Open Access Article
Open Access ArticleWaste not want not: life cycle implications of gold recovery and recycling from nanowaste†
Paramjeet
Pati
a,
Sean
McGinnis
bc and
Peter J.
Vikesland
 *bde
*bde
aPicker Engineering Program, Smith College, USA
bVirginia Tech Institute of Critical Technology and Applied Science (ICTAS) Sustainable Nanotechnology Center (VTSuN), USA. E-mail: pvikes@vt.edu; Tel: +540 231 3568
cDepartment of Material Science and Engineering, Virginia Tech, USA
dCivil and Environmental Engineering, Virginia Tech, USA
eCenter for the Environmental Implications of Nanotechnology (CEINT), Duke University, USA
First published on 24th August 2016
Abstract
Commercial-scale applications of nanotechnology are rapidly increasing. Enhanced production of nanomaterials and nano-enabled products and their resultant disposal lead to concomitant increases in the volume of nanomaterial wastes (i.e., nanowaste). Many nanotechnologies employ resource-limited materials, such as precious metals and rare earth elements that ultimately end up as nanowaste. To make nanotechnology more sustainable it is essential to develop strategies to recover these high-value, resource-limited materials. To address this complex issue, we developed laboratory-scale methods to recover nanowaste gold. To this end, α-cyclodextrin facilitated host–guest inclusion complex formation involving second-sphere coordination of [AuBr4]− and [K(OH2)6]+ was used for gold recovery and the recovered gold was then used to produce new nanoparticles. To quantify the environmental impacts of this gold recycling process we then produced life cycle assessments to compare nanoparticulate gold production scenarios with and without recycling. The LCA results indicate that recovery and recycling of nanowaste gold can significantly reduce the environmental impacts of gold nanoparticle synthesis.
Nano impactMany nanotechnologies employ resource-limited materials, which ultimately end up in nanomaterial waste streams (nanowaste). Nano-manufacturing infrastructures must therefore develop recycling methods within for recovering high-value metals from nanowaste. We report a method for selective recovery of gold from nanowaste using second-sphere coordination of tetrabromoaurate anion and hexaaquo potassium cation, facilitated by alpha-cyclodextrin. Technical innovations for improving sustainability must be analyzed within a life cycle framework to identify hidden environmental burdens. Therefore, we also conducted a life cycle assessment of the recovery process. Currently, there are no best practices to address the specific challenges in managing nanowaste. Our study is therefore timely and can provide new insights into the life cycle considerations in nanowaste recycling and inform future waste management policies. |
Introduction
In recent years, there has been rapid growth in the number of nanomaterial-based consumer products, resulting in a concomitant increase in waste streams containing nanomaterials (e.g., nanowaste). Several nanotechnologies depend on resource-limited precious metals and rare earth elements (REEs). Supply risks related to these critical raw materials may one day threaten the sustainable growth of nano-industries. In light of the declining global reserves of high-value metals,1–3 the recovery and recycling of critical materials is of paramount importance. Current waste treatment practices do not have specific provisions for nanomaterials,4 and uncertainties regarding end-of-life scenarios currently hinder the formulation of regulations for nanomaterial waste management and resource recovery from waste streams.5Conventional metal recovery methods (e.g., solvent extraction,6 ion-exchange,7 electrolysis,8 plasma technology,9 and microbiological methods10,11) offer strategies to recover high-value metals and REEs from nanowaste. The diversity and complexity of nanowaste, however, make it difficult to develop universally applicable methods for waste management.12 Fortunately, recent studies have developed novel approaches to metal recovery that may address these challenges. Nano-SnO2 was recovered from industrial electroplating waste sludge using the selective crystallization and growth of acid-soluble amorphous SnO2 into acid-insoluble SnO2 nanowires.13 In another study, adsorption-induced crystallization of uranium rich nanocrystals was used for uranyl enrichment.14 Thermo-reversible liquid–liquid phase transition12 and cloud point extraction15–18 also hold promise for the successful separation and recovery of critical, high-value and resource-limited materials from nanowaste. One such critical raw material is gold.
The concentration of gold in the continental crust is estimated to be about 1.5 μg kg−1.19 Gold ore grades have declined over the last decade;20 the cost of extracting gold has steadily increased21 and there are concerns about having reached peak gold.22 The current demand for gold for nanotechnology-based applications is small compared to other demands (e.g., jewelry23). Nonetheless, given the rapid growth of the nanotechnology industry,24,25 the application and market share for gold-based nanotechnologies is expected to increase exponentially in areas such as medical diagnostics and imaging, high-efficiency compact storage devices, and photovoltaics.26 The global market value of gold nanoparticles (AuNPs) was estimated to be 1.34 billion US dollars in 2014, with the medical industry consuming nearly 1.9 tons of gold. The market for gold-based nanotechnologies is expected to grow to 8 billion US dollars by 2022, requiring over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg of gold flowing into the nanotechnology industry by that time.26
000 kg of gold flowing into the nanotechnology industry by that time.26
In the future, a limited supply of the raw material may pose a substantial challenge to the development of gold-based nanotechnologies. Therefore, novel approaches for gold recovery from waste streams are being actively researched today.16,27,28 Recently, Liu et al.27 reported one approach to recover gold using α-cyclodextrin (α-CD). This method involves the formation of a host–guest inclusion complex involving tetrabromoaurate anion [AuBr4]−, α-CD, and hexaaquo potassium cation [K(OH2)6]+, followed by rapid co-precipitation at room temperature. This method has been reported to have high separation efficiencies (>75%) and recovery yields (>90%), and unlike traditional methods for selective gold recovery, does not involve the use of toxic cyanide29 or mercury.30 In this study, we investigated the applicability of such an approach for the recovery of gold from nanowaste. We undertook this study with the following goals: i) to recover gold from nanowaste; ii) to use the freshly recovered gold to synthesize new AuNPs; and iii) to assess the environmental performance of the overall process from a life cycle perspective.
