Carboxylation of multiwalled carbon nanotubes reduces their toxicity in primary human alveolar macrophages†
Abstract
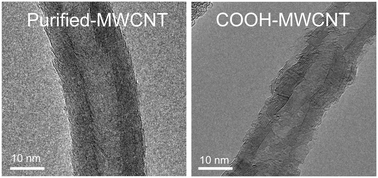
Surface functionalisation of multiwalled carbon nanotubes (MWCNT) is commonly used to facilitate their various and diverse applications. Inhaled nanomaterials, such as MWCNTs, have a high deposition rate in the alveolar units of the deep lung, where alveolar macrophages (AM) provide the front line of cellular immune defence by removing foreign matter (microbes, particles etc.). The toxicity of MWCNTs (with or without functionalisation) towards primary human AMs is not known. We investigated the physicochemical characteristics and toxicity of two MWCNT materials: acid purified ‘Purified-MWCNT’ and concentrated acid functionalised ‘COOH-MWCNT’. We hypothesised that the bioreactivity with primary human AM would differ between the materials. Full characterisation of the MWCNTs revealed that –COOH functionalisation yielded shorter MWCNTs, accompanied by a greater occurrence of framework defects, in comparison to Purified-MWCNT. In agreement with our hypothesis that the bioreactivity would differ, Purified-MWCNT were significantly more toxic as measured by reduced cell viability and increased inflammatory mediator release. For example, IL-1β and IL-8 release by AMs significantly increased 3.5- and 2.4-fold, respectively (P < 0.05), 24 hours after treatment with Purified-MWCNT. In contrast, IL-1β and IL-8 release by AMs did not significantly change 24 hours after treatment with COOH-MWCNT. We determined that the mechanism of this toxicity is likely due to activation of the inflammasome, as lipopolysaccharide priming of primary human AMs was necessary to see the inflammatory response and this was accompanied by lysosomal disruption and increased generation of reactive oxygen species. This study contributes further to our understanding of the effects of MWCNTs and surface modification on highly relevant human lung AMs; the findings have important implications for the manufacture, application and use of MWCNTs. In particular, this is relevant where applications prefer biocompatible MWCNTs.

 Please wait while we load your content...
Please wait while we load your content...