Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impacts of metal-based engineered nanomaterials on soil communities†
Moira S.
McKee
* and
Juliane
Filser
University of Bremen, UFT, General and Theoretical Ecology, Leobener Str., D-28359 Bremen, Germany. E-mail: moira.mckee@uni-bremen.de; Tel: +49 421 218 63472
First published on 28th April 2016
Abstract
As use and emission of metal-based engineered nanomaterials (MENM) is steadily increasing, concern of adverse effects on soil communities is rising. MENM are not only toxic to various organisms in soil, but can bioaccumulate, trophically transfer and even biomagnify in some systems. Negative effects of MENM on plant-fungi and plant-bacteria interactions have been shown in various studies, while further research on other forms of interactions (e.g. competition, predation) is needed to assess potential risks. Negative effects of MENM on nitrogen turnover and increased carbon emissions have been shown in numerous studies, and other biogeochemical cycles potentially at risk are addressed here. Most data to date has been collected on the consequences of MENM exposure for microorganisms and particle dependent changes in their community composition have been shown; data on other organism communities is however not available. In this review we summarize community interactions and soil ecosystem processes affected by MENM exposure and show how soil organisms influence MENM properties. Based on short- and long-term toxic effects, multiple inter- and intraspecific interactions and chemical processes we develop a conceptual framework. We postulate that cascading and potentially catalytic effects of MENM in soil might explain toxic effects at low concentration after longer exposure. Therefore, risk assessment of MENM relying solely on acute single species tests might be insufficient, and major research efforts are still needed in the area of soil communities and MENM exposure.
Nano impactMetal-based nanomaterials (MENM) emitted to the soil environment affect not only single species but rather the entire soil community and to date little is known on how interactions, community composition and ecosystem functions are impacted. This review summarizes findings and points out trends detected for most MENM: biogeochemical cycles (e.g. nitrogen) are affected negatively and carbon emissions increase because microorganisms involved and subsequent consumers are hampered; the ratio of bacteria to fungi may increase; trophic transfer and biomagnification within the food web are possible. Some of these strong effects were found at low concentrations that are environmentally relevant. Further research is needed to gain an understanding of the complexity of interactions, in particular on soil animals. |
Introduction
The applications of nanotechnology have been constantly increasing over the past decades, especially of metal-based engineered nanomaterials (MENM).1 OECD defines nanomaterial as material confined to 1–100 nm in one, two or three dimensions. This also includes material with internal or surface structure at this scale.2 Since a large variety of MENM products run a high risk of release into the environment, concern about potential negative impacts on the environment have been first raised 10 years ago.3 Since then, the number of publications dealing with this aspect has increased exponentially, although research into the technology and its applications clearly dominates.4Most authors have investigated the potential impact of MENM on organisms in the aquatic environment (e.g.ref. 5), whereas research on MENM risk for the terrestrial environment gained momentum only recently.6 Due to possible human exposure via food, MENM toxicity, uptake and accumulation in plants have received considerable attention.6,7 Far less is known about the site from which plants get their water, nutrients and find hold; namely the soil. This is astounding since soil is the environmental compartment where the majority of MENM will end up, via atmospheric deposition, inundation from rivers, sewage sludge application in agriculture, waste disposal and targeted application of locally huge amounts of iron-based MENM for remediation purposes.8–10 Although the quantity of MENM currently reaching the soil is relatively small, production volumes are steadily increasing.1,11 This increase could become dramatic due to the amount of research activity, especially in developing countries, into directly applying MENM in agriculture.12–15 Thus it is all the more important to explore whether MENM could compromise soil organism communities16 and the important functions maintained by them.
The vast majority of studies on the environmental impact of MENM in soils has been restricted to single-species tests and ecosystem processes17 or has dealt with abiotic processes such as dissolution, speciation, sorption or transport.18,19 Only recently soil microbial community structure came into focus, e.g.ref. 20 and 21. Some studies have also examined the effects of size and coating of MENM on their behaviour and bioavailability in soil.19 Tourinho et al.18 reviewed the fate, behaviour and effects of metal-based MENM on invertebrates in soil. The 28 studies on invertebrates compiled by these authors address only a handful of different metals or oxides and few standard test species (earthworms, nematodes and collembola) plus one isopod species. Studied endpoints were usually survival, growth and reproduction, in single cases gene expression. Community testing with various trophic levels in soil ecotoxicology has only recently gained increased attention,22–24 but is becoming increasingly important for environmental risk assessment and regulation,25 in order to meet the protection goal biodiversity. During the past decades a large body of evidence on the importance of biodiversity for the functioning of all kinds of different ecosystems has been collected (see e.g.ref. 26 for a recent review). For instance, merely by manipulating tiny soil organisms such as bacteria, fungi, or nematodes by size (using filters for the inoculum of grassland mesocosms), Wagg et al.27 demonstrated how increasing soil biodiversity positively affected multiple ecosystem functions such as plant productivity and diversity, nutrient retention or N2O release.
Navarro et al.28 were among the first to point out the relevance of interactions with both the abiotic and the biotic environment for MENM bioavailability and toxicity. In this review we focus on research on the impact of MENM on soil communities, biotic interactions in these and associated ecosystem processes. Predicted environmental concentrations of MENM are shortly summarized before briefly introducing the different trophic levels in soil. The topics of bioaccumulation and -magnification, community interactions, soil biodiversity and ecosystem functioning in relation to MENM are then discussed in more detail. We also raise some methodological points and summarize how soil organisms change their environment and thus MENM properties. Finally, we come up with a longer synthesis in which we put up a hypothetical worst-case scenario that delivers community-based explanations for negative effects of MENM at realistic, low concentrations.
Methods
To find scientific papers related to the topic of this review, various search requests were run on the Web of Science™ (Thomson Reuters™) in October 2015. Each search included soil* and nanoparticle* OR nanomaterial* combined individually with the following list of terms: parasite*, predator*, mutualis*, competit*, interaction*, herbivor*, symbio*, infochemical*, function*, communication, pheromone*, biodiversity, trophic level, antibiotic*, allelopath*. It became clear that these search terms did not adequately cover literature on isopods, therefore the main search terms were additionally combined with isopod*. Wildcards were used to maximize the search output. After reading the abstracts of the search results, the relevant papers were chosen and studied in more depth. If these papers included references to other papers important for the topic of the present review, these were additionally included. Further articles were retrieved from literature in our own files and by following selected links offered via research platforms such as ScienceDirect, ResearchGate and Google Scholar.We set up a scheme to rate the quality of original papers based on particle characterization. Papers including basic information on the origin (producer, synthesis), chemistry (core material), size (nominal, DLS, UV-vis, filtration/centrifugation, REM, TEM) and surface properties (coating/dispersant) of the particles and the used control (zero, salt, bulk, dispersant) were selected to ensure citing only papers of high quality. For details refer to the ESI.†
Rather than focusing on a specific group of organisms in the soil community, the goal of this review is to discuss data of MENM effects on several groups, especially various forms of interactions among them, and biodiversity. As our search revealed that published evidence thus far has a strong bias towards soil microorganisms we here present only a selection of studies on these and point out the gaps. Our analysis is supported by relevant examples from soil ecology, ecotoxicology and ecosystem research.
In the following, abbreviations for the different types of engineered nanoparticles (NP) and nanomaterials are used: Ag – silver; AgS2 – silver sulphide; Al2O3 – aluminium oxide; Au – gold; CdSe QD – cadmium selenide quantum dots; CdTe – cadmium tellur; CdZnS – cadmium zinc sulphide; CeO2 – cerium oxide; Cu – copper; CuCO3 – copper carbonate; CuO – copper oxide; Fe3O4 – magnetite; FePt – iron platinum; IONP – iron oxide; La2O3 – lanthanum oxide; Mn – manganese; nZVI – nanosized zero valent iron; SnO2 – tin dioxide; TiO2 – titanium oxide; WO3 – tungsten oxide; ZnO – zinc oxide.
Environmental concentrations of MENM
Currently it is not possible to measure MENM concentrations in the environment due to analytical restrictions, so modelling predicted environmental concentrations (PEC) is used instead. Keller and Lazareva29 estimated global mass flow of the MENM with the largest production volumes. SiO2 has the largest global production volume followed by TiO2, Fe, ZnO and Al2O3, Ag and CeO2 and Cu. Based on these and other factors, such as the products MENM are incorporated into, it is predicted that 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 metric tons of these will end up in the soil compartment.29 The estimated primary recipient compartment for photostable and photocatalytic TiO2, CuCO3, Ag and CeO2 will be the soil, receiving more than 50% of the release.30 Due to population size and density, the release of MENM to the environment is highest in Asia, followed by Europe and then North America.29
600 metric tons of these will end up in the soil compartment.29 The estimated primary recipient compartment for photostable and photocatalytic TiO2, CuCO3, Ag and CeO2 will be the soil, receiving more than 50% of the release.30 Due to population size and density, the release of MENM to the environment is highest in Asia, followed by Europe and then North America.29
Gottschalk et al.31 focused on modelling PEC for Europe and the US and their findings for TiO2, Ag and ZnO are summarized in Table 1. They predict the highest increase per year for TiO2 both in untreated and sludge treated soil and estimate that concentrations in the soil for all three MENM will steadily rise due to increase in production and use. A higher worldwide yearly increase of AgNP in sewage sludge-treated soil was calculated by Massarsky et al.32 (1.407–6.36 μg kg−1 a−1) and they also predict the yearly increase to rise with production volume. It is interesting to model not only potential concentrations of MENM in sewage-treated soil but also natural soil and agricultural soil to which MENMs are not directly applied. The predictions for these are also summarized in Table 1 and they show that also soils without sewage sludge input experience an increase in MENM concentrations.30 This is important to consider when evaluating the potential hazards of MENM to soil communities because it shows that not only agricultural soil communities might be influenced. Gottschalk et al.30 calculated concentrations of MENM that accumulated in environmental compartments between 2000 and 2014 while Gottschalk et al.31 estimated increases in concentrations per year. The authors of the first paper point out that ZnONP and AgNP are almost completely removed or transformed in WWTP and this model specifically calculates concentrations of MENM forms. Because no data on the transformation processes is available for CuCO3 the environmental concentrations of this MENM might be overestimated.30 There are not only models estimating the continental and regional concentrations of MENM but Keller & Lazareva29 also produced local data for the San Francisco Bay area. Biosolid concentrations from WWTP are predicted to be 10–170 mg TiO2NP kg−1, 1–8 mg AgNP kg−1, 10–80 mg ZnONP kg−1 and 10−6 mg CeO2NP kg−1 (Table 1). This results in a yearly release of 10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg TiO2 in the San Francisco Bay area.