Current end-of-life treatment facilities may not be well-suited to handle nanowaste, because nanomaterials may differ significantly in their properties (e.g., specific heat capacities, melting temperatures, etc.) compared to their corresponding bulk materials. For example, current high temperature metal recovery processes used for battery recycling may be inadequate for nano-enabled lithium ion batteries. The nanomaterials in those batteries may require smelting temperatures that are significantly higher than current operating conditions, resulting in higher energy consumptions and overall emissions.31 In such cases, thermodynamic analysis based on life cycle approaches can help inform the modifications that current waste management facilities need to make when dealing with nanowaste. The life cycle considerations are therefore integral to the development of future nanowaste management. Moreover, technical innovations aimed at improving sustainability may have hidden environmental burdens. These hidden impacts and the overall sustainability of a product or a process can be identified and analyzed using life cycle assessment (LCA), which is a quantitative framework used to evaluate the cumulative environmental impacts associated with all stages of a material – from the extraction of raw materials (‘cradle’) through the end-of-life (‘grave’).32 Novel methods for nanowaste recycling may involve hidden environmental costs that can only be identified through development of comprehensive LCAs. For example, an LCA of gold nanoparticle synthesis showed that the use of benign reagents during nanomaterial synthesis, while intuitively ‘green’, can have significant life cycle impacts.33 In another study, a new method for recovery of gold from sewage sludge ash recently claimed that the method “eliminates the need for water” during metal recovery.28 However, the process involves temperatures of ∼800 °C for optimum gold retrieval. High temperature processes typically have substantial water footprints because they involve considerable fuel consumption and generally employ high-pressure steam. Any reduction in direct water use during material recovery may be negated by such indirect water uses. It is therefore imperative to analyze potential environmental burdens from a life cycle perspective when developing new nanowaste management strategies. By coupling novel recycling approaches with LCA modeling, we can develop next-generation closed- and open-loop processes for sustainable nanotechnology. The novelty of our study lies in the combination of LCA modeling with an innovative approach involving α-CD-facilitated selective recovery of gold.
Experimental
Selective gold recovery by α-CD
We prepared a simulated nanowaste comprised of citrate-reduced gold nanoparticles (AuNPs).34 The simulated nanowaste suspension was precipitated by adding KCl and was then dissolved using a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v
1 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v mixture of HBr and HNO3. HBr was employed to ensure that gold was present as the square planar complex AuBr4−. The pH of the resultant clear red solution was adjusted to ∼5 using KOH. Assuming that all the gold originally in the AuNP suspension was precipitated and redissolved in the HBr–HNO3 mixture, we added a calculated mass of α-CD sufficient to achieve a 2
v mixture of HBr and HNO3. HBr was employed to ensure that gold was present as the square planar complex AuBr4−. The pH of the resultant clear red solution was adjusted to ∼5 using KOH. Assuming that all the gold originally in the AuNP suspension was precipitated and redissolved in the HBr–HNO3 mixture, we added a calculated mass of α-CD sufficient to achieve a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of gold
1 molar ratio of gold![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α-CD. Almost immediately the clear red solution became turbid. After 30 minutes, the reaction mixture was filtered through a 0.22 μm PTFE filter. The retentate was then resuspended in deionized water by sonication, which yielded a clear brown solution. An aliquot of 50 mM of sodium metabisulfite (Na2S2O5) was then added to precipitate and recover gold. The volume of 50 mM Na2S2O5 required was based on the amount of gold originally present in the simulated nanowaste. All steps involved in the recovery process occurred at room temperature. The percent recovery of gold for three samples was determined by inductively coupled plasma mass spectrometry (ICP-MS) using standard method 3125-B.35
α-CD. Almost immediately the clear red solution became turbid. After 30 minutes, the reaction mixture was filtered through a 0.22 μm PTFE filter. The retentate was then resuspended in deionized water by sonication, which yielded a clear brown solution. An aliquot of 50 mM of sodium metabisulfite (Na2S2O5) was then added to precipitate and recover gold. The volume of 50 mM Na2S2O5 required was based on the amount of gold originally present in the simulated nanowaste. All steps involved in the recovery process occurred at room temperature. The percent recovery of gold for three samples was determined by inductively coupled plasma mass spectrometry (ICP-MS) using standard method 3125-B.35
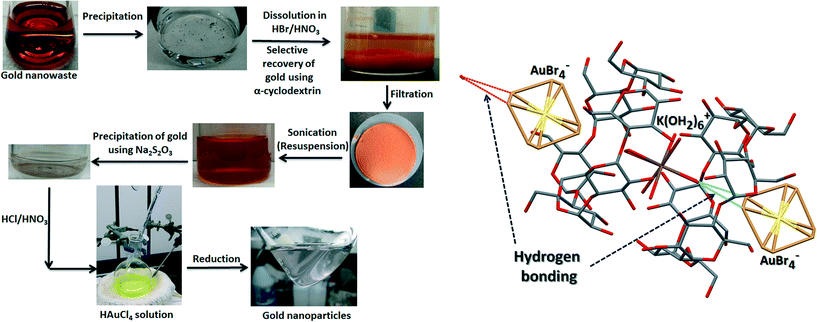
The solid precipitate obtained after adding Na2S2O5 was recovered by filtration, analyzed using powder X-ray diffraction (XRD; Fig. S1, ESI†). All steps up to this point were focused on recovering gold from nanowaste. To recycle the freshly recovered gold for synthesis of new AuNPs. the gold was dissolved in aqua regia, and the resulting yellow solution was boiled to remove HNO3 (observed as brown nitrogen dioxide gas leaving the flask), while adding HCl intermittently. Boiling was stopped after ebullition of brown gas concluded. The final solution was analyzed by UV-vis spectroscopy (Fig. S2†) and used to synthesize new citrate-reduced AuNPs. The AuNPs were characterized for size and chemical composition using high-resolution transmission electron microscopy (TEM) and selected area electron diffraction (SAED) (Fig. S3†). The overall recovery process is summarized in Fig. 1.
