Inactivation of bacteria from contaminated streams in Limpopo, South Africa by silver- or copper-nanoparticle paper filters
Theresa A.
Dankovich†
*abc,
Jonathan S.
Levine
c,
Natasha
Potgieter
d,
Rebecca
Dillingham
b and
James A.
Smith
a
aDepartment of Civil and Environmental Engineering, University of Virginia, Thornton Hall, P.O. Box 400742, Charlottesville, VA 22904, USA. E-mail: dankovich@cmu.edu; Tel: (315) 559 2135
bThe Center for Global Health, Carter-Harrison Research Building, MR-6, Room 2526, 345 Crispell Drive, P.O. Box 801379, University of Virginia Health System Charlottesville, VA 22908-1379, USA
cPage Drinking Paper, Pittsburgh, PA 15221, USA
dDepartment of Microbiology, University of Venda, Thohoyondou, South Africa
First published on 30th September 2015
Abstract
There is an urgent need for inexpensive point-of-use methods to purify drinking water in developing countries to reduce the incidence of illnesses caused by waterborne pathogens. Previously, our work showed the deactivation of laboratory-cultured bacteria by percolation through a thick paper sheet containing either silver (Ag) or copper (Cu) nanoparticles (NP). In this study, these paper filters containing AgNPs or CuNPs have been tested with water sourced from contaminated streams in Limpopo, South Africa. Following the percolation of the contaminated stream water through the metal nanoparticle (MNP) papers, the water quality of the filtered effluent was evaluated with respect to the colony counts of total coliform and E. coli bacteria, turbidity, and either silver or copper ions. Influent total coliform bacteria concentrations from the stream water in Limpopo ranged from 250 CFU per 100 mL to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750
750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CFU per 100 mL. With the less contaminated stream water (250–15
000 CFU per 100 mL. With the less contaminated stream water (250–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CFU per 100 mL), both AgNP and CuNP papers showed complete inactivation of the coliform bacteria. With the surface water with higher coliform bacteria levels (500
000 CFU per 100 mL), both AgNP and CuNP papers showed complete inactivation of the coliform bacteria. With the surface water with higher coliform bacteria levels (500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–1
000–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CFU per 100 mL), both the AgNP and CuNP papers showed similar results with a slightly higher bacteria reduction of log10
000 CFU per 100 mL), both the AgNP and CuNP papers showed similar results with a slightly higher bacteria reduction of log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.1 for the AgNP papers than the log10
5.1 for the AgNP papers than the log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.8 reduction for the CuNP papers. E. coli results followed similar trends. For most water purification experiments, the metal release from the sheets was minimal, with values under 0.1 ppm for Ag and 1.0 ppm for Cu (the current US EPA and WHO drinking water limits for Ag and Cu, respectively). These results show good potential for the use of paper embedded with silver and/or copper nanoparticles as effective point-of-use water purifiers.
4.8 reduction for the CuNP papers. E. coli results followed similar trends. For most water purification experiments, the metal release from the sheets was minimal, with values under 0.1 ppm for Ag and 1.0 ppm for Cu (the current US EPA and WHO drinking water limits for Ag and Cu, respectively). These results show good potential for the use of paper embedded with silver and/or copper nanoparticles as effective point-of-use water purifiers.
Water impactMicrobial-contaminated water causes the spread of preventable water-borne diseases, such as giardiasis, cholera, cryptosporidiosis, etc. For the first time in the field, we demonstrate a novel and affordable technology: nano-enabled paper filters effectively inactivated coliform bacteria from contaminated water sources in Limpopo, South Africa. This technology has great potential for purifying drinking water in the developing world. |
Introduction
The lack of access to clean, potable water sources has contributed to the spread of preventable water-borne diseases by microbial contaminants, such as giardiasis, cholera, cryptosporidiosis, viral gastroenteritis, etc.1 Point-of-use (POU) water treatment produces clean drinking water by eliminating pathogenic microbial pollutants through filtration and/or chemical disinfection. POU systems are typically used in the household for improving water quality. Antimicrobial nanotechnology, e.g. silver and copper nanoparticles, are commonly used as the sole disinfectant agent in POU water treatment.2–5 A novel and affordable technology for eliminating bacteria from contaminated water is a thick filter paper embedded with silver or copper nanoparticles.2,6,7Silver and copper nanoparticles are well known to be broad spectrum, potent antimicrobial agents8–10 and are easily incorporated into cellulosic materials.11,12 Our previous work demonstrated that thick paper sheets embedded with silver or copper nanoparticles are bactericidal by passing model bacterial suspensions through a MNP paper sheet, and analyzing the effluent water for viable bacteria.2,6,7 These MNP paper sheets are used in a manner similar to a filter paper ,and, for convenience's sake, they are referred to as “filters”. However, their primary function is not sieving. Rather the paper ensures that the bacteria come into contact with silver or copper ions released from the nanoparticles on the paper fiber surfaces. As the bacteria absorb the lethal silver or copper ions from the nanoparticles, the bacteria cells are inactivated and dead bacteria are passed through the “filter” into the effluent.2 However, the MNP papers do act to filter out larger dirt particles and protozoan organisms based on size exclusion, as the smallest particle retention size of these filters is typically 3 to 10 microns.
Both the AgNP and CuNP paper filters have showed successful reduction of E. coli by 9 log10 under ideal laboratory conditions,2,6,7 but overall bactericidal performance under field conditions has not previously been presented. The primary objective of this paper is to examine the effectiveness of bacterial inactivation by papers containing AgNPs or CuNPs for bacteria from surface water sources in the largely rural province of Limpopo, South Africa. Past research from this field site has indicated a high risk of coliform bacteria contamination in surface water sources and an ineffective water supply and distribution system.13 Due to the poor reliability of the municipal water supply system, community members often use surface water, i.e. local streams, as their primary source for drinking water; furthermore, recontamination of purified water often occurs due to lengthy storage times.13,14 In this study, we examined the antibacterial performance of AgNP and CuNP paper sheets with two different surface water sources, which contained differing levels of total coliform and E. coli bacteria. The surface water samples were passed through the AgNP and CuNP paper sheets in a simple gravity-flow percolation experiment, which has been described previously.2,6,7 In this work, for the first time, the bactericidal performance of AgNP and CuNP sheets is evaluated with natural water sources with substantial bacterial contamination.
Although previous proof-of-concept experiments demonstrated high levels of bacterial inactivation with these metal nanoparticle papers, the transition from the lab bench to a field trial is not a trivial step. Many aspects of natural water sources can reduce the effectiveness of water filters and chemical disinfectants, e.g. turbidity, dissolved organic matter, various ions, etc. Researchers have demonstrated changes in silver ion release and chemical transformations with models of silver nanoparticles in harsh environmental exposures, and some chemical species, such as chlorides and carbonates, enhance the silver ion release, while others such as sulfides, inhibit the release.15,16 Typically, the levels of these ions are fairly low in natural water sources; however, the long-term effectiveness of filters containing silver nanoparticles may be impacted. Another important consideration for this study is variation of the paper void space, i.e., porosity, to achieve an acceptable flow rate with highly turbid waters. To evaluate for this, our study used four different papers with varying thicknesses and porosities. The same filter holder design was used for all experiments allowing for direct comparison of the flow rates and thus effective permeabilities of the filter papers.