000 kg TiO2 in the San Francisco Bay area.
| ENM | Region | PEC in soil | PEC in sludge-treated soil | PEC in sewage treatment sludge |
|---|---|---|---|---|
| a Photostable and photocatalytic TiO2 not separated. b Agricultural soil. c Cu not CuCO3. d Natural soil. | ||||
| Ag | Denmark | 13–61 ng kg−1d (ref. 30) | 20–350 ng kg−1 (ref. 30) | 4.2–250 μg kg−1 (ref. 30) |
| 6–21 ng kg−1b (ref. 30) | ||||
| Europe | 17.4–58.7 Δ ng kg−1 a−1 (ref. 31) | 1209–4091 Δ ng kg−1 a−1 (ref. 31) | 1.31–4.44 mg kg−1 (ref. 31) | |
| San Francisco Bay | NA | NA | 1–8 mg kg−1 (ref. 29) | |
| USA | 6.6–29.8 Δ ng kg−1 a−1 (ref. 31) | 526–2380 Δ ng kg−1 a−1 (ref. 31) | 1.29–5.86 mg kg−1 (ref. 31) | |
| Worldwide | NA | 1.407–6.36 μg kg−1 a−1 (ref. 32) | NA | |
| CeO2 | Denmark | 24–1500 ng kg−1d (ref. 30) | 94–5100 ng kg−1 (ref. 30) | 44–2300 μg kg−1 (ref. 30) |
| 10–530 ng kg−1b (ref. 30) | ||||
| Europe | NA | NA | NA | |
| San Francisco Bay | NA | NA | 10−6 mg kg−1 (ref. 29) | |
| USA | NA | NA | NA | |
| CuCO3 | Denmark | 39–130 μg kg−1d (ref. 30) | 32–70 μg kg−1 (ref. 30) | 5.2–17 mg kg−1 (ref. 30) |
| 18–41 μg kg−1b | ||||
| Europe | NA | NA | NA | |
| San Francisco Bay | NA | NA | 0.01–0.9 mg kg−1c (ref. 29) | |
| USA | NA | NA | NA | |
| TiO2a | Denmark | NA | NA | NA |
| Europe | 1.01–4.45 Δ μg kg−1 a−1 (ref. 31) | 70.6–310 Δ μg kg−1 a−1 (ref. 31) | 100–433 mg kg−1 (ref. 30) | |
| San Francisco Bay | NA | NA | 10–70 mg kg−1 (ref. 29) | |
| USA | 0.43–2.13 Δ μg kg−1 a−1 (ref. 31) | 34.5–170 Δ μg kg−1 a−1 (ref. 31) | 107–523 mg kg−1 (ref. 31) | |
| Photocatalytic TiO2 | Denmark | 0.2–4.9 μg kg−1d (ref. 30) | 17–480 μg kg−1 (ref. 30) | 9.3–230 mg kg−1 (ref. 30) |
| 0.1–1.7 μg kg−1b (ref. 30) | ||||
| Europe | NA | NA | NA | |
| USA | NA | NA | NA | |
| Photostable TiO2 | Denmark | 0.024–1.1 μg kg−1d (ref. 30) | 130–3100 μg kg−1 (ref. 30) | 69–1500 mg kg−1 (ref. 30) |
| 0.01–0.39 μg kg−1b (ref. 30) | ||||
| Europe | NA | NA | NA | |
| USA | NA | NA | NA | |
| ZnO | Denmark | 0.018–0.9 μg kg−1d (ref. 30) | 0 (ref. 30) | NA |
| 0.008–0.35 μg kg−1b (ref. 30) | ||||
| Europe | 0.085–0.661 Δ μg kg−1 a−1 (ref. 31) | 2.98–23.1 Δ μg kg−1 a−1 (ref. 30) | 13.6–57 mg kg−1 (ref. 31) | |
| San Francisco Bay | NA | NA | 10–80 mg kg−1 (ref. 29) | |
| USA | 0.041–0.274 Δ μg kg−1 a−1 (ref. 31) | 1.62–10.9 Δ μg −1 kg a−1 (ref. 30) | 17.4–57.7 mg kg−1 (ref. 31) | |
When considering any predicted environmental concentrations it is important to bear in mind that every model has its limitations. Many of the studies reviewed here examined much higher concentrations than currently estimated for soils and therefore test conditions not found in the environment; however concentrations in all environmental compartments are expected to increase. The use of higher concentrations in ecotoxicological tests than in the environment is common practice for various types of chemicals and is not unique to MENM.33
Trophic levels in soil
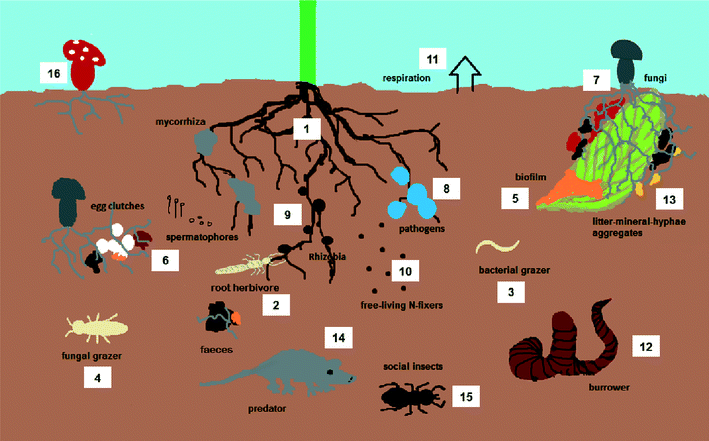
A soil community is composed of several trophic levels that are all interconnected and therefore strongly influence one another and together make up a complex food web (Fig. 1). The primary producers of biomass are mainly plants which root into the soil habitat. Roots and their exudates are important food sources for many primary consumers (bacteria, fungi and herbivores). Plants also produce litter that is decomposed by litter feeding animals and microorganisms, producing soil organic matter and releasing nutrients to the soil. Examples of animal decomposers are earthworms, collembola and isopods that greatly differ among and between the groups in life history traits. Predators such as coleopteran larvae, millipedes, spiders and some acari prey on the grazers and other decomposers and are themselves consumed by higher trophic levels like birds, amphibians, small mammals and top predators aboveground.The soil environment consists of three phases: the solid phase (minerals and organic matter), the liquid phase (soil solution) and the gaseous phase (soil air). Within these, several microhabitats (spheres according to ref. 34) can be distinguished that clearly differ both in physicochemical properties and in their associated organism communities. For instance, the detritusphere consists of more or less decomposed dead organic matter, has a low bulk density and is inhabited by litter dwellers such as fungi or isopods whereas the aggregatusphere deeper in the soil is characterized by mostly minerals, bacteria, protozoa and nematodes living in the water film surrounding soil aggregates.34 Evidently such different conditions (and exposure routes) will mean pronounced differences in both fate and effects of MENM. As previously described, MENM are released to soil habitats via various pathways and there can affect each exposure route and component of the food web. Numerous studies have been conducted to assess the effects various MENM have on soil organisms.