LCA modeling
The focus of the LCA modeling was to identify and analyze the impacts associated with the AuNP synthesis process with and without recycling. The system boundary for this study included cradle-to-gate processes (AuNP synthesis) and end-of-life treatment processes (selective recovery of gold from nanowaste followed by the synthesis of new AuNPs (i.e., recycling). (Fig. 2) The functional unit for our study was 1 mg of AuNP. The inventory for chemical precursors used in the AuNP synthesis and gold recovery was modeled using the EcoInvent 3.0 database.36 For the inventory, sodium hydrogen sulfite was used in place of sodium metabisulfite, since the latter was not available in the EcoInvent 3.0 database. The energy use during AuNP synthesis and gold recycling was recorded for LCA model development. The energy requirement inventories were obtained from direct measurements in the laboratory. Within the EcoInvent 3.0 database, the average medium-voltage electricity mix for the U.S. Northeast Power Coordinating Council was used to model energy use. The uncertainty for energy use was modeled as a uniform distribution with the maximum and minimum values being ∼20% of the calculated energy use as per measurements performed in our laboratory, following the method used in a previous study.33 LCA models were constructed using SimaPro 8, and life cycle impact assessment (LCIA) was done using the ReCiPe method37 (version 1.08), using midpoints and the hierarchist (H) perspective with European normalization. Uncertainty analyses were performed using Monte-Carlo simulations for 1000 runs. Four scenarios were simulated for 0%, 10%, 50%, and 90% recovery of gold from nanowaste, meaning that the percentage of the nanowaste gold put back into the synthesis process was 0% (i.e., no-recycle scenario), 10%, 50%, and 90%. In these four scenarios, all of the recovered gold was assumed to be recycled to make new AuNPs, and the unrecovered gold along with other chemicals was simulated as hazardous waste for incineration. The inventories used for LCA modeling are presented in Tables S1–S3.† Table S1† shows those inputs that were not available in the EcoInvent database and had to be custom defined (e.g., chloroauric acid, α-cyclodextrin). For clarity, the inputs used to model AuNP synthesis and AuNP recycling are shown separately in Tables S2 and S3† respectively.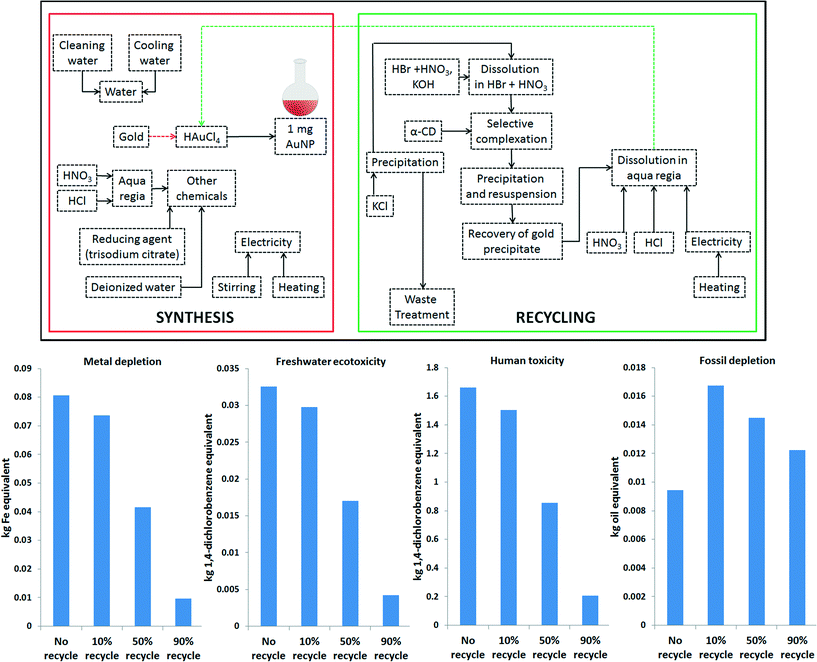 | ||
| Fig. 2 (Top) Schematic of LCA model for AuNP synthesis and recycling, (Bottom) – Life cycle impacts of 10%-, 50%-, 90%- and no-recycle scenarios. | ||
The different inputs – namely acids, deionized water, energy, cyclodextrin, and sodium hydrogen sulfite – were varied to estimate how sensitive the overall recycling process was to changes in individual inputs. In the initial phase, each input was increased by five times keeping other inputs unchanged. After identifying the input parameters that the recycling process was most sensitive to, those parameters were varied in increments of 25%, ranging from 50% to 150% of the amounts used in the baseline scenario. The results of sensitive analysis are presented in Fig. 3, 4, S8, and S9 (ESI†).
Results and discussion
Selective recovery of gold using α-CD
Host–guest inclusion complexes involving cyclodextrin (host) and metal ions (guest) have been reported to form rod- and chain-like nanoscale supramolecular assemblies.38,39 These inclusion complexes form when water molecules are displaced from the cyclodextrin cavity by more hydrophobic guest molecules, thus resulting in energetically favorable reduced ring-strain.40 The selective recovery of gold using α-CD is made possible by the perfect molecular recognition between [AuBr4]− and α-CD, causing square planar [AuBr4]− to orient axially with respect to the α-CD channel.27 Moreover, the cavity between two α-CD molecules is ideally suited to accommodate the octahedral [K(OH2)6]+ ion, thereby restricting its solvation by water molecules in the bulk solution. The specific orientation of the [AuBr4]− ion and the favorable location of [K(OH2)6]+ within the α-CD dimer facilitates second-sphere coordination between [K(OH2)6]+ and [AuBr4]−. A rod-shaped nanostructure forms due to the equatorial [C–H⋯Br–Au] hydrogen bonds between α-CD and [AuBr4]−, and axial [O–H⋯Br–Au] hydrogen bonds between [K(OH2)6]+ and [AuBr4]−. Individual rods bind to each other radially due to hydrogen bonding, forming a supramolecular assembly that ultimately precipitates due to colloidal instability.27 Each unit of this assembly is comprised of an [AuBr4]− anion and an α-CD dimer enclosing a [K(OH2)6]+ cation (Fig. 1B). The superstructure shown in Fig. 1B does not form in the case of β- or γ-CD due to the unfavorable cavity size of the CD dimer. The second-sphere coordination is highly specific to [AuBr4]−. Other gold complexes (e.g., [AuCl4]−) or square planar anions of other metals, (e.g., [PtBr4]− or [PdBr4]−) cannot form the rod-like assembly.27 Therefore this method is highly suitable for selective recovery of gold from mixed nanowaste.The gold contained within the precipitated superstructure can be isolated by Na2S2O5 reduction. XRD analysis of the reduced precipitate confirmed that it contained gold along with some unidentified peaks that indicate impurities. UV-vis spectroscopy of this precipitate following dissolution in aqua regia showed that the absorbance spectrum closely matches that of chloroauric acid. ICP-MS analysis showed that the percent recoveries of gold for three replicate samples were 59.6%, 60.2% and 77.4%. We synthesized citrate-reduced AuNPs using this recovered gold. The AuNPs were, however, colloidally unstable and coalesced soon after synthesis, presumably due to the as yet unidentified impurities in the recovered gold. Nonetheless, the d-spacings in the diffraction patterns confirmed that the nanoparticles were AuNPs (Fig. S3†).
Nanowaste from laboratories can be expected to be in complex matrices with extremely low gold concentrations. Several parameters need to be precisely controlled for optimum recovery. For example, the co-precipitation of the rod-like supramolecular assembly is pH-dependent. In the pH range 2.5–5.9, most of the [AuBr4]− is bound through complexation and associated with the supramolecular assembly; the residual concentration of [AuBr4]− in the reaction mixture is lowest in this pH range.27 Thus, the maximum recovery of gold occurs within this pH range. Depending on the pH (and the residual concentration of [AuBr4]−), the moles of α-CD required for optimum recovery will vary. Consequently, depending on the concentration of gold recovered in the resuspended retentate, the moles of Na2S2O5 needed to precipitate and recover gold will also vary. With this information, the recovery process can be optimized to improve the yield and purity of the recovered gold.