This study aims to evaluate the effectiveness of the AgNP and CuNP paper filter materials with more complex and challenging field-sampled waters relative to previous laboratory experiments that used well-controlled synthesized dirty water.6 Therefore, for consistency, the filter holder used in previous laboratory research studies was used for filter paper testing. This study does not aim to evaluate the effectiveness of the filter holder system, which requires additional design work incorporating human centered design principles and evaluated through user feedback and more complex user testing. This study represents some of the early challenges in moving an inexpensive and appropriate technology for providing clean drinking water for developing countries out of the research laboratory and into the field for practical use.
Materials and methods
Materials
Three types of absorbent blotting papers made from bleached softwood kraft pulp (made by Domtar Inc. and kindly supplied by FP Innovations, Pointe-Claire, QC), high viscosity cellulose ether pulp, and cotton linter pulp, grade 512 (supplied by GP Cellulose, Memphis, TN) were used. The sheets are free from sizing agents, fluorescent agents and chemical additives. The sheets were chosen due to their differences in the type of cellulose, thickness, and basis weight (grammage), which are reported in Table 1. Silver nitrate (AgNO3), β-D-glucose, copper sulfate (CuSO4), 30% hydrogen peroxide (H2O2), concentrated nitric acid (HNO3), sodium hydroxide (NaOH), ascorbic acid, and M-coliblue culture media were purchased from Fisher Scientific and used as received. For the filter paper preparation and atomic absorption analyses, ultra pure water (18.2 MΩ cm) treated with a Barnstead Nanopure system was used. For all other analyses, water purified by reverse osmosis, UV, and ozonation was obtained from Oasis Water, South Africa17 and sterilized in an autoclave at 121 °C for 15 minutes prior to use.| Paper label | Metal type | Metal content in paper (mg g−1) | Paper thickness (mm) | Grammagea (g m−2) | Porosity (%) | Permeability (m2) | Filtration time for 1 literb (min) | Liters per hour |
|---|---|---|---|---|---|---|---|---|
| a As indicated by the supplier. b Filtration times are for 1 L of water passing through a 4.7 cm × 4.7 cm filter paper with the indicated thickness. Filter head height was 9 cm. | ||||||||
| Thin | — | — | 0.472 | 250 | 65% | 1.5 × 10−14 | 270 | 0.2 |
| Thick | — | — | 1.462 | 732 | 67% | 3.5 × 10−13 | 36 | 1.7 |
| Thin | Ag | 3.21 | 0.473 | 250 | 65% | 7.4 × 10−14 | 55 | 1.1 |
| Thin | Cu | 64.9 | 0.625 | 250 | 73% | 2.0 × 10−13 | 27 | 2.2 |
| Medium | Ag | 1.39 | 1.139 | 569.5 | 67% | 4.5 × 10−13 | 22 | 2.8 |
| Thick | Ag | 1.89 | 1.667 | 732 | 71% | 9.7 × 10−13 | 15 | 4.1 |
Filter paper preparation
Filter papers containing either silver nanoparticles (AgNP) or copper nanoparticles (CuNP) were prepared in the laboratory at the University of Virginia. As previously described, the AgNP papers were prepared by an in situ reduction of silver nitrate using glucose in an oven at 70 °C.6 Similarly, the CuNP papers were prepared by paper saturation with a copper hydroxide solution followed by an in situ reduction of the copper ions absorbed into fibers using a hot bath (85 °C) of ascorbic acid.7The metal content of the NP papers was determined via Flame Atomic Absorption (Perkin Elmer, AAnalyst 200) following an acid digestion of the papers and is reported in Table 1. The acid digestion method is as follows: ~0.05 grams of MNP paper was added to 2 mL concentrated sulfuric acid (CuNP papers) or 2 mL concentrated nitric acid (AgNP papers) and heated in a sand bath to between 50 °C and 60 °C and was followed by the addition of 2 mL 30% hydrogen peroxide. The metal content is reported for four replicates per sample concentration with standard error reported.
Paper thickness measurements were completed using a micrometer caliper. Grammage was reported by the supplier. Filter paper porosities were determined indirectly from the dry paper volume, calculated from the measured area and thickness, and the density of cellulose (1.52 g cm−3).18 This method is only approximate, as the apparent thickness measurements using a caliper can be inaccurate, potentially overestimating the thickness by 10–20% as compared to the average surface profile of the paper.19 However, these approximations are held constant for the various papers in this study, and can provide some insight into how the nanoparticle synthesis steps and the filtration processes affect the porosity of the papers. That is, the measurement process errors should be identical between all samples and thus while absolute error is on the order of 10–20%, relative changes should have minimal errors.
Field study setting
In South Africa, 95% of the population is reported to have access to potable drinking water as of 2012. In the last twenty years, the drinking water quality has greatly improved in South Africa, with 6.9 million people without access in 1990 declining to 2.6 million people in 2012. Despite major gains in access to potable water in South Africa, 12% of rural residents do not have reliable access to potable water.20 Limpopo province, the setting for this field study, is the most rural province in South Africa (90% of its people live in rural areas), and is one of the most affected by the lack of access to potable water in South Africa.21 Rates of diarrhea cases are 1.7 times higher in Limpopo province than the national average.22Water samples were collected from two streams in the Luvuvhu stream catchment in the Venda region of Limpopo province, South Africa. Land use in this region is both agricultural and residential. The first water source was a cement-lined irrigation canal diverted from the Tshala stream in a rural village, designated as “rural”. Community members use this irrigation canal as a primary source for drinking water.13,14 The second water source was a wastewater-impacted stream near the University of Venda campus in Thohoyondou, which ultimately flows into the Nandoni Dam Reservoir, the main municipal source for drinking water in the Thohoyandou area. This stream was polluted with raw sewage from a nursery school upstream of our sample collection point,18 and is designated as “urban”. The data from the urban water source is subdivided into “urban high” and “urban medium” due to drop in the coliform bacteria count in the stream of a factor of about 160 (2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10) in the middle of the field sampling study. This drop was due to an abrupt change in the level of raw sewage discharged into the stream.23
log10) in the middle of the field sampling study. This drop was due to an abrupt change in the level of raw sewage discharged into the stream.23
Surface water samples were collected twice a week for a period of five weeks in the dry season of July and August 2013. Water samples were collected aseptically into sterile 532 mL (18 oz) Whirlpak plastic collection bags and transported on ice to the laboratory at the University of Venda for water purification tests, which were performed within 2–3 h of collection. Surface water collection occurred at 11AM and was analyzed between 1PM–2PM. The turbidity of the surface water and effluent water was determined using a turbidimeter (Hach, 2100A Laboratory Turbidimeter).