Plants
As primary producers, plants are key for any community to function as they are responsible to transforming solar energy into carbohydrates that can be used by other trophic groups. Human food supply is largely based directly and indirectly on plants and we therefore generally have great interest in ensuring suitable conditions for plants to thrive. Plant roots prevent erosion, enhance soil structure and give off exudates. Their rhizosphere offers habitat to many soil organisms which have a large impact on plant growth.35 If this trophic group is harmed it influences all other trophic groups. As an emerging group of chemicals that is being increasingly used in fertilizers and pesticides for agriculture15,36 MENM have been tested on their effects on various plants.Both positive and negative effects were found, depending on plant species, particle properties, soil and test conditions. A review by Gardea-Torresdey et al.37 summarizes some of the findings of full-life cycle and long-term studies (≥4 weeks) with plants and MENM. 28 studies were conducted under laboratory conditions with various growth media; two field studies are, however, also included. We present the results from nine of these studies here. Concentrations between 500 and 3000 mg AgNP kg−1 increased fruit yield in cucumber and 20 to 60 mg kg−1 increased the seed yield of borage. However AgNP (100, 1000 mg kg−1) reduced chlorophyll content and increased superoxide dismutase activity in tomato. AuNP caused higher seed yield (25 mg kg−1) while at the same time amplifying oxidative stress (≥10 mg kg−1) in Brassica juncea. Only one reviewed study showed positive effects of ZnONP on crop plants. ZnONP amplified pod yield of peanuts (133 mg kg−1) while it reduced biomass in wheat (45 mg kg−1), decreased chlorophyll and caused oxidative stress in green peas (125–500 mg kg−1).38 Cowpeas either showed reduced growth or no physiological effects in the presence of ZnONP (500 mg kg−1) depending on particle size and application type. TiO2NP lead to increased superoxide dismutase activity in tomatoes (50–5000 mg kg−1).37 In natural soil, TiO2NP (40–60 nm, mainly anatase) at < 200 mg kg−1 fresh soil did not affect growth or nutrient content of maize and soybean.39 The fruit weight of tomatoes rose when CeO2NP (1.3–130 mg kg−1) was applied; while shoot growth of soybean decreased (100–1000 mg kg−1). IONP increased grain yield in soybean (250–750 mg kg−1). Mostly negative effects on the growth and physiology of crop plants in the reviewed papers were caused especially by AgNP and ZnONP.37
Another review summarizes that AgNP, ZnONP and CuONP caused both positive and negative effects on crop plants while CeO2NP had more subtle effects on the physiological level.16 Almost all studies reviewed by Gardea-Torresdey et al.37 and Dimkpa16 focus on crop plants, which are in most cases only grown until harvest and therefore only exposed to MENM for a relatively short period. Colman et al.40 examined the effects of AgNP on five meadow plants in a mesocosm for 50 days and detected a biomass decrease for Microstegium vimineum. All plant species generally showed stronger root growth in the top soil layer; 0.14 mg Ag kg−1 in form of AgNP had been applied to the soil as biosolid slurry.40 The exposure period for meadows and permanent crops such as fruit, wine or asparagus would however be much longer, and also exposure of adjacent unmanaged land via surface runoff or wind must be taken into consideration. Therefore more studies of potential chronic effects are needed. In summary, no overall statement on the sensitivity or robustness of plants as a trophic group to MENM is possible due to the great variety of impacts MENM have.
Microorganisms
At the base of the soil food web are microorganisms – archaea, bacteria and fungi exploiting energy either from inorganic or, primarily, from organic sources, mostly plant roots, their exudates and litter. By decomposing organic material, providing minerals, fixing atmospheric nitrogen, yet also attacking living organisms (pathogens and nematode-trapping fungi) they play a prime role in any soil, especially for plant growth. The microbial biomass serves as food for soil animals (see consumers below). Fungi are important in first colonizing leaf litter and for mineralization of e.g. carbon.41Bacteria are generally considered a trophic group at risk of MENM because several metal-based MENM, particularly Ag- and Cu-based NP, exhibit antimicrobial characteristics.16,42–44 These effects differ between metals and are not necessarily negative, and they may be nano-specific. For instance, AgNP were twice as toxic for microbial growth than Ag+ ions45 (see Table 3 for details). In their review on microbial toxicity of ENM, Suresh et al.46 concluded that parent material, particle size and shape could be related to bacterial toxicity (mostly pure cultures of single species) whereas no general conclusions can be made for the respective coating. They described toxic effects for Ag, Al2O3, TiO2, CeO2, CuO, CdSe, CdTe, FePt and ZnO NP whereas studies with Si, Fe, Au, Pd, Ag2S and Pt NP frequently found no or only little effect. Yet, these effects were not always consistent, depending on particle configuration and coating: for instance, Ag-oleate (4 nm) and Ag2S NP (2–20 nm) with protein coating were non-inhibitory.46 However, biogenically prepared AgNP with a protein/peptide coating were more toxic than chemically prepared AgNP.46 Upon comparing the toxicity of 0–200 mmol g−1 CuONP (40–80 nm) and ZnONP (20 nm) for microbial soil communities to their bulk and ionic counterparts, Rousk et al.47 related the toxicity of both to dissolved ions. The initial inhibitory effect of agglomerated CuONP (primary size <50 nm; 200 mg L−1) on the growth of Pseudomonas chlororaphis was not seen any more after 72 h, which was not the case for agglomerated ZnONP (primary size <100 nm; 500 mg L−1).48 Exposure of the bacteria to the corresponding ions (at 2 and 5 mg L−1) had a similar effect in this study for Cu2+ but not for Zn2+. In his review, Dimkpa16 concluded that soil microbial communities in general are either “decimated or modified” by CuONP, ZnONP, CeO2NP, Fe3O4NP, SnO2NP and AgNP. The most recent review on this topic comes to similar conclusions for AgNP, CuONP and ZnONP but not for Fe3O4NP.43 These authors also report negative effects of nZVI and TiO2NP in certain soils and point out more research need for most MENM.
Due to their different cell wall structure, Gram-negative bacteria are often less sensitive towards MENM than Gram-positive ones, which has intensively been studied in medical research.44,46 However, this is not universally true. For instance, CuNP have a negative impact on both groups, and in one study with kill-time experiments Gram-negative strains were more susceptible.44 AgNP are more effective against Gram-negative bacteria than Gram-positive: e.g., various types of fungal-produced AgNP (5–56 nm) completely inhibited Pseudomonas putida at 0.8 mg L−1.49,50 Pawlett et al.51 report bactericidal effects of nZVI on Gram-positive bacteria in soils. Premanathan et al.52 showed higher sensitivity of Gram-positive bacteria towards ZnONP. Thus, generalizations are difficult, and given the huge bacterial diversity in soils53 we do not consider this morphological distinction too informative in an ecosystem context.
Ecologically more relevant would be a shift in the ratio of bacteria to fungi as the latter are able to degrade more complex organic compounds, including anthropogenic pollutants. If this takes place in the presence of MENM is not clear, as to date our knowledge on effects of MENM on soil fungi and Archaea is very limited.43,54 The generally higher metal susceptibility of bacteria due to their prokaryotic nature49 is supported by a study with mineral silver compounds including metal-accumulating fungi.55 On the other hand, MENM are also highly efficient fungicides.56 MENM effects on microbial community structure and ecosystem functioning will be discussed in more detail later on.
Consumers
This trophic group is composed of a huge variety of different life forms and taxonomic groups that all have in common that they feed on living and dead organic material, this way contributing to decomposition and nutrient cycling. Within this group collembola, acari (oribatida), myriapoda, insecta, isopoda and oligochaeta are important representatives that make major contributions both directly and indirectly to preventing litter build-up and ensuring soil fertility.57–59 Many litter feeding invertebrates are important for forming pore structures in soils. This increases water holding capacity, aeration and root penetration.59 Earthworms are regarded as “ecosystem engineers” and they are essential for loosening the soil and moving organic plant detritus to deeper soil layers.60 After protozoa and nematodes, collembola and acari are the detritivores with the highest abundances found in soil and they play a key role in breaking up litter and this way making it available for microorganisms to further decompose it and mineralize nutrients. They also stimulate the growth of fungi by grazing on them.59 Bacterivorous invertebrates regulate and stimulate microorganisms involved in nutrient cycling. Due to their life form below ground or in close association with soil, primary consumers are exposed to toxins mainly via dermal contact with contaminated soil and uptake from food.57,58 Because of their important role in the soil community litter decomposing invertebrates should receive considerable attention in the risk assessment of metal-based NP as a loss of the functions of this trophic group can both directly and indirectly negatively affect many processes in soils. However, compared to the number of studies on plants and microorganisms published to date, only few studies have examined the effects of MENM on litter decomposing invertebrates. A search on Web of Science™ in November 2015 rendered 369 articles for plants, 124 for microorganisms and 34 for invertebrates when combined with soil* and (nanoparticle* OR nanomaterial*).Tourinho et al.18 recently reviewed effects of MENM on soil invertebrates. They report toxic thresholds of Ag, Al2O3, Au, CeO2, Cu, TiO2 and ZnO NP tested with representatives of invertebrate groups (nematodes, earthworms, enchytraeids and collembola), yet also point out more research need in this area. Table 2 gives an overview of the papers published on MENM effects on soil invertebrates since Tourinho et al.'s18 review from 2012.