LCA of AuNP recycling process
LCA models were constructed to assess the environmental performance of the recycling process. Using the models, we were able to compare between different recycle scenarios, identify the primary drivers of impacts, and assess the sensitivity of the overall recycling process to changes in different inputs. Within the EcoInvent database, several inputs required for building the life cycle inventory were unavailable for US based processes. Therefore, most of the inventories were inputs chosen from Europe. Since the direct measurement of energy consumption was done in a US-based laboratory, the electricity mix used for building the inventories was chosen as medium-voltage for the U.S. Northeast Power Coordinating Council. Since most of the inventory was based on European geography, we chose to use the ReCiPe impact assessment.41 Endpoint impact assessment makes interpretation of the results easier by aggregating impact categories. For example, midpoint impacts such as ozone depletion potential, freshwater toxicity etc., can be aggregated into three damage categories: human health (measured in disability life adjusted years (DALYs)), ecosystems (measured in species × year), and resources (measured in dollars). The endpoint characterization values are obtained by multiplying midpoint characterization factors by damage factors. However, doing so increases the uncertainty in the results.42 Knowing that the lack of nano-specific characterization factors in impact already introduces uncertainties that cannot be accounted for in our LCA study, we chose the midpoint impact assessment method. We note that the TRACI impact assessment method, which is a US-based midpoint method, may serve as an alternative to the ReCiPe midpoint method.Weighting choices (individualistic (I), hierarchist (H), or egalitarian (E)) are value based. The individualistic weighting leans towards technological optimism and human adaptability, and involves lower uncertainties due to the shorter time frames involved (typically 20 years). At the other end, the egalitarian weighting approach entails a strongly precautionary perspective, and involves longer time frames (typically >500 years). We chose the hierarchist perspective for the impact assessment because this type of weighting involves mid-range time frames (typically 100 years) and is based on commonly used policy principles that are both politically and scientifically accepted.43
A schematic of the LCA models for this study is shown in Fig. 2. The life cycle impacts of four different gold recycling scenarios (0%, 10%, 50%, and 90% recycling) show that recycling can significantly reduce the environmental impacts of AuNP synthesis across several impact categories. The normalized impact assessment results show that the most important impact categories are freshwater and marine eutrophication, freshwater and marine ecotoxicity, human toxicity, and metal depletion. Fig. 2 also shows the environmental impacts in the categories of metal depletion, freshwater ecotoxicity, human toxicity and fossil fuel depletion. Scenarios involving recycling outperform the no-recycle scenario in most cases (as confirmed by uncertainty analysis [Fig. S4–S7†]), except for climate change potential, fossil fuel depletion, and water depletion. However, based on the normalized impact assessment results, the environmental burdens of recycle scenarios in these three impact categories were found to be negligible when compared to the benefits of recycling for the other impact categories.
The high fossil fuel depletion in the recycle scenarios is due to inefficiencies in the laboratory-scale boil-off of HNO3. As seen from the inventory in Table S3,† only 4.2 kJ of energy was required for the gold recovery steps, whereas the boil-off step during recycling required 70 kJ. It is important to note that the dissolution of freshly recovered gold in aqua regia, and subsequent boil-off of HNO3 is only necessary for closed-loop recycling, where the recovered gold is used for synthesizing new AuNPs. The dissolution in aqua regia followed by HNO3 boil-off may not be necessary for an open-loop recycle scenario, where the objective is to simply recover the gold, and not use it for AuNP synthesis. In this study, the dissolution of every batch of recovered gold in aqua regia and subsequent boiling were done in parallel. The energy footprint of this boiling step can be substantially reduced by first dissolving several batches of recovered gold in aqua regia and then boiling off HNO3 in a single step. Detailed analysis of scale-up scenarios may indicate additional reductions in the energy footprint.
In this study, we focused on investigating the potential for recovering gold from nanowaste, and identifying the means for improving recovery processes. Having pinpointed the boil-off of HNO3 as a key inefficiency in the current recycling approach, we then conducted sensitivity analysis to improve future redesign approaches. Sensitivity analysis is used for identifying the most influential parameters in a model, and is helpful for narrowing down the key parameters to control when improving the design of products and processes.44 In assessing our model's sensitivity to input parameters, we first compared the baseline recycle scenarios (10%, 50% and 90% recovery) with scenarios in which an individual input (e.g., deionized water) was increased fivefold.
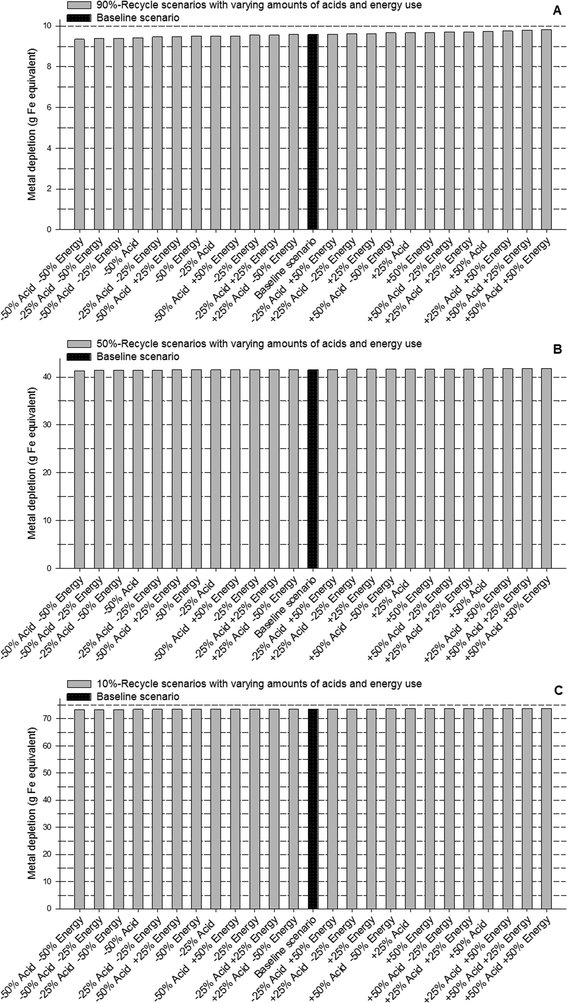
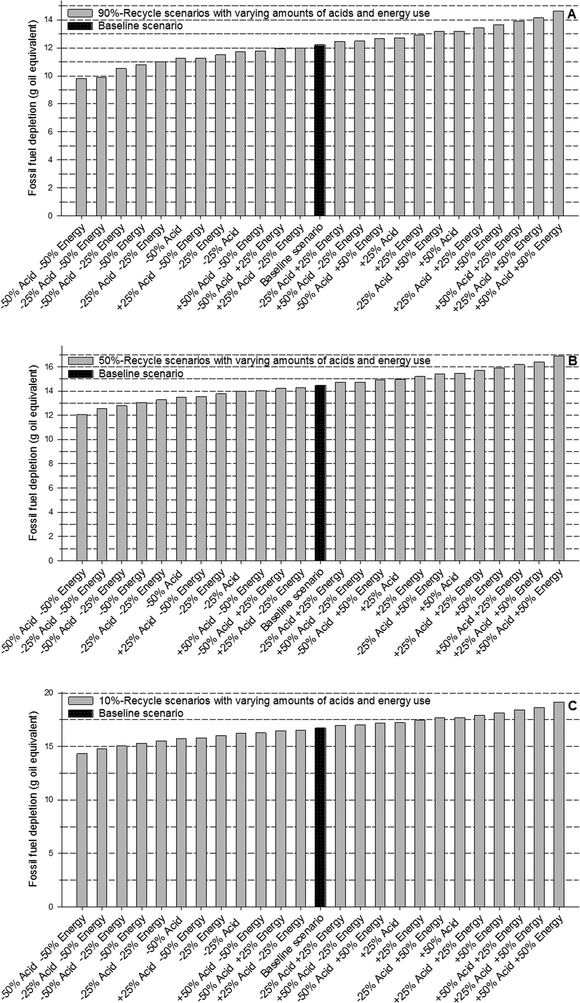
Our results showed that cyclodextrin, sodium hydrogen sulfite, and deionized water were not as influential parameters as acid use and energy consumption. For example, compared to the baseline scenario with 90% recovery, fivefold increases in the amounts of cyclodextrin, sodium hydrogen sulfite, and deionized water increased the metal depletion, freshwater ecotoxicity, human toxicity by <1%, and fossil fuel depletion by <3% (Table S4†). On the other hand, a fivefold increase in acids increased metal depletion by 13%, freshwater ecotoxicity by 7.5%, human toxicity by 5%, and fossil fuel depletion by 64%. Similarly, direct energy use was found to be substantially more influential than deionized water, cyclodextrin, or sodium hydrogen sulfite. A five-time increase in direct energy use resulted in a 7% increase in metal depletion, 11% increase in freshwater ecotoxicity, 4% increase in human toxicity, and 94% increase in fossil fuel depletion. Having identified that acids and energy consumption were key influential parameters, we modeled 24 scenarios of each of the recovery cases (10%, 50%, and 90% recovery) in which the two parameters were varied from 50% to 150% of the amounts used in the baseline scenarios in increments of 25% (Fig. 3, 4, S8, and S9, ESI†).