Water purification tests
The AgNP and CuNP papers were evaluated for bactericidal effectiveness by a simple water filter test, which has previously been reported.2 New fresh sheets of AgNP or CuNP papers were used for each test. Typically, the papers were cut into 6.5 cm by 6.5 cm squares. In the Microbiology laboratory at the University of Venda, South Africa, the collected surface water was dripped through the horizontal paper held in a simple plastic filter unit supported by a plastic grid, i.e. gravity-fed filtration (Fig. 2).6 About 2–3 liters of water were typically filtered through each paper, with collected samples diverted into different beakers at discrete intervals, typically 500, 750, 1000 mL, etc. The influent and permeate water samples at a given filtered volume were analyzed for the presence of viable total coliform and E. coli bacteria by the membrane filtration method. Briefly, the water samples were filtered either as an undiluted or diluted sample through a 47 mm diameter, 0.45 um pore size cellulose ester membrane in sterile membrane filter funnels. Following this, the membranes were incubated on M-coliblue media soaked absorbent pads at 35 °C for 24 h, and colonies were counted visually. A total of 250 filtered water samples were evaluated, approximately evenly divided between the rural and urban water sources. For each paper type, approximately 55–70 filtered water samples were evaluated. Periodic blank samples testing for contamination due to procedural errors all showed zero colonies.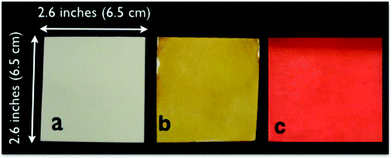 | ||
| Fig. 1 Blotter papers (a) untreated, (b) with silver nanoparticles, and (c) with copper nanoparticles. Note the scale: each paper is 6.5 cm by 6.5 cm and the filter cross section is 4.7 cm by 4.7 cm. | ||
During the water purification tests, filtration times were recorded to determine flow rates and paper permeabilities. In a typical filtration test, the filter head of 9 cm of water was kept close to constant by continuously adding contaminated water to keep the filter holder full. Filter paper permeabilities were calculated using Darcy's equation for a hydrostatic constant head:
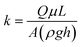 | (1) |
Metal release and retention
After filtration through the AgNP or CuNP paper, the permeate water samples were analyzed for metal content by graphite furnace atomic absorption spectrometry (GF-AA, Perkin Elmer AAnalyst 200 with HGA 900, Charlottesville, VA). This value was expressed as a percentage of the total metal mass contained in the papers, as determined by the flame atomic absorption measurements. The total metal release from each paper type was determined through integration of the metal leachate data with respect to the volume filtered. The metal release rate was found to asymptote to an approximately steady-state value after the first 500 mL of filtered water and this steady-state value was used to calculate the approximate maximum capacity in liters of a single sheet of NP-containing paper. Other factors, such as water composition variation and nanoparticle size, could potentially alter the overall metal release and are not accounted for with this approximation.Results
Filter paper characterization
A total of 110 paper filters were evaluated, including 23 thin AgNP, 22 medium AgNP, 28 thick AgNP, 28 CuNP, and 9 untreated papers. Thicknesses, varying from 0.472–1.667 mm, and porosities, varying from 65–73%, of the AgNP and CuNP paper filters were chosen to explore the impact of the filter void space on the filtration speed and subsequent retention time necessary for bacterial inactivation (Table 1). Measured porosities are similar to typical paper grades (70%), though filter papers typically have higher porosities, as high as 87%.24 Porosity measured in this manner varied by only ~3% (absolute) between samples, significantly less than the ~10% error of the method. The permeabilities of the filter papers ranged from 7.4 × 10−14–9.7 × 10−13 m2, which falls in the range of fibrous filter media with similar solid volume fractions, 0.27–0.35, (1-porosity).25 Permeability varied by 1–1.5 orders of magnitude despite relatively small differences in porosity; this is because differences in permeability between paper samples are due to the geometrical arrangement of cellulose fibers, not their relative amount as would be typical of granular media. For example, for the AgNP papers, flow rates were fastest for the thick AgNP filters. Its higher permeability more than compensated for the increased path length. Compared to granular filter media, which are typically 60–70% solids, the solid volume fractions of fibrous materials can vary by a few orders of magnitude from as low as 0.01% to 50%.25 Relative to many fibrous filter media, the papers evaluated in this study have a higher fibrous concentration, resulting in relatively lower permeabilities. The NP-containing papers have higher permeabilities relative to the untreated filter papers, indicating an effect from material processing. The NP synthesis methods require additional rewetting and re-drying processing steps, and the permeability increase is therefore likely due to hornification: the shrinking of intra-fiber pores and resulting expansion of the inter-fiber pores that control flow.26 Despite similar porosities, the AgNP paper filters generally filtered faster and thus had higher effective permeabilities than the CuNP papers. The lower effective permeability is (by definition) caused by either physical modifications changing flow paths or residual air blocking flow paths due to increased capillarity caused by the higher hydrophobicity of CuNP papers.7The average turbidity level of influent water samples was 8.1 NTUs, with 45% of the influent samples being less than 5 NTUs, the World Health Organization recommendation for turbidity in drinking water.1 After filtration, the average turbidity level dropped to 4.9 NTUs, with 65% of the filtered samples being less than 5 NTUs. The rural irrigation canal had lower influent turbidity levels, an average of 0.73 NTUs, and a reduction of 27% was observed after filtering. The urban stream had higher influent turbidity, an average of 13.1 NTUs, and a reduction of 43% was observed after filtering. The average pH values for the water sources was 6.8, where the rural stream's average pH was 6.3 and the urban stream's average pH was 7.3.
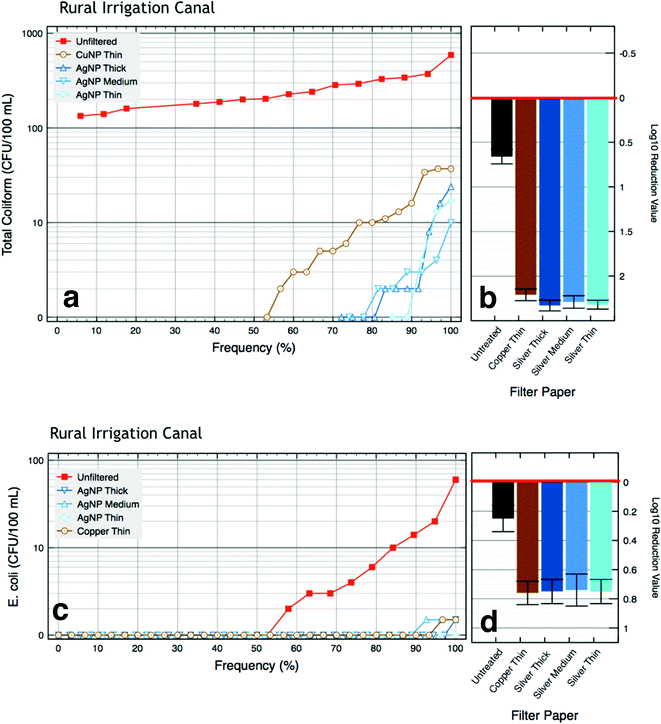
Surface water purification with AgNP and CuNP papers
The microbiological water quality was improved by passing contaminated surface water from rural South Africa through silver or copper nanoparticle papers: 90% of AgNP paper filtrate samples were under 10 E. coli per 100 mL, and 72% of AgNP paper filtrate samples were under 10 total coliform per 100 mL (Table 3; Fig. 3 and 4). Eighty-four percent (84%) of CuNP paper filtrate samples were under 10 E. coli per 100 mL, and 64% of filtrate samples were under 10 total coliform per 100 mL (Table 3; Fig. 3 and 4). In Fig. 3(a and c) and 4(a and c), the cumulative frequency of the bacteria counts of water pre- and post-filtration to show all the data from these studies were plotted on a log10 scale. From these figures, it is clearly evident that filtered water samples have much lower levels of bacteria than influent water samples. To further support these cumulative frequency plots, more standard logarithmic reduction values for each of the different paper filters tested was reported (Fig. 3(b and d) and 4(b and d)).A summary of the microbiological water quality data for untreated surface water and AgNP and CuNP paper effluent samples including mean total coliform and E. coli bacteria is presented in Table 2. Control experiments performed by filtering the surface water through untreated thin and thick papers indicated that paper alone does not eliminate bacteria from contaminated water: bacteria passing through the untreated paper filters were still viable, with counts ranging from 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 640
000 to 640![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CFU per 100 mL for the urban stream and 2 to 60 CFU per 100 mL for the rural irrigation canal (Table 2). These bacterial counts are somewhat lower than the untreated surface water, indicating that some bacteria were retained in the filter, demonstrating physical filtration removal.