| Particle 38 | Size | Organism | Concentration range | Exposure media | Duration | Endpoints | Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ag | 3–8 nm paraffin coated | Folsomia candida | Toxicity: 850 mg kg−1 AgNP, 50–380 mg kg−1 AgNO3; toxicokinetics: 168 mg kg−1 AgNP, 30–60 mg kg−1 AgNO3 | Lufa 2.2 soil | Toxicity: 28 days; toxicokinetics: 14 days exposure + 14 days elimination | Reproduction, survival, internal concentrations | AgNP-no effects on reproduction, survival, eliminated faster than AgNO3; AgNO3-reduced survival, reproduction | Waalewijn-Kool et al. 2014100 |
| Ag | 3–8 nm alkane coated | Porcellionides prunosus | 1–800 mg kg−1 | Lufa 2.2; spiked alder leaves | Avoidance: 48 hours; feeding inhibition: 14 days | Avoidance; biomass, internal concentrations | Avoidance of NP and AgNO3; more biomass loss with AgNO3 than NP | Tourinho et al. 201582 |
| Ag | 3–8 nm alkane coated | Porcellionides prunosus | 30–4499 mg kg−1 | Lufa 2.2 soil; spiked alder leaves | 21 days exposure + 21 days elimination phase | Biomass, internal concentration | Ag accumulation from both forms | Tourinho et al. 2016102 |
| Ag | 15 nm | Eisenia andrei | 60–200 mg kg−1 (test 1) 15–200 mg kg−1 (test 2) | RefeSol 01A soil | 28 days adult exposure + 28 days cocoon incubation | Reproduction | Dose-dependent reproduction decrease; AgNO3 twice as toxic as AgNP | Schlich et al. 201374 |
| Ag | 15 nm | Lumbricus rubellus | 1.5–154 mg kg −1 | 4 weeks;10 months (chronic) | Weight gain, number of cocoons, juvenile survival; histological observations, modelled population growth | Reduced weight gain, produced cocoons, juvenile survival and population growth rate | van der Ploeg et al. 201476 | |
| Ag | 30–50 nm PVP coated | Enchytraeus albidus | 100–1000 mg kg−1 | OECD soil | 6 weeks | Survival, reproduction, gene expression | Reduced reproduction, AgNO3 more toxic than AgNP | Gomes et al. 201378 |
| Ag | 50 nm PVP coated | Eisenia fetida | 18–1758 mg kg−1 | Lufa 2.2 soil | 28 days adult exposure + 4 weeks cocoon incubation | Survival reproduction gene expression | Reduced reproduction; transcription related to endocytosis and cilia different than in AgNO3 | Novo et al. 201570 |
| CeO2 | 10–50 nm | Porcellionides pruinosus; Folsomia candida | 10–1000 mg kg−1 | Lufa 2.2 soil | P. pruninosus: 14 days; F. candida: 28 days | P. pruninosus: survival, food consumption, biomass; F. candida: survival, reproduction | Both species: no effects | Tourinho et al. 201583 |
| Cu | <50 nm oxide coated | Porcellio scaber | 2000–5000 mg kg−1 | Spiked hazel leaves | 14 day exposure + 14 days elimination phase | Food assimilation efficiency, internal concentrations | Highest Cu concentrations in digestive system, similar uptake of Cu from NP and salt | Golobič et al. 2012103 |
| Cu | 66–80 nm; 419 nm agglomerates | Enchytraeus albidus | 30–1030 mg kg−1 | Field soil | 48 h (avoidance) and 42 days (reproduction) | Avoidance, survival, reproduction | Survival reduced >600 mg kg−1, dose-dependent reduced reproduction, CuNP avoidance even at low concentrations | Amorim & Scott-Fordsmand 201279 |
| nZVI | <50 nm sodium polyacrylic acid coated | Caenorhabditis elegans | 10% | Soil (ISO 10872) Pb and Zn contaminated, moistened with M9 medium | 96 h | Survival, growth | Decreased growth in Pb & NZVI-treated soil; Zn toxicity reduced when NZVI treated | Fajardo et al. 201565 |
| nZVI | <50 nm | Caenorhabditis elegans | 0.5–10 mg mL−1 (in vitro) 17 mg g−1 (soil test) | Soil (ISO 10872), moistened with M9 medium; Lufa 2.2, Lufa 2.4 (soil test) | 96 h (growth, survival) 72 h (reproduction); 7 days (soil test) | Growth, survival, reproduction | Reduced growth, survival and reproduction; increased growth and reproduction (soil test) | Saccá et al. 201464 |
| TiO2 | <25 nm | Caenorhabditis elegans | 7.7 and 38.5 μg mL−1 | MilliQ water; nematode growth medium | 24 h (MilliQ water); 5 days (reproduction test) | Metabolites, ROS production; reproduction | Nine metabolic pathways affected; ROS production increased; reproduction reduced | Ratnasekhar et al. 201563 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1), however not in a dose response manner. In a 4 week test with artificial soil E. fetida's cocoon production decreased stronger in the ZnONP treatment, reaching zero at 1000 mg kg−1; weight loss was also observed.71 Seven day exposure to ZnONP and TiO2NP in concentrations above 1 g kg−1 negatively affected E. fetida's activities of antioxidant enzymes and cellulose, and damaged DNA and mitochondria of gut cells.72 Al2O3NP caused reduced cocoon production above 3000 mg kg−1 in a 28 day test with E. fetida while this effect was not found with micro sized Al exposure. 5000 mg kg−1 and higher contamination with micro- and nano-sized Al lead to avoidance of this earthworm.73
000 mg kg−1), however not in a dose response manner. In a 4 week test with artificial soil E. fetida's cocoon production decreased stronger in the ZnONP treatment, reaching zero at 1000 mg kg−1; weight loss was also observed.71 Seven day exposure to ZnONP and TiO2NP in concentrations above 1 g kg−1 negatively affected E. fetida's activities of antioxidant enzymes and cellulose, and damaged DNA and mitochondria of gut cells.72 Al2O3NP caused reduced cocoon production above 3000 mg kg−1 in a 28 day test with E. fetida while this effect was not found with micro sized Al exposure. 5000 mg kg−1 and higher contamination with micro- and nano-sized Al lead to avoidance of this earthworm.73
E. andrei avoided soil contaminated with 120 and 200 mg AgNP kg−1 in a 28 day reproduction test. The reproduction was adversely affected in a dose–response manner by AgNP and the calculated EC50 value is 74.3–80 mg kg−1.74 In this test Schlich et al.74 also found that the silver concentration in this earthworms was higher when exposed to AgNP than to the same concentration of AgNO3 while AgNO3 was twice as toxic. TiO2NP were also avoided by the earthworm E. andrei in concentrations of 1000 mg kg−1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 while micro-sized TiO2 was not.75
000 mg kg−1 while micro-sized TiO2 was not.75
When the earthworm Lumbricus rubellus was exposed to 154 mg AgNP kg−1 for four weeks the weight gain and number of produced cocoons was significantly reduced.76 None of the juveniles produced survived when the adults were exposed to this AgNP concentration and even 15.4 mg kg−1 significantly reduced the juvenile survival. The long term effects of 15.4 mg AgNP kg−1 on this earthworm were worse than for the same concentration of Ag in the form of AgNO3. In a population model a significant decrease in population growth rate of L. rubellus was seen for all tested concentrations of AgNP (1.5–154 mg kg−1).76 TiO2 nanocomposites caused a significant increase in apoptosis of cuticle and intestinal epithelium cells at 100 mg L−1 in Lumbricus terrestris when exposed via water for seven days.77
Gomes et al.78 showed that the enchytraeid Enchytraeus albidus reproduces less at concentrations above 225 mg AgNP kg−1. At 100 and 200 mg kg−1 CuNP (66 nm) reduced the reproduction of E. albidus by more than 70% and 95%, respectively, while the corresponding salt concentrations only caused a reduction of about 20% and 45%.79 In this study, also avoidance towards CuNP was much more pronounced – the difference to CuCl2 only disappeared at 600 mg Cu kg−1 and higher.
So generally it appears that several litter feeding invertebrates are sensitive to MENM; however more research is needed to further validate this (see also the review by Tourinho et al.18). This is particularly important because many invertebrates play a vital role in organic matter breakdown. The effects of high MENM concentrations on earthworms have received most attention because they are well established test organisms and play key roles in soil communities. Tests with nematodes, collembola and enchytraeids, groups which also interact which various other soil organisms, have, in contrast, only been studied by few scientists and were confined to one single standard test species per group. Some groups such as soil insects and millipedes have not been studied up to now, however, play an important role in the soil community. All concentrations with effects on primary consumers presented here are higher than current modelled environmental concentrations of MENM (Table 1) which means to date it is not expected that such effects are found in the environment unless bioaccumulation occurs. However, ecotoxicological tests with higher concentrations give indications what effects are to be seen if release of MENM to the environment further increase as predicted. Below we will show that low, realistic MENM concentrations do raise concern when ecological interactions are taken into account.
MENM can pose a potential risk not only to trophic groups in soil but also to functional ones, therefore this is discussed when assessing effects of MENM on ecosystem functions.
Bioaccumulation and -magnification
Many persistent or non-degradable substances are found in higher concentrations in living organisms than in their environment. Such bioaccumulation occurs through adsorption at the organisms' surface or uptake via food or water, which often increases with increasing trophic level (biomagnification). Among the best known examples are plants: more than 1% of the shoot biomass of metal hyperaccumulators may consist of metals.85 Bioaccumulation in soil organisms has gained infamy with the Chernobyl disaster in 1986: especially fungi86 and lichens accumulated the radioactive 137Cs, posing a large risk to humans and animals consuming them. Numerous fungi are known to drastically enrich metal concentrations in their tissue even in unpolluted environment,55,87 and also for soil invertebrates considerable heavy metal accumulation has been reported.88 MENM therefore have bioaccumulation potentials which is highly relevant for hazard assessment.When MENM enter the environment it is seldom via a single input in time but rather a continuous or reoccurring application that can potentially lead to a long-term exposure and, therefore, accumulation of MENM in organisms.37 Even when soil concentrations are below the threshold for toxic effects, accumulation of MENM in the organisms can eventually lead to the build-up of MENM concentrations that cause adverse effects. Hou et al.17 define bioaccumulation as the uptake of a contaminant via food as well as through ambient sources. In soil systems uptake from food, pore water, soil gas and solid soil components is possible.17 Metal uptake from MENM in several forms (ionic and/or particulate) is possible depending on particle characteristics dissolution and aggregation behaviour, which vary with concentration. Based on this, Cornelis et al.19 surmised that ENM are bioavailable at low concentrations while uptake would decrease with increasing concentration. When measuring internal metal concentrations of organisms the physical form as well as the possibility of transformation of MENM after uptake should be taken into account.17 The question we ask in this section is, do any soil organisms specifically enrich MENM?