As seen from the results of this sensitivity analysis, variations in acid and (direct) energy consumption influence fossil fuel depletion more strongly than metal depletion, freshwater ecotoxicity, or human toxicity. Fig. 3 and 4 also show that a decrease in the amount of acids results in marginally greater reduction in metal depletion, compared to a decrease in direct energy consumption by the same amount. Decreases in energy consumption, on the other hand, exert a greater influence on fossil fuel depletion. Also, by comparing the results in Fig. 2–4 we see that although acid consumption and (direct) energy use are important parameters, the percentage of gold recycled is even more influential in reducing the impacts across all impact categories.
The sensitivity of the recycling process to acid use and direct energy consumption offers an important insight into improving the overall process. The key steps in the recycling process that use acids and (direct) energy are: i) the dissolution of nanowaste in HBr and HCl, ii) the resuspension of CD-complex (which involves sonication), and iii) the dissolution of the recovered gold followed by HNO3 boil-off. As discussed earlier, energy consumption can be reduced by using more efficient methods for separating HNO3 (compared to inefficient laboratory-scale boil-off). The key role of acid indicates that processes that incorporate recycling of acids (primarily used in this method as solvents) will substantially improve the environmental performance of the overall recycling.
Broader impacts and challenges
Although this study focused on recovering gold from AuNP nanowaste, the method is also applicable for more complex nanowastes. As mentioned earlier, the rod-like superstructure involved in the selective recovery of gold does not form in the case other square planar tetrahalides (e.g., [PtBr4]− or [PdBr4]−)27 because the second-sphere coordination is specific to [AuBr4]−. This method can therefore be applicable for selective recovery of gold from mixed nanowaste as well.In this study, we focused on the closed-loop recycling of gold for AuNP synthesis, in which the recovered gold was put back into the original process to synthesize the same end product (i.e., gold nanoparticles). However, this technique can also be applied for separation, pre-concentration, and open-loop recycling in which the recovered gold, instead of being used in the original process to make nanoparticles, may instead be used in other processes (e.g., gold-plated jewelry, contacts for relay switches or connectors). Of course, the decision to employ a closed- or open-loop recycling strategy will depend on the source from which gold is being recovered. Moreover, with this method, we can recover gold not only from nanowaste (e.g., nanoparticulate gold in consumer electronics, spent point-of-use sensors and medical devices, etc.), but also from other waste streams that contain gold (e.g., gold alloy scraps27 and e-waste). For example, the amount of gold in mobile phones and personal computers sold in 2007 was estimated to be 3% of the gold mined globally.45 Given the growing concerns about e-waste management46 and the urgent need to establish best practices,47 this novel approach for recovering gold is especially important. By recovering precious metals and REEs from waste streams, we can reduce the adverse impacts of mining metals and REEs, mitigate the environmental burdens of toxic waste, decrease the cost of critical raw material procurement, and improve the resilience of the material supply chain.48
Economic as well as thermodynamic considerations will determine the feasibility of recovering gold from nano-enabled applications. For recycling to be profitable, the financial gains from obtaining the recycled material should exceed the cost of interim steps (separation, collection, processing). From a thermodynamic standpoint, the work required to separate a critical material from a mixture increases monotonically as the mixture becomes more dilute.49 These factors pose challenges for recovery and recycling of precious metals and REEs from nano-enabled products; some applications of AuNPs will be more amenable for material recovery that others. For example, AuNPs incorporated in consumer electronics with modular designs may be easier to recover than AuNPs in medical waste from imaging or targeted drug delivery applications.
Modern products have a higher degree of material mixing, raising the cost of material recovery, and miniaturization further decreases the value of the recycled material per unit (i.e., per device, e.g., flash drive, wearable sensor, etc.),50 Moreover, the heterogeneity of waste containing nano-enabled products may pose additional challenges. For example, in the case of smart clothing, the nanoelectronics can hinder the recyclability of the textiles51 by being dispersed as a contaminant in the heterogeneous mixture. Increased heterogeneity and mixed nanowaste streams are possible in applications where two nano-enabled properties may be combined. For example, if wearable AuNP-based nanoelectronics were incorporated in athletic clothing that also utilize the antimicrobial properties of silver nanoparticles,52,53 the waste stream would be heterogenous mixture of textile and mixed nanowaste. Therefore, in addition the selective recovery approaches (such as the one presented in this study), innovations in separation techniques of solid waste streams in the end-of-life phase of nano-enabled products can help mitigate this issue.54
Of course, to have large-scale impacts, recovery and recycling approaches must be economically feasible in scaled-up scenarios, which in turn depend on the concentrations of the precious metals and REEs in in the nanowaste specific to the system (e.g., AuNPs embedded in paper-based sensors,55 AgNPs in clothing56,57), process (AuNP embedding using wax printing,55,58 AgNPs in filter materials59), and material (e.g., gold, silver, platinum etc.) under consideration. Nonetheless, our work provides a starting point for developing future material recovery processes and nanowaste management strategies (e.g., judicious separation nanomaterial waste containing precious metals, instead of single-stream disposals).
