen-Laakso, I. Laakso and R. Hiltunen, Anal. Chim. Acta, 2002, 465, 39–62 CrossRef CAS.
- K. Ichihara and Y. Fukubayashi, J. Lipid Res., 2010, 51, 635–640 CrossRef CAS PubMed.
- L. Beyer, C. Wachendorf, D. Elsner and R. Knabe, Biol. Fertil. Soils, 1993, 16, 52–56 CrossRef CAS.
- S. M. Lee, J. Y. Jung and Y. C. Chung, Biotechnol. Lett., 2000, 22, 991–994 CrossRef CAS.
- M. M. Alves, M. A. Pereira, D. Z. Sousa, A. J. Cavaleiro, M. Picavet, H. Smidt and A. J. M. Stams, Microb. Biotechnol., 2009, 2, 538–550 CrossRef CAS PubMed.
- M. A. Pereira, O. C. Pires, M. Mota and M. M. Alves, Biotechnol. Bioeng., 2005, 92, 15–23 CrossRef CAS PubMed.
- D. A. Salam, N. Naik, M. T. Suidan and A. D. Venosa, Environ. Sci. Technol., 2012, 46, 2352–2359 CrossRef CAS PubMed.
- A. P. Desbois and V. J. Smith, Appl. Microbiol. Biotechnol., 2010, 85, 1629–1642 CrossRef CAS PubMed.
- J. Palatsi, R. Affes, B. Fernandez, M. A. Pereira, M. M. Alves and X. Flotats, Water Res., 2012, 46, 5268–5278 CrossRef CAS PubMed.
- A. Rinzema, M. Boone, K. Vanknippenberg and G. Lettinga, Water Environ. Res., 1994, 66, 40–49 CrossRef CAS.
- K. Bober and M. Garus, J. Liq. Chromatogr. Relat. Technol., 2006, 29, 2787–2794 CrossRef CAS.
- M. A. Pereira, O. C. Pires, M. Mota and M. M. Alves, Water Sci. Technol., 2002, 45, 139–144 CAS.
- D. Z. Sousa, M. A. Pereira, A. J. M. Stams, M. M. Alves and H. Smidt, Appl. Environ. Microbiol., 2007, 73, 1054–1064 CrossRef CAS PubMed.
- K. Y. Cho and M. R. Salton, Biochim. Biophys. Acta, 1966, 116, 73–79 CrossRef CAS.
- E. A. Bishop and M. A. C. Bermingham, Antimicrob. Agents Chemother., 1973, 4, 378–379 CrossRef CAS PubMed.
- X. He, J. Osborne and F. L. de los Reyes, Water Environ. Res., 2012, 84, 195–201 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ew00013d |
| This journal is © The Royal Society of Chemistry 2016 |