Bioaccumulation
At concentrations of 10 and 30 mg TiO2NP L−1 in a nutrient solution, wheat, beans and the wetland species Rumex crispus TiO2NP had significantly higher Ti concentrations in their roots than the control.92 Wheat accumulated 4.97 mg Ti g−1 dry weight and bean roots had a concentration of 1.46 mg g−1 d.w. after four weeks of exposure to 30 mg TiO2NP L−1. In R. crispus Ti taken up from the roots was translocated in significant concentrations to the shoot, reaching 0.215 μmol g−1 d.w. at 30 mg TiO2NP L−1 exposure.92 Accumulation of CdSe/CdZnS QDs in roots and root hairs of Arabidopsis thaliana grown in hydroponic cultures was shown at a concentration of 10 mg Cd L−1.93 Bean plants exposed to 100, 250 and 500 mg CuONP kg−1 soil accumulated Cu in the shoot in concentrations of 225, 131 and 125 mg kg−1 respectively.94 Cornelis et al.19 reviewed and summarised various other studies on uptake of MENM by plants, their focus however lies more on mechanistic aspects at the cellular level.
Even though plant species, particle characteristics and test conditions differed between studies, all the presented findings indicate that plants can take up and accumulate metals when exposed to metal-based MENM. As also the rhizosphere is an important food source for the entire soil food web35 and soil animals are preyed upon by many smaller vertebrates aboveground the risk of MENM entering the trophic chain via plant litter and living roots should not be underestimated. It is important to further deepen our understanding of the speciation of MENM taken up by plants and to assess whether the accumulated metals are passed on to other trophic levels.
Whiteside et al.98 detected that the soil fungi Penicillium solitum can accumulate CdSe QD conjugated to an amino acid; however without the amino acid no uptake of CdSeQD was seen. Also ectomycorrhizal fungi accumulate very high concentrations of bulk metals such as silver.55 In the 1980's it was detected that wood-decaying fungi accumulate several metals from the wood including Cd, Fe, Zn, Cu and Rb.99 This raises concern that bioaccumulation of metals from MENM is possible as well. The number of studies dealing with accumulation of MENM in fungi is very limited and further research is needed to assess whether MENM can enter the soil food web via fungi.
Data on bioaccumulation in soil invertebrates are still very scarce, yet the mentioned studies show that lower level trophic groups in soil can accumulate metals from MENM and, therefore, potentially pass them on to predators. As shown, many studies have detected the uptake and accumulation of metals from MENM, however the form of the metal (ionic or particulate) is often not clear due to methodological limitations. Knowing the form of a metal in the organism can be important in understanding how MENM cause toxicity and how they are bioaccumulated and are transferred to other trophic levels. More research in this field is needed to allow an adequate evaluation of the potential risks for the entire soil community posed by the exposure to MENM.
Trophic transfer
Unrine et al.104 examined the trophic transfer of AuNP (tannic aid capped, 12 nm) from Eisenia fetida to bullfrogs and detected bioaccumulation of Au, but not -magnification. The frogs were fed with earthworms for 14 days that had been exposed to 200 mg AuNP kg−1 contaminated soil for 60 days. Au concentrations were higher in the frogs when trophic transfer took place than when they were fed directly with AuNP via oral gavage. This indicates that Au from AuNP is more bioavailable through trophic transfer. In a test system including three trophic levels, zucchini exposed to 1228 μg CeO2NP g−1 soil (155 nm diameter) was fed to crickets and these were fed to wolf spiders. All three trophic levels showed elevated Ce levels. The accumulation of Ce was higher from NP than from the bulk material, however no biomagnification was detected. Instead a 2-order magnitude decrease of Ce concentration with each trophic transfer was observed.105 De la Torre Roche et al.106 exposed lettuce to 500 mg La2O3NP kg−1 soil and the plants took up La during the 50 day treatment; the uptake of La was higher from bulk La than from NP. When these two lettuce types were fed to crickets the uptake of La was significantly higher from bulk (0.53 mg kg−1) than from NP (0.33 mg kg−1) treated-lettuce. The same trend was found when the lettuce was consumed by darkling beetles. However elimination of La in the insects was slower from La2O3NP treated plants than from bulk La. When the crickets which had fed on contaminated lettuce were consumed by mantis the trophic transfer factor was higher for La2O3NP than for bulk La. No biomagnification took place in this system because the concentration of La in the mantises was 5–10 times lower than in the crickets.106 Intact CdSe/CdZnS quantum dots were taken up both by Arabidopsis plants and caterpillars feeding on these, causing damage in both. The uptake and degree of damage were strongly affected by the two different types of QD coating studied. Younger Arabidopsis leaves transported CdSe/CdZnS QD considerably faster than older ones,93 which demonstrates that life stage of an organism must not be ignored when it comes to the fate of MENM. Unlike the studies reviewed by Ma et al.,107 the trophic transfer factor of QD to faeces was only 0.28,93 which might hint at accumulation in the herbivore. When fed Rhicinus communis leaves surface-treated with either PVP-coated AgNP or AgNO3, two species of lepidoptera larvae excreted most silver through the faeces. Relative body uptake of Ag was higher for AgNO3 than for AgNP in Achaea janata while this was not so clear for Spodoptera litura. Accordingly, for A. janata Ag concentrations in faeces of AgNP-treated animals were mostly higher than in animals treated with AgNO3. However, the Ag concentrations tested in this study were extremely high (500–4000 mg L−1).108Judy et al.109 found that hornworms (Manduca sexta) can take up and accumulate Au from tobacco leaves that were previously sprayed with AuNP. Tannic acid coated AuNP were used in the diameters 5, 10 and 15 nm. The concentration of Au in the tissue of the hornworms was 6.2, 11.6 and 9.6 times higher than in the tobacco leaves, respectively, for the three particle sizes. This clearly shows that biomagnification takes place in this system. Using 3 kDa membranes as filters before performing the chemical analysis of the internal Au concentration of the hornworms indicate that Au was taken up in form of AuNP from the tobacco leaves. A similar experimental setup with AuNP sprayed tomato leaves and hornworms as primary consumers was also performed by Judy et al.110 The indirect trophic exposure of 12 nm diameter tannate coated AuNP to hornworms led to a bioaccumulation of AuNP, however lower than in the previous study and no biomagnification was observed. The elimination efficiency of AuNP was low after the gut of the hornworms was emptied.110 As noted before, citrate-capped, 5 nm diameter CdSe QD accumulate in Pseudomonas aeruginosa bacteria and, when the latter were consumed by the protozoa Tetrahymena thermophila, the QD were passed on to this trophic level within a 16 h exposure. X-ray spectroscopy showed that the CdSe QD were taken up by the protozoans in intact form. The trophic transfer factor, which is based on the ratio of metal mass to dry body mass, was about 5.4 for CdSe QD, indicating substantial biomagnification.97
These are the only studies on trophic transfer of MENM in soil systems we are aware of. More is known about the passing on of MENM from one trophic level to the next in aquatic settings. The nematode C. elegans does not only internalise citrate-coated AgNP (7 nm, 54 mg L−1) from its bacterial food but also transfers them to its offspring.66 In a simplified rice paddy microcosm, TiO2NP and 9 nm TiO2 nanotubes were taken up by water plant roots and transferred to nematodes and snails feeding on the roots. The highest Ti concentration was detected in a biofilm consumed by rice fish which also accumulated Ti.111
Due to limited data it is difficult to predict if, where and which MENM might bioaccumulate in natural soil communities. In their review on metal-based nanotoxicity in higher plants, Ma et al.107 identified major research gaps with respect to field experiments at realistic concentrations including implications to the food chain. They concluded that in the few studies thus far apparently no biomagnification of MENM occurred, yet (if measured) a high percentage was found in faeces. This implies a large exposure and bioaccumulation potential to the decomposer community. It is concerning that MENM can be transferred between trophic levels in nano form because MENM are specifically introduced to products due to their changed or new characteristics in this form. This hinders predictions based solely on previous experiences with metal accumulation from non-nano forms in food webs because of potential differences in behaviour and fate of the particles within organisms.18 Research in the area of MENM accumulation should be intensified to assess potential risks posed for soil food webs.