Conclusions
This study serves as proof-of-concept for lab-scale recovery of gold from nanowaste using a benign chemical, α-cyclodextrin, at room temperature. The LCA results show recycling gold can reduce the environmental impacts of the AuNP synthesis process, which makes a compelling case for incorporating recycling strategies for nanotechnologies involving precious metals and high value REEs. The life cycle inventory showed that the HNO3 boil-off step required for synthesizing new AuNPs consumed ∼17 times the energy required for gold recovery. Therefore, redesigning the HNO3 removal process can reduce the energy footprint of the recycling process. The reductions in impacts strongly depend on the percent gold recovered, and the environmental performance of the overall recycling process was most sensitive to the energy consumption and the use of acids.Recycling strategies such as the one we present in this paper can help mitigate the supply risk of critical raw materials in the future.60 Recently there has been a surge in research on recovery and recycling of precious metals and REEs from anthropogenic waste streams61 (e.g., cellphones,62 hard drives,63 printed circuit boards,64 liquid crystal displays,65 used fluorescent lamps,66 sewage sludge ash28) as well as the natural environment (e.g., seawater67). However, we must analyze recycling approaches from a life cycle perspective to identify unanticipated environmental burdens. Caballero-Guzman et al.68 reported that current recycling approaches do not significantly increase the incorporation of recycled nanomaterials in new products or applications. LCA models such as those presented in this study can help us analyze how effectively we incorporate recovered materials into the supply chain. Although a single nano-enabled application or product may involve only dilute amounts of a given metal or REE, the projected markets for nano-based products suggest that in the aggregate, nano-based applications may account for substantial use of high value materials. Therefore, it is important to develop material recovery and recycling methods (such the one reported in this study) that dovetail with nano-manufacturing technologies in the early phases of the design process.
Acknowledgements
This work was supported by grants from the Virginia Tech Graduate School (Sustainable Nanotechnology Interdisciplinary Graduate Education Program), the Virginia Tech Institute of Critical Technology and Applied Science (ICTAS), and NSF Award CBET-1133746. Nanoparticle characterization was conducted using the facilities of the ICTAS Nanoscale Characterization and Fabrication Laboratory (NCFL). The efforts of Jennifer Kim and Leejoo Wi in gold recovery experiments, and Chris Winkler in the collection of TEM images are much appreciated. We are also grateful to the two anonymous reviewers who helped us make this article better through their comments and suggestions.References
- D. P. van Vuuren, B. J. Strengers and H. J. M. De Vries, Long-term perspectives on world metal use—a system-dynamics model, Resour. Policy, 1999, 25(4), 239–255 CrossRef.
- R. B. Gordon, M. Bertram and T. E. Graedel, Metal stocks and sustainability, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(5), 1209–1214 CrossRef CAS PubMed.
- J. Busch, J. K. Steinberger, D. A. Dawson, P. Purnell and K. Roelich, Managing critical materials with a technology-specific stocks and flows model, Environ. Sci. Technol., 2014, 48(2), 1298–1305 CrossRef CAS PubMed.
- L. Breggin and J. Pendergrass, PEN 10 - Where Does the Nano Go? End-of-Life Regulation of Nanotechnologies. http://www.wilsoncenter.org/publication/pen-10-where-does-the-nano-go-end-life-regulation-nanotechnologies.
- N. Musee, Nanowastes and the environment: Potential new waste management paradigm, Environ. Int., 2011, 37(1), 112–128 CrossRef CAS PubMed.
- A. Shibayama, Y. Takasaki, K. Haga, H. Umeda, A. Sasaki and K. Takahashi, in Metal recovery from effluent water of metallurgical refining processes by cementation and solvent extraction, GDMB-Informationsgesellschaft, 2009, pp. 375–384 Search PubMed.
- N. Badawy, M. A. Rabah and R. Hasan, Separation of some heavy metal species from electroplating rinsing solutions by ion exchange resin, Int. J. Environ. Waste Manage., 2013, 12(1), 33–51 CrossRef CAS.
- L. Zhang, L. X. Qiu, X. L. Xi, L. W. Ma, L. W. Zhang, Z. H. Wang and J. F. Zhou, in Recovery of copper from waste printed circuit boards by suspension electrolysis, Trans Tech Publications Ltd, 2013, pp. 452–455 Search PubMed.
- E. Gomez, D. A. Rani, C. R. Cheeseman, D. Deegan, M. Wise and A. R. Boccaccini, Thermal plasma technology for the treatment of wastes: A critical review, J. Hazard. Mater., 2009, 161(2–3), 614–626 CrossRef CAS PubMed.
- G. M. Gadd, Bioremedial potential of microbial mechanisms of metal mobilization and immobilization, Curr. Opin. Biotechnol., 2000, 11(3), 271–279 CrossRef CAS PubMed.
- B. Volesky, Detoxification of metal-bearing effluents: biosorption for the next century, Hydrometallurgy, 2001, 59(2–3), 203–216 CrossRef CAS.
- O. Myakonkaya, C. M. Guibert, J. Eastoe and I. Grillo, Recovery of Nanoparticles Made Easy, Langmuir, 2010, 26(6), 3794–3797 CrossRef CAS PubMed.
- Z. Zhuang, X. Xu, Y. Wang, Y. Wang, F. Huang and Z. Lin, Treatment of nanowaste via fast crystal growth: With recycling of nano-SnO2 from electroplating sludge as a study case, J. Hazard. Mater., 2012, 211–212, 414–419 CrossRef CAS PubMed.
- Z. Chen, Z. Zhuang, Q. Cao, X. Pan, X. Guan and Z. Lin, Adsorption-induced crystallization of U-rich nanocrystals on nano-Mg(OH)2 and the aqueous uranyl enrichment, ACS Appl. Mater. Interfaces, 2014, 6(2), 1301–1305 CAS.
- J.-F. Liu, J.-B. Chao, R. Liu, Z.-Q. Tan, Y.-G. Yin, Y. Wu and G.-B. Jiang, Cloud Point Extraction as an Advantageous Preconcentration Approach for Analysis of Trace Silver Nanoparticles in Environmental Waters, Anal. Chem., 2009, 81(15), 6496–6502 CrossRef CAS.