000 CFU per 100 mL for the urban stream and 2 to 60 CFU per 100 mL for the rural irrigation canal (Table 2). These bacterial counts are somewhat lower than the untreated surface water, indicating that some bacteria were retained in the filter, demonstrating physical filtration removal.
| Urban stream – high | Urban stream – moderate | Rural irrigation canal | |
|---|---|---|---|
| a 95% confidence intervals. b Only one sample evaluated. | |||
| Total coliform (CFU per 100 mL) | |||
| Untreated water | 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (500 000 (500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–1 000–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (7300–15 000 (7300–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
250 (195–300) |
| Control paper thin | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (50 000 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–175 000–175![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
— | 18 (0–46) |
| Control paper thick | 640![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
— | 60 (50–70) |
| CuNP thin | 40 (0–80) | 18 (6–26) | 7 (3–11) |
| AgNP thin | 100 (10–200) | 14 (4–22) | 1 (0–3) |
| AgNP med | 155 (40–260) | 20 (4–24) | 1 (0–2) |
| AgNP thick | 110 (0–260) | 14 (5–24) | 2 (0–3) |
| E. coli (CFU per 100 mL) | |||
| Untreated water | 375![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (85 000 (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–665 000–665![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
5000 (100–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
13 (8–16) |
| Control paper thin | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (2400–53 000 (2400–53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
— | 2 (0–2) |
| Control paper thick | 310![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
— | 5 (1–8) |
| CuNP thin | 70 (0–140) | 6 (0–9) | 0 |
| AgNP thin | 8 (0–20) | 4 (0–8) | 0 |
| AgNP med | 70 (15–120) | 4 (0–7) | 0 |
| AgNP thick | 60 (0–130) | 4 (0–9) | 0 |
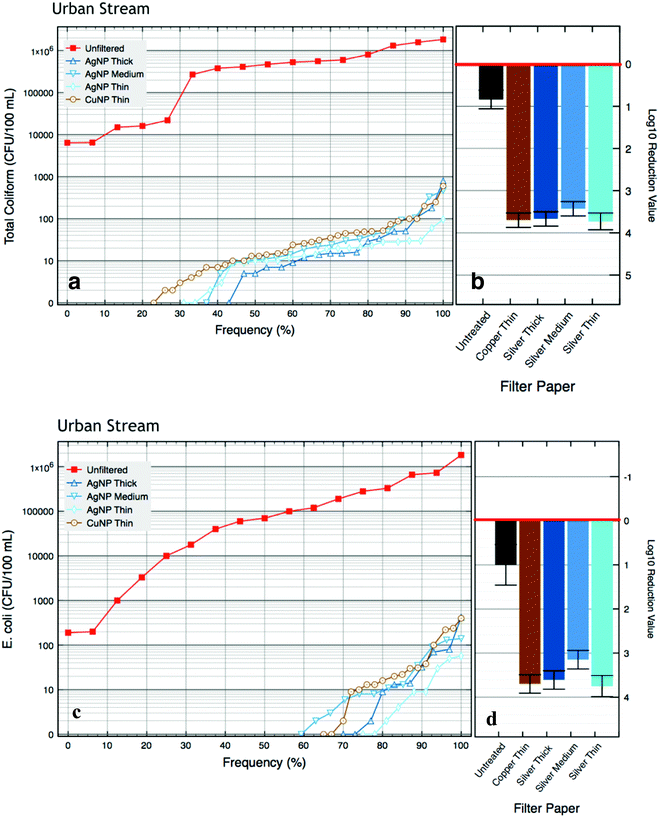
All of the AgNP and CuNP paper filters greatly reduced total coliform and E. coli as compared to the untreated influent water, as nearly all of the total coliform and E. coli bacteria were eliminated for the rural and urban medium samples (Table 3). The urban high influent had the highest initial mean bacteria count, 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CFU per 100 mL, and had a mean log10 reduction value (LRV) of 4.6 for total coliform bacteria after passing through the NP papers (Fig. 4b). The urban medium water had LRVs of 3.2 for total coliform bacteria (Fig. 4b). Both total coliform and E. coli bacteria showed similar reductions in viable bacteria count (Fig. 4d). No statistical differences in microbiological reduction were observed between the MNP paper filters. In Fig. 3 and 4, the order of magnitude between the influent and filtered bacteria counts is a graphical representation of the bacterial log10 reduction due to the bacterial inactivation from the AgNP and CuNP papers. In addition to presenting the complete dataset rather than just the more typical summary statistics, the graphical presentation of bacterial reduction also enables the presentation of several features common in water treatment of field samples: (1) log10 reduction of water samples is limited to- and is typically proportional to- influent bacterial concentrations; (2) the majority of samples are below or near a meaningful limit of quantification, i.e. the difference between CFU counts of 0–10 or even slightly higher are not substantively meaningful; and (3) summary statistics such as the mean or log10 reduction blend a handful of larger bacterial counts with many negative or near-negative samples (CFU < 10), and thus hide the salient results: that most samples were cleaned while a handful were not.