Community interactions
In addition to herbivory and predation, competition, mutualism, allelopathy, symbiosis and parasitism also play major roles in forming the dynamics of communities. When contaminants influence one or various organisms in a community, this can indirectly affect other species due to changes in interactions. To date little is known on how MENM influence soil community interactions; the focus of research has been mainly on plants and their interactions with fungi and bacteria in the soil. The latter has been summarized in a review by Dimkpa.16 Ma et al.107 provide a good overview on what is known thus far concerning other interactions. We therefore only report main conclusions, point out single aspects of some studies cited in these reviews and focus on literature not covered there.Plants and microorganisms
Clover (Trifolium repens) roots are mycorrhized by arbuscular mycorrhizal fungi (AMF) which increases nutrient uptake for the plant. The fungi are supplied with carbohydrates from the plant. In the presence of 3.2 mg IONP kg−1 soil the glomalin content and nutrient acquisition of AMF decreased, which led to a decrease in biomass of the mycorrhizal clover.54 A more complex reaction was seen when AgNP were in the soil; at low concentrations (0.01 mg kg−1) the growth of the mycorrhized clover was inhibited. At high AgNP concentrations (>0.1 mg kg−1) the ability of AMF to attenuate AgNP stress on the plant was enhanced and, therefore, Ag content and the activity of antioxidant enzymes were reduced. This suggests that the plant is harmed less by high AgNP concentrations because AMF buffer the negative effects above a certain AgNP threshold.54 In contrast, the presence of root-associated bacteria could not alleviate the negative effect of 500 mg Zn kg−1 in form of ZnONP on root growth of beans (Phaseolus vulgaris), and shoot growth was even only compromised in their presence. The authors showed that also the uptake of Fe and Mn in bean shoots was reduced by ZnONP when bacteria were present.112Nitrogen-fixing bacteria, which are symbionts of legumes, are also closely associated with plant roots. Legumes form nodules which are inhabited by nitrogen-fixing bacteria that in return supply the plant with nitrogen, which is often the limiting nutrient in plant growth. Rhizobium bacteria associated with Pisum sativum showed damaged membranes after 48 h exposure to 250 mg TiO2NP L−1 and nodule size was decreased after 7 day exposure. The pea plants treated with TiO2NP began nodule formation and N2 fixation later than the control plants.113 Exposure of the legume Pisum sativum to <500 mg L−1 ZnONP led to significantly shorter and fewer first- and second-order lateral roots compared to the control. The same was seen in the Zn2+ treatment. The legume was inoculated with the symbiosis partner Rhiziobium leguminosarum. When treated with 250 mg ZnONP L−1 rhizobia morphology changed from rod shaped to round cells. 750 mg ZnONP L−1 exposure led to damage or complete destruction of the bacteria cells. Exposure to equivalent Zn2+ concentrations also caused changes in morphology but no lysis occurred. TEM images showed that the size of nodules decreased with increasing ZnONP concentration, the infection of the nodules by rhizobia was delayed and nodule senescence was earlier. The bacteroid densities in the nodules and therefore the nitrogen fixation were lower than the control when treated with ZnONP.114 Dimkpa et al.94 detected an inhibition of bean shoot and root growth in presence of CuONP; however, when plant-associated bacteria were in the soil, the inhibition was reduced. The accumulation of Cu was also lower with bacteria in the soil. Dimkpa's16 review reports negative effects of AgNP, CeO2NP, CuONP, IONP, TiO2NP, WO3NP and ZnONP on nitrogen-fixing bacteria. There are, however, also cases where the opposite phenomenon was seen (see also mechanistic aspects below). For more information on how nitrogen fixation is influenced by MENM refer to “Ecosystem functioning – nitrogen turnover”.
Other interactions
Competition can also be indirectly affected by MENM; however to the best of our knowledge only one study has assessed how plant communities react to AgNP. Pure and mixed cultures of eleven wetland plants were sown out and the effects of 40 mg AgNP L−1 on the germination and early growth were observed. In the pure cultures AgNP increased germination of three species and reduced it in one plant species. The leaf length of six species decreased in the pure culture with AgNP treatment. When grown together, the germination effects were less pronounced and all but one taxa had reduced growth in presence of AgNP. Only Lolium muliflorum in the mixed culture treated with AgNP showed improved growth. The differences in reactions depending on culture type might be due to indirect AgNP effects mediated by altered species interactions. AgNP toxicity to several wetland plant species might have freed Lolium muliflorum from competition, causing improved growth.115In summary, most studies involving microorganisms reduced the toxicity of MENM to plants, whereas the presence of other plant species aggravated the toxic effects observed in the only study to date investigating this type of interactions. The ameliorating effect of soil microorganisms, however, does not seem to be nano-specific.16
Examining the effects MENM have on interactions between organisms in the soil community allows evaluation of more realistic scenarios than single species tests. Under natural conditions when species are integrated into a complex system of interactions (Fig. 1), toxins can cause effects that cannot be foreseen by evaluating toxicity to single species. As MENM in most cases will occur in very low concentration in the environment it is noteworthy that community reactions are differential and do not always follow a typical dose–response curve. In a study with AgNO3 and pulverized fruit bodies of a silver-accumulating fungus, bacteria and fungi showed an opposite reaction, irrespective of Ag source: relatively high doses (0.5 mg Ag kg−1) and the control favoured fungi and depauperated bacteria whereas the opposite was true at 0.008 mg Ag kg−1.55 Overall very little is known to date on how MENM affect interactions between various soil organisms and more research is needed, in particular on interactions with soil animals and their residues: excreta, faeces, dead bodies and egg clutches provide a lot of organic matter that is a very important food source for microorganisms.116 Recent experiments in our group have shown that MENM effects in single-species tests with collembola can be significantly aggravated in presence of interacting species (Hackmann, in preparation).
Mechanistic aspects
Mechanistic aspects of direct MENM action have been studied extensively at the beginning of MENM hazard assessment, especially in toxicology.117 These include, among others, interference with cell membranes, DNA replication or gene expression (see ref. 16 and 107 for examples in terrestrial microorganisms). Here we refer to the mechanisms of interactions.One important mechanism by which bacteria foster plant growth is the production of Fe(III) biochelators, siderophores. Dimkpa et al.48 showed that CuONP (200 mg Cu L−1) and ZnONP (500 mg Zn L−1) had completely different effects on the production of siderophores by Pseudomonas chlororaphis. Whereas ZnONP (although inhibiting bacterial growth) increased siderophore production, this was dramatically decreased by CuONP (which only initially inhibited bacterial growth). Effects of the corresponding ions were similar for Zn2+ but not for Cu2+. The effect could be explained by CuONP suppression of the transcription of a transport gene.48 CuONP increased the production of indole-3-acetic acid (IAA) and ZnONP increased siderophore production by bacteria – both components involved in plant growth promotion.16 In his review, Dimkpa16 provides some information on possible interference of these two types of MENM with other elements relevant for plant nutrition.
Hayashi and Engelmann118 reviewed the immune response of the earthworm Eisenia fetida, where coelomocytes play an important role, engulfing, for example bacteria. Based on studies with in vitro cultures of these cells with AgNP they suggest that coelomocytes seem to be a susceptible target of MENM. This is an example of one mode of action within an invertebrate – yet where could MENM interfere with community interactions in soils?
Chemistry is the key language of communication within and between organisms. An interference with substances relevant for chemical communication is especially relevant in a dark environment. Due to their high surface area and reactivity, MENM are prone to easily interfere with the countless small molecules involved in chemical communication.119 Such effects are known from in vitro cultures, for instance AgNP inhibited quorum sensing, biofilm formation and several factors involved in virulence.120 Such effects will not be limited to agar plates and organisms' cells but also occur in their environment – especially in soils, where orientation and communication are almost entirely based on chemical cues at often very low concentrations. Biofilms are involved in the formation of soil aggregates and an important food source for smaller soil fauna (protists, nematodes, microarthropods).35 What if MENM interfered also with their receptors? And what if MENM that are sorbed to mineral particles or organisms' surfaces bind the signalling molecules themselves, thus directly blocking their communication function? Would toxic fungi no longer be avoided or would ants and termites not find the way back to their mounds? Despite these obvious probabilities and potentially associated problems, based on our research virtually no literature on soils has dealt with this topic.