- J.-F. Liu, R. Liu, Y.-G. Yin and G.-B. Jiang, Triton X-114 based cloud point extraction: a thermoreversible approach for separation/concentration and dispersion of nanomaterials in the aqueous phase, Chem. Commun., 2009,(12), 1514–1516 RSC.
- A. R. Mustafina, J. G. Elistratova, O. D. Bochkova, V. A. Burilov, S. V. Fedorenko, A. I. Konovalov and S. Y. Soloveva, Temperature induced phase separation of luminescent silica nanoparticles in Triton X-100 solutions, J. Colloid Interface Sci., 2011, 354(2), 644–649 CrossRef CAS PubMed.
- M. F. Nazar, S. S. Shah, J. Eastoe, A. M. Khan and A. Shah, Separation and recycling of nanoparticles using cloud point extraction with non-ionic surfactant mixtures, J. Colloid Interface Sci., 2011, 363(2), 490–496 CrossRef CAS PubMed.
- H. E. Frimmel, Earth's continental crustal gold endowment, Earth Planet. Sci. Lett., 2008, 267(1–2), 45–55 CrossRef CAS.
- G. M. Mudd, Global trends in gold mining: Towards quantifying environmental and resource sustainability, Resour. Policy, 2007, 32(1–2), 42–56 CrossRef.
- R. Schodde, In Recent trends in gold discovery, NewGenGold Conference, 2011/11/22/23, 2011 Search PubMed.
- R. A. Kerr, Is the World Tottering on the Precipice of Peak Gold?, Science, 2012, 335(6072), 1038–1039 CrossRef CAS PubMed.
- M. Starr and K. Tran, Determinants of the physical demand for gold: Evidence from panel data, World Econ., 2008, 31(3), 416–436 CrossRef.
- M. Roco, C. Mirkin and M. Hersam, Nanotechnology research directions for societal needs in 2020: summary of international study, J. Nanopart. Res., 2011, 13(3), 897–919 CrossRef.
- A. Wiek, L. Gasser and M. Siegrist, Systemic scenarios of nanotechnology: Sustainable governance of emerging technologies, Futures, 2009, 41(5), 284–300 CrossRef.
- Gold Nanoparticles Market Size | Industry Report, 2022, GMI358, Global Market Insights Inc., 2016 Search PubMed.
- Z. Liu, M. Frasconi, J. Lei, Z. J. Brown, Z. Zhu, D. Cao, J. Iehl, G. Liu, A. C. Fahrenbach, Y. Y. Botros and O. K. Farha, Selective isolation of gold facilitated by second-sphere coordination with α-cyclodextrin, Nat. Commun., 2013, 4, 1885 Search PubMed.
- J. Kakumazaki, T. Kato and K. Sugawara, Recovery of gold from incinerated sewage sludge ash by chlorination, ACS Sustainable Chem. Eng., 2014, 2(10), 2297–2300 CrossRef CAS.
- J. Stenson, Disaster management as a tool for sustainable development: a case study of cyanide leaching in the gold mining industry, J. Cleaner Prod., 2006, 14(3–4), 230–233 CrossRef.
- O. Malm, Gold Mining as a Source of Mercury Exposure in the Brazilian Amazon, Environ. Res., 1998, 77(2), 73–78 CrossRef CAS PubMed.
- S. Olapiriyakul and R. J. Caudill, Thermodynamic Analysis to Assess the Environmental Impact of End-of-life Recovery Processing for Nanotechnology Products, Environ. Sci. Technol., 2009, 43(21), 8140–8146 CrossRef CAS PubMed.
- J. B. Guinée, R. Heijungs, G. Huppes, A. Zamagni, P. Masoni, R. Buonamici, T. Ekvall and T. Rydberg, Life Cycle Assessment: Past, Present, and Future†, Environ. Sci. Technol., 2011, 45(1), 90–96 CrossRef PubMed.
- P. Pati, S. McGinnis and P. J. Vikesland, Life cycle assessment of “green” nanoparticle synthesis methods, Environ. Eng. Sci., 2014, 31(7), 410–420 CrossRef CAS.
- G. Frens, Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions, Nature, 1973, 241(105), 20–22 CAS.
- APHA, American Public Health Association, American Water Works Association, and Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 1998 Search PubMed.
- R. Frischknecht, N. Jungbluth, H.-J. Althaus, G. Doka, R. Dones, T. Heck, S. Hellweg, R. Hischier, T. Nemecek and G. Rebitzer, The ecoinvent database: Overview and methodological framework (7 pp), Int. J. Life Cycle Assess., 2005, 10(1), 3–9 CrossRef CAS.
- M. Goedkoop, R. Heijungs, M. Huijbregts, A. De Schryver, J. Struijs and R. van Zelm, ReCiPe 2008. A life cycle impact assessment method which comprises harmonised category indicators at the midpoint and the endpoint level, 2009, vol. 1 Search PubMed.
- Y. Liu, L. Li, H.-Y. Zhang, Y.-L. Zhao and X. Wu, Bis(pseudopolyrotaxane)s Possessing Copper(II) Ions Formed by Different Polymer Chains and Bis(β-cyclodextrin)s Bridged with a 2,2′-Bipyridine-4,4′-Dicarboxy Tether, Macromolecules, 2002, 35(27), 9934–9938 CrossRef CAS.
- Y. Liu, Y.-L. Zhao, H.-Y. Zhang and H.-B. Song, Polymeric Rotaxane Constructed from the Inclusion Complex of β-Cyclodextrin and 4,4′-Dipyridine by Coordination with Nickel(II) Ions, Angew. Chem., Int. Ed., 2003, 42(28), 3260–3263 CrossRef CAS PubMed.
- J. Szejtli, Introduction and General Overview of Cyclodextrin Chemistry, Chem. Rev., 1998, 98(5), 1743–1754 CrossRef CAS PubMed.
- M. Owsianiak, A. Laurent, A. Bjørn and M. Z. Hauschild, IMPACT 2002+, ReCiPe 2008 and ILCD's recommended practice for characterization modelling in life cycle impact assessment: A case study-based comparison, Int. J. Life Cycle Assess, 2014, 19(5), 1007–1021 CrossRef CAS.
- O. Jolliet, R. Müller-Wenk, J. Bare, A. Brent, M. Goedkoop, R. Heijungs, N. Itsubo, C. Peña, D. Pennington, J. Potting, G. Rebitzer, M. Stewart, H. U. D. Haes and B. Weidema, The LCIA midpoint-damage framework of the UNEP/SETAC life cycle initiative, Int. J. Life Cycle Assess., 2004, 9(6), 394 CrossRef.