000 CFU per 100 mL, and had a mean log10 reduction value (LRV) of 4.6 for total coliform bacteria after passing through the NP papers (Fig. 4b). The urban medium water had LRVs of 3.2 for total coliform bacteria (Fig. 4b). Both total coliform and E. coli bacteria showed similar reductions in viable bacteria count (Fig. 4d). No statistical differences in microbiological reduction were observed between the MNP paper filters. In Fig. 3 and 4, the order of magnitude between the influent and filtered bacteria counts is a graphical representation of the bacterial log10 reduction due to the bacterial inactivation from the AgNP and CuNP papers. In addition to presenting the complete dataset rather than just the more typical summary statistics, the graphical presentation of bacterial reduction also enables the presentation of several features common in water treatment of field samples: (1) log10 reduction of water samples is limited to- and is typically proportional to- influent bacterial concentrations; (2) the majority of samples are below or near a meaningful limit of quantification, i.e. the difference between CFU counts of 0–10 or even slightly higher are not substantively meaningful; and (3) summary statistics such as the mean or log10 reduction blend a handful of larger bacterial counts with many negative or near-negative samples (CFU < 10), and thus hide the salient results: that most samples were cleaned while a handful were not.
| Number and percentage of ALL samples by bacteria concentration of filtered water | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Filter paper | 0 CFU per 100 mL | 1–10 CFU per 100 mL | 11–100 CFU per 100 mL | 101–1000 CFU per 100 mL | Total samples | ||||
| Total coliform | |||||||||
| AgNP thin | 31 | 51% | 12 | 20% | 17 | 28% | 1 | 2% | 61 |
| AgNP med | 27 | 48% | 11 | 20% | 13 | 23% | 5 | 9% | 56 |
| AgNP thick | 36 | 51% | 18 | 26% | 14 | 20% | 2 | 3% | 70 |
| CuNP thin | 22 | 34% | 19 | 30% | 22 | 34% | 1 | 2% | 64 |
| Total | 116 | 46% | 60 | 24% | 66 | 26% | 9 | 4% | 251 |
| E. coli | |||||||||
| AgNP thin | 51 | 85% | 6 | 10% | 3 | 5% | 0 | 0% | 60 |
| AgNP med | 39 | 71% | 9 | 16% | 5 | 9% | 3 | 5% | 55 |
| AgNP thick | 57 | 81% | 6 | 9% | 3 | 4% | 1 | 1% | 70 |
| CuNP thin | 46 | 73% | 7 | 11% | 7 | 11% | 2 | 3% | 63 |
| Total | 193 | 78% | 28 | 11% | 18 | 7% | 6 | 2% | 248 |
Metal release and retention
Due to possible human health effects from silver or copper exposure,27,28 we analyzed the silver and copper contents of the permeate water. Fig. 5a and b show that the general trends of silver and copper release are initially high values that drop to nearly steady state values after the first 1 L of water filtered. No significant variation is detectable between the different water sources or paper types. From all AgNP paper types tested, the average silver content in the permeate water was 55.59 (±31.76) ppb, as measured by graphite furnace atomic absorption, which measures total silver content including both silver ions and nanoparticles. The average amount of silver leaching from the filter papers thus meets the US-EPA guideline for drinking water of less than 100 ppb (Fig. 5a).29 The average copper content in the permeate water from the rural surface water was 562 (±100) ppb, which meets the US-EPA guideline for drinking water of less than 1000 ppb.24 Problematically, the average copper in the permeate from the urban surface water was over the limit with an average of 1497 (±242) ppb. However all of the excessive copper release occurred over the initial 0.5 L of stream water filtered, after which copper levels were consistently less than the 1000 ppb limit (Fig. 5b).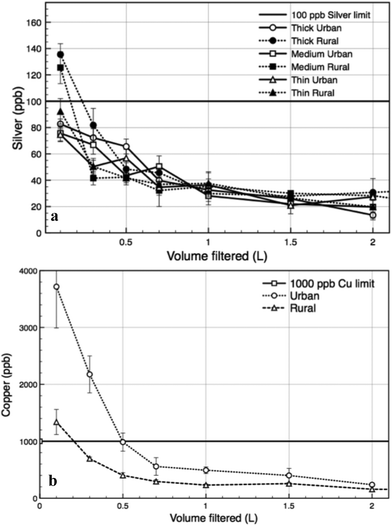 | ||
| Fig. 5 (a) Silver release from AgNP papers and (b) copper release from CuNP papers with respect to volume of water filtered for the two water sources. | ||
The papers' antimicrobial functionality requires metal ion release, thus below some level the papers will cease to perform satisfactorily. The theoretical filter paper lifetime can therefore be estimated from the metal leaching rate. For the purposes of a preliminary estimate, we assume the antibacterial activity of paper filters declines at around 50% of the metal mass remaining in the paper, though this needs to be experimentally determined in subsequent development testing.
| Mr = Mi + MsV | (2) |
| Paper | Source | Initial metal releasea (μg) | Metal release per volume filteredb (μg L−1) | Estimated capacity (L) | Percent metal loss per L |
|---|---|---|---|---|---|
| a The first 0.5 L filtered through the MNP papers. b Slope of the graph for x > 0.5 L. Estimated capacity assumes each sheet to contain the metal content listed in the paper details table. | |||||
| AgNP thin | Urban | 7 | 30 | 70 | 0.7% |
| Rural | 20 | 33 | 63 | 0.8% | |
| AgNP medium | Urban | 13 | 35 | 56 | 0.9% |
| Rural | 33 | 31 | 63 | 0.8% | |
| AgNP thick | Urban | 7 | 34 | 97 | 0.5% |
| Rural | 35 | 26 | 127 | 0.4% | |
| CuNP thin | Urban | 1116 | 488 | 80 | 0.6% |
| Rural | 235 | 222 | 176 | 0.3% | |
Discussion
Here we describe a simple POU filter paper to purify drinking water that is not dependent upon energy and is simple to use. The filter papers require only a plastic support and a vessel to collect the filtered water. This is the first field trial of such a filter paper, and our results show that these AgNP and CuNP papers are highly effective at reducing bacterial contamination from filtering natural water sources. This work represents a first step from a laboratory demonstration to a potential new alternative purifier for providing clean drinking water in developing-world communities.The stream water sources used are typical of those found in other parts of Africa and other developing regions. In these sets of experiments, we observed significant variations in microbial concentrations in the stream water sources, ranging from the tens to millions CFU per 100 mL. Other researchers have previously reported total coliform values to be an average of 938 CFUs per 100 mL for rural stream water sources in the same general area of the rural irrigation canal.13,30 In the larger geographic area of the Venda region of Limpopo province in South Africa, where both the urban and rural surface water sources are located, studies have reported total coliform and fecal coliform bacteria levels to be 600–3.7 × 104 CFU per 100 mL and 18–6.3 × 104 CFU per 100 mL, respectively.31 The bacteria levels we report in this article are comparable with the exception of the “urban high” samples, and are representative of this region of South Africa and can be generalized to other similar settings in the developing world.
In comparison to typical stress tests in laboratories to evaluate antibacterial filtration capabilities, these natural water sources tended to have lower levels of bacteria. For laboratory testing of novel antibacterial water filters, bacterial concentrations are typically cultured to be 106 CFU per mL or higher, chosen to be represent worst-case levels of coliform bacteria in highly polluted stream waters.32 Our previous laboratory research demonstrated the thin AgNP papers showed 100% inactivation of ~109 lab-cultured E. coli bacteria per mL (LRV = 8.7).6 Due to these natural water sources having lower concentrations of coliform bacteria (the highest E. coli count was 1.8 × 106 CFU per 100 mL), and with such high antibacterial effectiveness in laboratory experiments, one would expect these filter papers to show complete inactivation of E. coli bacteria in all samples. In this field study, the same thin AgNP papers from the previous laboratory study showed high levels of E. coli inactivation, 99.994%, for natural waters with an average of 3.8 × 105E. coli per 100 mL (LRV = 5.1), representing data for the “urban high”. While these are still high levels of antibacterial inactivation, it suggests than some complications may arise with the increased variability of the source water. For example, laboratory studies of antibacterial agents generally limit the number of variables to the influent bacteria concentration, species of microbe, disinfection concentration, filtration time, and specific dissolved salts (i.e. phosphate buffers to maintain osmotic pressure). This performance trend is not unique to these filter papers; typically, all point-of-use water purifiers, including ceramic water filters, biosand filtration, solar disinfection, free chlorine, and coagulation/chlorination, fail to perform to their maximum performance in field settings.33 Some of the environmental challenges that arise from the complexity of local stream water sources include variability in microbial levels and species, temperature, pH, dissolved solids, organic matter, and turbidity.