Soil biodiversity and ecosystem functioning
Soil communities are extremely abundant and diverse: 1 g of soil may contain thousands to ten thousands different taxa of microorganisms and small invertebrates such as protozoans, nematodes and microarthropods.27 Wardle et al.121 showed the multiple connections between these organisms and plants, underpinning the vital role soil organisms and their diversity play in ecosystem functioning. This has been demonstrated in countless studies since, reviewed for instance by Hooper et al.26 More recently, Lavelle et al.60 pointed out the relevance of soil invertebrates for ecosystem services. For an ecosystem to function properly and to deliver the ecosystem services it is essential that each functional group contributes to material and energy flows within the system. An understanding of whether and to what extent MENM might compromise soil biodiversity and ecosystem functioning is therefore urgently needed.Biodiversity and community structure
Effects on biodiversity can be expressed in various ways. The simplest approach is to count the number of taxa revealed, while keeping in mind the respective methodology (see methodological remarks below) – which is especially relevant in microbiology. Briefly, community analysis in microbiology is based either on physiological profiles (culture-dependent methods) or on culture-independent methods that are applied on soil extracts of either DNA or structural components such as fatty acids. Phospholipid fatty acids (PLFA) are important components of microorganisms' cell walls. Since they are only present in active microorganisms and a number of marker PLFA for specific groups has been found they are often used in soil community analysis (e.g.ref. 122). Soil invertebrates are usually identified by microscopy and morphological differences, more recently also by DNA-based methods.Aside from the number of taxa, community size (overall abundance or biomass), activity, composition (exchange of single taxa by others) and/or abundance of single taxa (shifts in dominance structure) can change. This may or may not have consequences on ecosystem functioning: on the one hand, redundancy in soil is high and omnivory widespread; on the other processes and subsequent cascades can be seriously affected. For instance, if ammonification is reduced, this will have negative effects on subsequent nitrifiers, plant growth and health, often also on aboveground herbivores.121
During 14 days of incubation the activity and community structure of methanogenic bacteria in anaerobic sewage sludge remained unaffected at concentrations up to 40 mg AgNP (29 nm) L−1, most likely due to the negligible dissolution of free Ag+ ions.123 However, AgNP-treated sludge aged in soil for up to 140 days became very toxic to bacteria at much lower concentrations11 (see also Table 3). At still lower concentration in a similar study, AgNP significantly altered microbial community structure and OTU richness only after one day, but not anymore after 50 days.40 The only study we are aware of that was conducted in a forest soil found a pronounced decrease of CNmic upon AgNP exposure for up to 90 days, strongly suggesting a higher sensitivity of bacteria95 (see also Table 3). This was confirmed by cultivable bacteria and fungi. DGGE profiles in this study rendered no impact of AgNP on overall genetic diversity of bacteria but rather distinct community shifts, also hinting on apparently more resilient taxa (Luteobacter rhizovicinos, Dyella sp., Edaphobacter modestus).95 Adding AgNP during the composting of municipal solid waste altered the structure of the highly diverse (more than 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 operational taxonomic units (OUT)) microbial community.124
000 operational taxonomic units (OUT)) microbial community.124
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cmic); qM – microbial quotient (Cmic
Cmic); qM – microbial quotient (Cmic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) total organic carbon); TMAH – tetramethylammonium hydroxide; Tween 20 – polyoxyethylene sorbitan monolaurate; NA – not available
total organic carbon); TMAH – tetramethylammonium hydroxide; Tween 20 – polyoxyethylene sorbitan monolaurate; NA – not available
| Process (specification) and effect (maximum significant or range) in % of control | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Particle type | Size primary (nm) | Coating/stabilizer | Concentration range) (mg kg dry mass−1 or L−1) | Control | N fix | N min | C flux | Other/specifications | Source |
| a Own measurements (Weis & Filser 2009; unpublished report). | |||||||||
| Ag | 9–21 | PVA | 3–26 | Zero (respiration) salt (nitrification) | Nitrification: −6–−63% | Nitrifyer respiration: −6–−63% | Choi & Hu 200845 | ||
| Ag | 10a | Traces of other elements and organic solventsa | 0.0032–0.32 | Zero | −13–−16 | Cmic: −14–−34 | Effects 120 d after application | Hänsch & Emmerling 20108 | |
| qCO2: +70–+89 | |||||||||
| Ag | 30 | Sodium citrate | 100–560 | Zero | Inhibition of AOB by stabilizer | García et al. 20129 | |||
| Ag | 29 | PVA | 10–40 | Zero | Methane production: 0 | Anaerobic sludge | Yang et al. 2012123 | ||
| Ag | 21 | PVP | 0.14 | Zero, slurry, salt (4 fold Ag concentration) all effects compared to slurry control | N2O emission on day 8: +350 | Aboveground plant biomass: 0; one plant species: −32 | Long-term field mesocosms, sludge application enzyme activity peptidase: −52, phosphatase: −27 | Colman et al. 201340 | |
| Cmic: −35 | |||||||||
| Ag | 15 | PGT + Tween 20 | 0.3–9 | Zero, salt | Nitrification (various endpoints):up to −51% | Cmic: up to −64 | Effect on respiration only after 3 h | Schlich et al. 201311 | |
| Ag | 1–20 | NA | 10 and 100 | Zero | Nmic: 0 | Cmic: −50 | Forest soil, 30–90 days | Carbone et al. 201495 | |
| qCO2: +100 | CNmic: +20 | ||||||||
| qM: ∼−70 | |||||||||
| Au | 20 | Sodium citrate | Zero | −13–−14 | 0 | García et al. 20129 | |||
| CeO2 | 8 | NA | 0–1000 | Zero | >−80 | Root nodule fixation potential | Priester et al. 201290 | ||
| CeO2 | 12 | HMTA | 30–640 | Zero | Up to −100 | Anaerobic biogas production: −90 | García et al. 20129 | ||
| aerobic respiration: up to −100 | |||||||||
| CeO2 | 50–105 | NA | 10 and 100 | Zero | qCO2: up to +60 | CNmic: varying effect, mostly decrease | Vittori Antisari et al. 2013134 | ||
| Cu | 25/NA | NA | 0.032–65.3 | 5 soils from a long-term trial with 3.8–86.2 mg Cu kg−1 | EC50 (Cmic): 0–+10 | Wakelin et al. 2014143 | |||
| CuO | 40 | — | 0–1000 | Zero | Cmic: +20–−43 | Urease: up to +71 | Xu et al. 201521 | ||
| PLFAtot: −20–−30 | Phosphatase: −28–−94 | ||||||||
| Dehydrogenase: −48–−97 | |||||||||
| IONP | 10.2–10.5 | Water | 420–1260 | Zero | Urease and invertase activity: up to ∼+12 | He et al. 2011127 | |||
| IONP | 25–46 | PVP | 0.1–100 | Zero | Anaerobic respiration in activated sludge: up to −87.5 | Dehydrogenase activity: up to −28 | Filser et al. 201310 | ||
| IONP (Fe3O4) | 20–30 | NA | 10 and 100 | Zero | qCO2: up to +118 | CNmic: mostly increase, sometimes no effect | Vittori Antisari et al. 2013134 | ||
| SiO2 | 80–100, amorphous | NA | 1 and 50 | Zero (activated sludge) | −33 at 50 mg L−1 | Activity of nitrite reductase: −20, nitrate reductase: −50 at 50 mg L−1 | Zheng et al. 2012126 | ||
| SnO2 | 61 | NA | 10 and 100 | Zero | qCO2: up to +109 | CNmic: mostly increase, sometimes no effect | Vittori Antisari et al. 2013134 | ||
| TiO2 (mainly anatase) | 10 | — | 1–150 | Zero | −100% at 1 mg per L | EC50 (GrR Anabaena variabilis): 0.15–13.98 | Cherchi & Gu 2010138 | ||
| TiO2 (anatase) | 70–90 | Water | 1–50 | Zero (activated sludge) | −70 at 50 mg L−1 | Anaerobic activated sewage sludge | Zheng et al. 2011144 | ||
| TiO2 (81% anatase) | 15–20/190–230 | Water | 0–2000 | Zero | Cmic: ∼−10–−20 | Ge et al. 2011137 | |||
| DNA: ∼−60 | |||||||||
| TiO2 | <100 | — | 0.001–10 | 0 | Allard et al. 2013174 | ||||
| TiO2 | 35 | — | 100–1000 | Zero | ∼−30–−50 | Various endpoints related to N fixation | Fan et al. 2014113 | ||
| TiO2 (80% anatase) | 21 | — | 1 and 500 | Zero | Cmic: −12–−22.7% at both concentrations in one out of six tested arable soils | Silty clay with high OM content | Simonin et al. 2015149 | ||
| TiO2 (anatase) | 20 | — | 0–1000 | Zero | Cmic: 0 | Urease: −70–−94 | Xu et al. 201521 | ||
| PLFAtot: −4–−6 | Phosphatase: up to −56 | ||||||||
| Dehydrogenase: 0–−70 | |||||||||
| TiO2 | 7.5 | TMAH | 560–1010 | Zero | 0 | Anaerobic biogas production: +10 | García et al. 20129 | ||
| WO3 | <100 | — | 0.001–10 | Zero/salt | −100% at 1 mg L−1 | Allard et al. 2013174 | |||
| ZnO | 10 | NA | 0–500 | Zero | 0 | Root nodule fixation potential | Priester et al. 201290 | ||
| ZnO | 20–30 | Water | 5–500 | Zero | Cmic: ∼−10–−20 | Ge et al. 2011137 | |||
| DNA: ∼−60 | |||||||||
| Mixture (Ag, ZnO, TiO2) | Ag: 52 ZnO: 30 TiO2: NA | Ag: PVP | Ag: 100 | Bulk/salt, zero | Nodulation frequency: −94 in MENM compared to bulk/salt | Cmic: −36 (MENM < bulk/salt) | Aged soil/sewage sludge | Judy et al. 201520 | |
| ZnO: none | ZnO: 1400 | Shoot BM: −23 (MENM < salt/bulk) | |||||||
| TiO2: 2400 | |||||||||
Xu et al.21 compared MENM at concentrations between 100 and 1000 mg kg soil−1 in flooded paddy soil to untreated controls. Based on PLFA patterns, CuONP were overall more toxic than TiO2NP, yet also these inhibited bacteria whereas fungi were not compromised by both MENM. When distinguishing aerobic and anaerobic bacteria, only CuONP had negative effects, and only these changed the overall community structure.21 In OECD soil incubated at 20 °C and 80% WHC with a 16 h light/8 h dark cycle for 30 days, gold nanorods (average size: 12.3 nm, 3.3 mg kg soil−1) significantly altered the bacterial community structure (based on DGGE profiling).125 This was also found for TiO2NP in the same study, yet at extremely high concentrations (5 g kg soil−1). Also exposing activated sludge for 70 days with 50 mg SiO2NP L−1 modified the microbial community, fostering e.g. Stenotrophomonas sp. and Rhodocyclaceae while reducing Thiotrix sp. and actinobacteria126 (Table 3).
nZVI (<50 nm, 3% sodium polyacrylic acid coating, 17 mg nZVI g−1) modified the phylogenetic microbial composition, and which taxa were affected varied with soil type.64 Fajardo et al.65 tested the same nZVI for the remediation of Pb- and Zn-polluted soils, which increased their Fe concentrations from 9.8 to 28.2 (Pb soil) and from 12.5 to 28.7 g kg soil−1 (Zn soil). Effects varied greatly with the type of pollution: no significant impact on the phylogenetic composition was found in the Zn soil whereas in the Pb soil β-proteobacteria increased from 7% to 21.8% while γ-proteobacteria decreased from 9.9% to 6.1%. Transcriptional biomarkers seemed more affected in Gram-negative than in Gram-positive bacteria in this study. Pawlett et al.51 found that nZVI reduced AMF fungi. Specific taxa were more sensitive in this study, responding to CuONP as well: at high concentrations (1%) proportions of some groups increased, for instance 1-bacillales. Interestingly, this group decreased upon CuONP exposure at just 0.1%. However, due to low replication and high variation these results should not be overrated.