- A. M. De Schryver, R. van Zelm, S. Humbert, S. Pfister, T. E. McKone and M. A. J. Huijbregts, Value Choices in Life Cycle Impact Assessment of Stressors Causing Human Health Damage, J. Ind. Ecol., 2011, 15(5), 796–815 CrossRef CAS.
- W. Wei, P. Larrey-Lassalle, T. Faure, N. Dumoulin, P. Roux and J.-D. Mathias, How to Conduct a Proper Sensitivity Analysis in Life Cycle Assessment: Taking into Account Correlations within LCI Data and Interactions within the LCA Calculation Model, Environ. Sci. Technol., 2015, 49(1), 377–385 CrossRef CAS PubMed.
- S. Mathias, H. Christian, K. Ruediger, M. Federico, M. Claudia, M. Christina, M. Esther and W. FengRecycling - From e-waste to resources, United Nations Environment Programme, 2009 Search PubMed.
- K. Zhang, J. L. Schnoor and E. Y. Zeng, E-Waste Recycling: Where Does It Go from Here?, Environ. Sci. Technol., 2012, 46(20), 10861–10867 CrossRef CAS PubMed.
- J. Li, X. Zeng, M. Chen, O. A. Ogunseitan and A. Stevels, “Control-Alt-Delete”: Rebooting Solutions for the E-Waste Problem, Environ. Sci. Technol., 2015, 49(12), 7095–7108 CrossRef CAS PubMed.
- B. Sprecher, I. Daigo, S. Murakami, R. Kleijn, M. Vos and G. J. Kramer, Framework for Resilience in Material Supply Chains, With a Case Study from the 2010 Rare Earth Crisis, Environ. Sci. Technol., 2015, 49(11), 6740–6750 CrossRef CAS PubMed.
- T. G. Gutowski, In Thermodynamics and recycling: A review, 2008, pp. 321–332 Search PubMed.
- J. B. Dahmus and T. G. Gutowski, What Gets Recycled: An Information Theory Based Model for Product Recycling, Environ. Sci. Technol., 2007, 41(21), 7543–7550 CrossRef CAS PubMed.
- A. R. Köhler, L. M. Hilty and C. Bakker, Prospective Impacts of Electronic Textiles on Recycling and Disposal, J. Ind. Ecol., 2011, 15(4), 496–511 CrossRef.
- Y. Li, P. Leung, L. Yao, Q. W. Song and E. Newton, Antimicrobial effect of surgical masks coated with nanoparticles, J. Hosp. Infect., 2006, 62(1), 58–63 CrossRef CAS PubMed.
- R. M. El-Shishtawy, A. M. Asiri, N. A. M. Abdelwahed and M. M. Al-Otaibi, In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation, Cellulose, 2010, 18(1), 75–82 CrossRef.
- X. Zhang, J. Guan, Y. Guo, X. Yan, H. Yuan, J. Xu, J. Guo, Y. Zhou, R. Su and Z. Guo, Selective Desoldering Separation of Tin–Lead Alloy for Dismantling of Electronic Components from Printed Circuit Boards, ACS Sustainable Chem. Eng., 2015, 3(8), 1696–1700 CrossRef CAS.
- R. H. Lahr, G. C. Wallace and P. J. Vikesland, Raman Characterization of Nanoparticle Transport in Microfluidic Paper-Based Analytical Devices (μPADs), ACS Appl. Mater. Interfaces, 2015, 7(17), 9139–9146 CAS.
- T. Walser, E. Demou, D. J. Lang and S. Hellweg, Prospective Environmental Life Cycle Assessment of Nanosilver T-Shirts, Environ. Sci. Technol., 2011, 45(10), 4570–4578 CrossRef CAS PubMed.
- D. Meyer, M. Curran and M. Gonzalez, An examination of silver nanoparticles in socks using screening-level life cycle assessment, J. Nanopart. Res., 2011, 13(1), 147–156 CrossRef CAS.
- P. Liang, H. Yu, B. Guntupalli and Y. Xiao, Paper-Based Device for Rapid Visualization of NADH Based on Dissolution of Gold Nanoparticles, ACS Appl. Mater. Interfaces, 2015, 7(27), 15023–15030 CAS.
- L. Mpenyana-Monyatsi, N. H. Mthombeni, M. S. Onyango and M. N. B. Momba, Cost-Effective Filter Materials Coated with Silver Nanoparticles for the Removal of Pathogenic Bacteria in Groundwater, Int. J. Environ. Res. Public Health, 2012, 9(1), 244–271 CrossRef CAS PubMed.
- J. H. Rademaker, R. Kleijn and Y. Yang, Recycling as a strategy against rare earth element criticality: a systemic evaluation of the potential yield of NdFeB magnet recycling, Environ. Sci. Technol., 2013, 47(18), 10129–10136 CAS.
- Q. Tan and J. Li, Recycling Metals from Wastes: A Novel Application of Mechanochemistry, Environ. Sci. Technol., 2015, 49(10), 5849–5861 CrossRef CAS PubMed.
- J. M. V. Navazo, G. V. Méndez and L. T. Peiró, Material flow analysis and energy requirements of mobile phone material recovery processes, Int. J. Life Cycle Assess., 2014, 1–13 Search PubMed.
- B. Sprecher, R. Kleijn and G. J. Kramer, Recycling potential of neodymium: the case of computer hard disk drives, Environ. Sci. Technol., 2014, 48(16), 9506–9513 CrossRef CAS PubMed.
- J. Wang and Z. Xu, Disposing and recycling waste printed circuit boards: Disconnecting, resource recovery, and pollution control, Environ. Sci. Technol., 2015, 49(2), 721–733 CrossRef CAS PubMed.
- X. Zeng, F. Wang, X. Sun and J. Li, Recycling indium from scraped glass of liquid crystal display: Process optimizing and mechanism exploring, ACS Sustainable Chem. Eng., 2015, 3(7), 1306–1312 CrossRef CAS.
- F. Yang, F. Kubota, Y. Baba, N. Kamiya and M. Goto, Selective extraction and recovery of rare earth metals from phosphor powders in waste fluorescent lamps using an ionic liquid system, J. Hazard. Mater., 2013, 254–255(1), 79–88 CrossRef CAS PubMed.
- M. S. Diallo, M. R. Kotte and M. Cho, Mining Critical Metals and Elements from Seawater: Opportunities and Challenges, Environ. Sci. Technol., 2015, 49, 9390–9399 CrossRef CAS PubMed.
- A. Caballero-Guzman, T. Sun and B. Nowack, Flows of engineered nanomaterials through the recycling process in Switzerland, Waste Manage., 2015, 36, 33–43 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6en00181e |
| This journal is © The Royal Society of Chemistry 2016 |