In this field study, the specific water chemistry was not analyzed, but certain dissolved solids and types of organic matter may impact the bactericidal effectiveness of the AgNP and CuNP papers. For the rural irrigation canal, based on the very low values of influent bacteria, turbidity, and metal release in the effluent water (Tables 2 and 4 and Fig. 5), it can be assumed that other contaminants such as dissolved solids and organic matter have a minimal impact. However, the urban stream had higher variability of influent bacteria, turbidity, and metal release, which is likely due to chemical contaminants in the highly-polluted water. For the urban stream, the total coliform bacteria concentration ranged from 7300 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CFU per 100 mL (Table 2), which falls in the bacterial concentration range of “weak” sewage water.34 Based on this estimation, we can assume that the other water pollutants are comparable to what is reported for “weak” sewage water. Potentially interfering chemicals found in sewage water include divalent cations, humic acids, and sulfide containing compounds such as proteinaceous materials. These chemical pollutants could reduce the bactericidal effectiveness of silver or copper by binding to or complexing with the metal ions, which limits bacterial absorption of the given metal ion. For example, the high levels of copper release with urban water samples could be due copper chelation with various compounds present in sewage water.35 However, it is not clear to what degree these added substances could affect the bactericidal effectiveness of the AgNP and CuNP papers; e.g., it has recently been shown that typical dissolved solids and organic matter did not have an impact on the bactericidal effectiveness of a silver nanoparticle ceramic disc filter.36 In our previous laboratory research, various contaminant proxies, including proteins, salts, and natural organic matter, were spiked into the influent water along with high levels of E. coli, and showed a reduced effectiveness of the AgNP paper in bacteria elimination with high levels of proteinaceous contaminants.6 However, the effectiveness of bacteria elimination by the AgNP paper was not affected by fulvic acid (a type of natural organic material) or dissolved salts (NaCl). Although the present study did not evaluate the influent water for chemical contaminants, it is possible the modest decline in bacterial reduction in the urban stream (Fig. 4) is due to high levels of proteinaceous material dissolved in the stream. The pollution of natural streams with untreated sewage increases not only coliform bacteria, but also dissolved organic materials, of which about half are proteins.34 To ensure eminant long-term effectiveness of these MNP filter papers, a pre-filteration step to remove proteinaceous material may be necessary, such as a coagulation or settling step.
000 CFU per 100 mL (Table 2), which falls in the bacterial concentration range of “weak” sewage water.34 Based on this estimation, we can assume that the other water pollutants are comparable to what is reported for “weak” sewage water. Potentially interfering chemicals found in sewage water include divalent cations, humic acids, and sulfide containing compounds such as proteinaceous materials. These chemical pollutants could reduce the bactericidal effectiveness of silver or copper by binding to or complexing with the metal ions, which limits bacterial absorption of the given metal ion. For example, the high levels of copper release with urban water samples could be due copper chelation with various compounds present in sewage water.35 However, it is not clear to what degree these added substances could affect the bactericidal effectiveness of the AgNP and CuNP papers; e.g., it has recently been shown that typical dissolved solids and organic matter did not have an impact on the bactericidal effectiveness of a silver nanoparticle ceramic disc filter.36 In our previous laboratory research, various contaminant proxies, including proteins, salts, and natural organic matter, were spiked into the influent water along with high levels of E. coli, and showed a reduced effectiveness of the AgNP paper in bacteria elimination with high levels of proteinaceous contaminants.6 However, the effectiveness of bacteria elimination by the AgNP paper was not affected by fulvic acid (a type of natural organic material) or dissolved salts (NaCl). Although the present study did not evaluate the influent water for chemical contaminants, it is possible the modest decline in bacterial reduction in the urban stream (Fig. 4) is due to high levels of proteinaceous material dissolved in the stream. The pollution of natural streams with untreated sewage increases not only coliform bacteria, but also dissolved organic materials, of which about half are proteins.34 To ensure eminant long-term effectiveness of these MNP filter papers, a pre-filteration step to remove proteinaceous material may be necessary, such as a coagulation or settling step.
This study shows that the vast majority of non-sewage samples have CFU < 10 or slightly higher, indicating successful treatment, while a handful of samples had significant bacterial reductions but were not entirely successful. This indicates that NP papers can achieve complete bacterial inactivation, but the laboratory filter holder design needs improvement. By comparison, the weak sewage samples (despite much higher LRVs) were not completely treated, and the effluent remained weakly contaminated though bacterial counts of 10–200 are still a vast improvement over 105–106. This indicates the need for a filter design that incorporates a pre-filter to remove confounding organic or inorganic materials and may point to the need for serial NP paper filtration for extremely dirty waters, i.e. a filter design that allows for stacking multiple papers. Future research must focus on the engineering and applied development necessary to move from a prototype to a practical implementation, with a particular emphasis on determining and avoiding failure modes whether due to the filter papers or the filter holder.
The levels of silver and copper released into the filtered effluent water were below the World Health Organization's recommendations for the majority of samples, including all samples taken after the initial 0.5 L. Ill health effects are rare from exposure to silver or copper ions, and if health effects are observed, they result from very high levels during occupational exposures. The analysis method for determining silver or copper concentration in the filtrate effluent does not distinguish between dissolved ionic metals or nano-sized metal particles, and the ratio of released nanomaterials to ionic silver or copper from these filters has not been specifically evaluated. Other researchers have examined the release of silver nanoparticles from silver containing textiles (some nano-silver, others silver salts or zeolites) into laundry washing liquids, where most fabrics showed less than 5% of the silver released was in nano-form (1–100 nm). Additionally, nano-silver textiles showed lower total silver releases than AgCl or Ag-zeolite fabrics and less than 1% of the silver released was in the nano-form.37 If these AgNP-containing filter papers have similar release behavior, then the overall risk of exposure to people from drinking water purified with silver nanoparticles is very low.
An additional requirement for a practical point of use water filter is a sufficiently high flow rate of purified water to appeal to the end user. In the case of a filter paper containing a chemical biocidal such as silver or copper NPs, the filtration flow rate must allow for sufficient contact time between influent bacteria and the metal ions. In this study, using a constant filter head height of 9 cm, filtration flow rates varied from 1 to 4 L h−1, depending upon the permeability of the papers (Table 3). Filtration flow rates may need to be limited by the contact time required for silver uptake by direct contact with the bacterial cells.
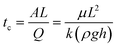 | (3) |
The contact time, tc for Darcy flow (eqn (1)) through a filter paper perpendicular to the flow is the inverse of volumetric flow rate Q [m3 s−1] through the area A [m2] of a paper of thickness L [m], i.e.: Table 1 shows the measured average times to filter 1 liter of water for the four MNP papers in this study. Filtration flow rate and thus the time to filter 1 L are proportional to filter head and area as well as paper permeability and thickness (eqn (3)). Thus, while the filter holder design or choice of paper can be modified to improve flow rates, antimicrobial effectiveness of the filter papers at shorter contact times must be verified in further studies. Furthermore, the relationship between filtration time, MNP-microbial contact time, and microbial death, needs to be further explored in future research.
Filter failure or equivalently a lack of acceptance and usage may ultimately be due to slow filtration flow rates, e.g. due to sediment clogging. In this field study, the turbidity levels were typical for streams, and did not detectably change filtration speeds. However, higher turbidity levels would be expected to slow filtration rates and interfere with the release of metal ions and thus the disinfection process. Potential filter failure from turbidity is the same as with other filters – some level of suspended sediments will lead to filter clogging, i.e. a decrease in the effective permeability of the filter. This failure mechanism is complicated by the paper-based filter media, which will undergo hornification processes due to wetting and drying cycles during repeated use. Paper hornification will provide some small increase in permeability,38,39 as seen in Table 1, where the untreated papers showed slower filtration times than their modified nanoparticle embedded paper, and possibly, hornification effects might be able to offset low levels of turbidity-induced clogging. However, the more significant effect will be that ever-larger diameter clay particles will be able to pass through the filter paper with repeated use. While clay is not pleasant to drink per se, in such a scenario it is already present in the water and filtering out pathogens without removing the clays is still a marked improvement. On the other hand, the filter papers have also been selected to achieve physical filtration of larger pathogenic protozoa such as Cryptosporidium, as well as species transported on clay particles such as cholera, which must still occur post-hornification. Future efforts on filter design and paper selection need to identify the optimal choice of paper, including pore size, to achieve acceptable flow rates and resilience to turbidity for both fresh papers as well as used papers that have undergone hornification. Other potential failure mechanisms include decreased tensile strength of paper due to hornification or paper damage as well as the exhaustion of MNPs.
This study was intended as a proof-of-concept study for natural water sources and necessarily has several important limitations. First, only 2–3 liters of water was filtered through each filter paper; greater volumes must be tested to determination longevity and filter failure modes. Typically, POU water filters are used to purify thousands of liters of water before filter replacements.40 However, if ongoing efforts to develop a cost-effective and reliable paper-based microbial live/dead test prove successful,41 the technology could be incorporated in the MNP filter papers, providing users an indicator of when to swap out filter papers. Another limitation is the lack of an evaluation of user preferences for the filter papers and a field-practical filter design, which will need to be addressed in future work.
Finally, development testing must determine failure modes to determine filter longevity. A single sheet of a silver or copper nanoparticle paper with a 42.25 cm2 cross-sectional area has been projected to produce tens to hundreds of liters of clean water before the metal content of the sheet has been exhausted. These projections are solely determined from the mass balance of metal ion release with respect to the volume of water filtered for relatively small volumes of water. These calculated filter capacities are meant to illustrate a potential filter capacity in order to design future experiments to elucidate the actual capacity. Various detrimental factors that are not taken into account include: chemical changes to the nanoparticles altering metal ion release rate, nanoparticle aggregation, insufficient antimicrobial activity of nanoparticles below some threshold concentration, and various other functional complications associated with field use. Based on MNP release calculations of filter paper capacities, if an individual drank 3 liters of water per day, these filters have the potential to last for about a month, while a family would need to replace the filter paper every few days. The filter capacity for household usage will depend upon the final filter design which could be selected to minimize up-front cost or to maximize filter capacity, e.g. by including multiple sheets of MNP paper in series or choosing larger filter paper cross-sectional areas.
These silver and copper nanoparticle papers present yet another option in the crowded point-of-use water purification field. The primary advantage of these papers may be due to their affordability – the material costs of each sheet is only a few pennies to produce at lab scale. At an industrial scale, filter paper media cost 0.22–0.37 USD per m2.42 The addition of MNPs are expected to increase the cost <20% because silver concentrations are 0.1–1% of the mass of the AgNP papers, while copper is added at 5–10% by mass but copper costs significantly less than silver. The processing required for the addition of the MNPs is in both cases similar to existing processes: application of an aqueous solution, rinsing, and drying. The driver of the cost of either the AgNP or CuNP paper filters is thus the paper filter itself, with a small increase for the MNP raw materials and treatment. Typically, the initial upfront costs for POU devices are high relative to local incomes, e.g. ceramic water filters are ~$15–30 USD43 and the Life Straw is ~$20 USD.43 The life-spans of both ceramic water filters and the Life Straw are much longer than these filter papers, and as a result, the projected overall costs per unit of water processed are likely to be more similar than the upfront initial costs. However, the fact still remains that the relatively high upfront cost of one of these other filters is too expensive for many people who do not have access to clean drinking water. For example, other researchers are exploring ways to reduce this initial costs for existing filters, by evaluating a pilot project in Kenya where ceramic water filters are being purchased on an installment plan through mobile technology.44 Other advantages of a disposable paper filter include: ease of distribution and portability, lower potential for biofilm growth on the filter media, and, unlike similarly cheap and transportable chlorine, treated water has no off-taste. The main drawback to using a paper filter is the limited lifespan, which would require frequent filter replacement compared to many other types of filters and therefore a reliable supply chain.
Conclusions
Metal nanoparticle-containing filter papers have been demonstrated to purify drinking water from stream water sources in South Africa, the first time these papers have been demonstrated in a field setting. The silver and copper nanoparticle filter papers studied show great promise as a new point-of-use water purification option for resource-limited countries and other places where drinking water becomes contaminated. The microbial contamination of water, turbidity, and other pollutants from the natural water sources studied are representative of many similar settings in the developing world. This work was limited to analysis of filter paper performance, and not the filter holder design. Future work will need to include evaluation of MNP paper performance over a greater quantity of water, optimization of paper characteristics with respect to filtration flow rates and antimicrobial effectiveness, and most importantly, the design of a practical and user-friendly filter holder. Future studies will also need to address the practical challenges of implementing this technology in rural households and emergency/disaster relief settings.Acknowledgements
This research was supported by the Fogarty International Center at the National Institutes of Health (Award Number D43TW009359) and by the National Science Foundation (Grant EEC 1156999). The views expressed in this publication are solely those of the authors, and the NIH and NSF do not endorse any products or commercial services mentioned in this publication. We thank Professor Vhonani Netshandama from the University of Venda and Chief Lucas of the village of Tshibvumo for facilitating the water sample collections in the rural community. We gratefully acknowledge the use of facilities at the University of Venda from Professor Natasha Potgeiter and Professor Paul Mojapelo. We also thank Research Experience for Undergraduates (REU) students Corinne Clinch and Hannah Weinronk for their laboratory assistance. Finally, we would like to thank the Water and Health in Limpopo collaboration without whose framework we could not have conducted this research.References
- World Health Organization, Guidelines for Drinking-water Quality, 4th edn, 2011, pp. 1–18 Search PubMed.
- T. A. Dankovich and D. G. Gray, Environ. Sci. Technol., 2011, 45, 1992 CrossRef CAS PubMed.
- P. Jain and T. Pradeep, Biotechnol. Bioeng., 2005, 90, 59 CrossRef CAS PubMed.
- T. Y. Klein, J. Wehling, L. Treccani and K. Rezwan, Environ. Sci. Technol., 2013, 47(2), 1065 CrossRef CAS PubMed.
- V. A. Oyanedel-Craver and J. A. Smith, Environ. Sci. Technol., 2008, 42, 927 CrossRef CAS PubMed.
- T. A. Dankovich, Environ. Sci.: Nano, 2014, 1, 367 RSC.
- T. A. Dankovich and J. A. Smith, Water Res., 2014, 63, 245 CrossRef CAS PubMed.
- G. Borkow and J. Gabbay, Curr. Chem. Biol., 2009, 3, 272 CAS.
- R. L. Davies and S. F. Etris, Catal. Today, 1997, 36, 107 CrossRef CAS.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramírez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346 CrossRef CAS PubMed.
- R. Bendi and T. Imae, RSC Adv., 2013, 3, 16279 RSC.
- J. He, T. Kunitake and A. Nakao, Chem. Mater., 2003, 15, 4401 CrossRef CAS.
- J. E. Mellor, J. A. Smith, A. Samie and R. A. Dillingham, J. Environ. Eng., 2013, 139, 1152 CrossRef CAS PubMed.
- J. B. Demarest, S. A. Pagsuyoin, G. P. Learmonth, J. E. Mellor and R. A. Dillingham, J. Artif. Soc. Soc. Simul., 2013, 16(4), 3 Search PubMed.
- C. Levard, B. C. Reinsch, F. M. Michel, C. Oumahi, G. V. Lowry and G. E. Brown, Jr., Environ. Sci. Technol., 2011, 45(12), 5260 CrossRef CAS PubMed.
- C. Levard, S. Mitra, T. Yang, A. D. Jew, A. R. Badireddy, G. V. Lowry and G. E. Brown, Jr., Environ. Sci. Technol., 2013, 47(11), 5738 CrossRef CAS PubMed.
- http://www.oasiswater.co.za/filtration, accessed July 7, 2015.
- W. E. Morton and J. W. S. Hearle, Physical Properties of Textile Fibers, The Textile Institute, Manchester, England, 3rd edn, 1993 Search PubMed.
- “Paper Physics”, Papermaking Science and Technology, Book 16, ed. K. Niskanen, Finnish Paper Engineers Association and TAPPI, Helsinki, Finland, 1998 Search PubMed.
- South African Department of Water Affairs and Forestry. Personal communication, July 2013.
- Handbook of Paper Science, ed. H. F. Rance, Elsevier, Amsterdam, The Netherlands, 1982 Search PubMed.
- G. W. Jackson and D. F. James, Can. J. Chem. Eng., 1986, 64, 364 CrossRef CAS.
- M. A. Hubbe, R. A. Venditti and O. J. Rojas, BioResources, 2007, 2(4), 739 CAS.
- P. L. Drake, Ann. Occup. Hyg., 2005, 49, 575 CrossRef CAS PubMed.
- Department of Health and Human Services, Public Health Service, Agency for Toxic Substances & Disease Registry, Division of Toxicology, Public health statement on Copper, 2004 Search PubMed.
- U.S. Environmental Protection Agency, Title 40: Protection of the Environment, 2002, 143.3 Search PubMed.
- World Health Organization, UNICEF, Progress on Drinking Water and Sanitation, 2014 Search PubMed.
- P. Lehohla, Gross Domestic Product Statistical Release P044, Statistics South Africa, 2011 Search PubMed.
- E. Sello, District and Province Profiles, South Africa National Burden of Disease Study, 2010, Available at: http://www.healthlink.org.za/uploads/files/dhb0708secBlp.pdf Search PubMed.
- L. S. Abebe, J. A. Smith, S. Narkiewicz, V. Oyanedel-Craver, M. Conaway, A. Singo, A. Samie, P. Mojapelo, J. Brant and R. A. Dillingham, J. Water Health, 2014, 12(2), 288 CrossRef PubMed.
- C. Obi, N. Potgieter, P. Bessong and G. Matsaung, Water Sci. Technol., 2003, 47, 59 CAS.
- Guide standard and protocol for testing microbiological water purifiers, ed. U.S. Environmental Protection Agency: Office of Pesticide Programs, US EPA, Washington, D.C., 1987 Search PubMed.
- M. D. Sobsey, C. E. Stauber, L. M. Casanova, J. M. Brown and M. A. Elliott, Environ. Sci. Technol., 2008, 42(12), 4261 CrossRef CAS PubMed.
- Metcalf and Eddy, Wastewater Engineering, Treatment Disposal Reuse, ed. G. Tchobanoglous and F. L. Burton, McGraw-Hill, 1991, p. 1820 Search PubMed.
- J. A. Davis, Geochim. Cosmochim. Acta, 1984, 48, 679 CrossRef CAS.
- J. Rayner, H. Zhang, J. Schubert, P. Lennon, D. Lantagne and V. Oyanedel-Craver, ACS Sustainable Chem. Eng., 2013, 1(7), 737 CAS.
- D. M. Mitrano, E. Rimmele, A. Wichser, R. Erni, M. Height and B. Nowack, ACS Nano, 2014, 8(7), 7208 CrossRef CAS PubMed.
- J. D. Lindsay and P. H. Brady, Tappi, 1993, 76(9), 119 CAS.
- S. J. Hashemi, V. G. Gomes and W. J. M. Douglas, Proceedings of 1995 International Paper Physics Conference, Niagara-on-the-lake, Ontario, Canada, 1995, p. 21 Search PubMed.
- M. Peter-Varbanets, C. Zurbrugg, C. Swartz and W. Pronk, Water Res., 2009, 43(2), 245 CrossRef CAS PubMed.
- F. Deiss, M. E. Funes-Huacca, J. Bal, K. F. Tjhung and R. Derda, Lab Chip, 2014, 14(1), 167 RSC.
- K. Sutherland, Filter and Filtration Handbook, 5th edn, 2008, p. 44 Search PubMed.
- J. Brown and M. D. Sobsey, J. Water Health, 2010, 8(1), 1 CrossRef CAS PubMed.
- J. E. Luoto, RAND Corporation. Testing Mobile Phone-based Sales to Increase Use of Safe Water Filters in Kenya, Available at: http://www.rand.org/labor/centers/rapid/projects/kenya-water-filters.html (accessed September 17, 2015) Search PubMed.
Footnote |
| † Presently: Department of Civil and Environmental Engineering, 5000 Forbes Avenue, Pittsburgh, PA, 15213, USA. |
| This journal is © The Royal Society of Chemistry 2016 |