IONP stimulated bacteria related to actinobacteria, and γ-Fe2O3-NP seemed to have a greater effect on bacterial community structure than Fe3O4NP127 (Table 3). In natural soil, TiO2NP (40–60 nm, mainly anatase) at <200 mg kg−1 fresh soil caused a clear structural shift in arbuscular mycorrhizal but not in bacterial community composition.39 Judy et al.20 compared aged soil amended with biosolids containing a mixture of Ag (100 mg kg−1), ZnO (1400 mg kg−1) and TiO2 (2400 mg kg −1) of either MENM or salts (Ag, Zn) or bulk material (TiO2) that had been continuously dosed at very realistic conditions, albeit ending up in rather high concentrations of the final mixtures (deviations from nominal concentrations in both treatments <10%). Based on PLFA patterns, both treatments had significant negative effects on anaerobic bacteria, Gram-positive bacteria and “actinomycetes”, which were more pronounced in the MENM than in the salt/bulk treatment for all groups except for the latter. Only the MENM treatment significantly reduced total biomass, Gram-negative bacteria, fungi and AM fungi whereas positive effects on fungi, AM fungi and eukaryotes were only found in the salt/bulk treatment.128 This study thus indicates a considerably higher hazard potential for MENM than for the bulk or ionic form of the three materials studied. Importantly, the authors stated that “operationally defined extraction methods used were unable to capture differences in bioavailability between the ENM and bulk/dissolved metal treatments”. The effect of ZnONP (29.8 nm, 2500 mg kg−1) was highly pH-dependent: its difference to bulk (300 nm) or salt (ZnCl) counterparts was more pronounced at lower pH.129 This was particularly apparent in Proteobacteria which were hardly affected by bulk or nano-ZnO at pH 7 but substantially reduced at lower pH. In turn, ZnCl changed the community composition towards Actinobacteria, almost unaffected by pH. Bacteroidetes largely increased at low and medium pH (6) in presence of bulk and nano ZnO, with ZnCl much less and only at low pH (4.8). More studies on MENM and microbial communities were summarized recently,43 see above under “Microorganisms”.
Overall, various MENM have caused alterations in microbial community structure. Changes in the community composition of soils upon environmental stress are not surprising, given the astronomical number of potential combinations in physiology and morphology in such extremely diverse systems. More interesting is the question which (combination of) trait(s) make taxa vulnerable or resistant to a given stressor. In bacteria, extracellular polymer substances (EPS) seem to play an important role, protecting them against toxicity of AgNP and CuONP.16,130 Using two strains of genetically engineered bacteria and a commercial polymer, Joshi et al.131 showed that EPS trapped AgNP outside the cells and also caused their aggregation in the medium. Their results were supported by measuring the dissolved Ag+ ions, which alone could not explain the observed toxicity. The authors remarked that differences in EPS characteristics may cause opposite results for aggregation and they pointed out that a reduced growth rate may also be considered a protective mechanism against AgNP toxicity in bacteria. A stimulation of EPS production by bacteria has also been shown for AgNP, ZnONP and Cu-doped TiO2NP.16
In their review on AgNP and microorganisms, Sweet and Singleton49 recommend that future studies focus on “biofilm communities as a more pertinent system when regarding food, medical and environmental systems” (see also ref. 120). They also demanded further attention be paid to the interactions between soil microorganisms and their invertebrate predators.
Ecosystem functioning
Frequently studied ecosystem functions are basic element cycles, in particular of carbon, nitrogen and phosphorous. Various standardized guidelines by OECD, ISO etc. have been performed for testing MENM. These guidelines are simple tests in which mineralization, e.g. release of carbon dioxide, nitrite or nitrate from entire communities, is measured. Another fairly simple approach is measuring activities of enzymes involved in organic matter breakdown and plant nutrition such as phosphatase, dehydrogenase or urease. Numerous studies (of which we show only a selection since this is not our focus) have shown negative impacts of nanoparticles on element mineralization and enzyme activities.11,16 These were mainly (but not exclusively, e.g.ref. 133) metal-based and soluble MENM, in particular Ag and Cu.Table 3 summarizes those studies referring to soil nutrient cycling which provided sufficient basic information on particle characterization and included at least a negative control, ideally also a salt or bulk control. Mostly negative effects of MENM were reported, yet for some endpoints or concentrations also neutral and positive effects were observed, even for otherwise toxic metals such as Ag or Cu. Positive effects are not necessarily beneficial but in most cases rather reflect a stress response such as increased growth, reproduction, moulting or metabolic activity (e.g.ref. 134) to dilute, excrete or detoxify the contaminants. This holds especially for the microbial metabolic quotient (MQ), the ratio between microbial respiration and microbial biomass, which often increased upon MENM exposure. One study with a positive effect of CuNP135 refers to the development of resistance: the particles were applied to a soil community adapted to Cu. “True” positive effects in Table 3 (e.g.ref. 127) mainly refer to iron-based MENM which are used for remediation purposes of contaminated soils. Note, however, that under both aerobic and anaerobic conditions Filser et al.10 found negative effects of IONP on bacteria, which increased with decreasing concentration. In their review, Simonin et al.43 raise concern also for nZVI.
In planktonic cultures, growth rates of nitrifying and denitrifying bacteria were much more sensitive to CuNP compared to when they were growing in biofilms, which was also reflected in ammonium oxidation and nitrate reduction. However, the opposite was found when measuring ATP contents in nitrifiers. In contrast, nitrogen fixing bacteria grew better in planktonic cultures than in biofilms. Dissolution behaviour, speciation and particularly the formation of an oxide layer at the NP surface strongly depended on the respective medium of the three different species of bacteria studied. Differences between the three species' reactions were also related to species traits such as speed of growth and nutrient requirements.140
In activated sewage sludge, nitrifying bacteria were compromised both in enzyme activity and abundance by SiO2NP, yet only at high concentration126 (Table 3). García et al.9 observed that CeO2NP and – considerably less – AuNP reduced the activity of ammonifying bacteria in a wastewater treatment plant, whereas no such effects were seen for TiO2NP and AgNP. AgNP, however, are inhibitory to the nitrifier Nitrosomonas europaea that oxidates ammonia.141 Choi and Hu45 (Table 3) found that the observed toxicity was best – even better than by Ag+ – explained by the AgNP fraction <5 nm. Dramatic effects on nitrifiers at rather low silver concentrations were found in various tests with long-term incubations (up to 180 d) of soil treated with sewage sludge containing AgNP – for instance, almost 100% inhibition after 140 d at 5.2 mg kg−1 dry soil.11 Colman et al.40 performed a realistic long-term field mesocosm experiment at a very low dose (final concentration 0.14 mg AgNP kg soil−1). A single application of AgNP-treated slurry resulted in a 4.5 fold increase of N2O emissions compared to the slurry only treatment, and 50 days after application the activity of the proteolytic enzyme peptidase was still 52% lower. Moreover, AgCl2 had similar or smaller effects, despite 4 fold Ag concentration.40 Still lower concentrations of AgNP (up to 100 μg Ag kg−1 dry soil) in an arable sandy loam soil significantly inhibited net N mineralization.8 Soil treated with aged sewage sludge (spiked with a mixture of Ag, ZnO and TiO2 in either MENM or bulk/ionic form, see Table 3) reduced nodulation frequency by 94% in the MENM compared to the salt/bulk treatment.20
Bacteria involved in the nitrogen cycle can, therefore, be considered a functional group that is specifically at risk of metal-based MENM. N and C cycle are closely interlinked. For example, the abovementioned reduced nodulation in the study by Judy et al.20 was accompanied by a 23% reduction in shoot biomass (Table 3) and a particularly large inhibition of fungi. As fungi (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N 5–15) have a higher C
N 5–15) have a higher C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio than bacteria (C
N ratio than bacteria (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N 3–6),134 this should have rendered an overall lower C
N 3–6),134 this should have rendered an overall lower C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio of the microbial biomass (CNmic), yet in that study fungi made up less than 20% of the total microbial biomass. An increased CNmic upon exposure to Fe3O4NP and SnO2NP in one study might suggest a community shift towards mycorrhizal fungi, but this was not found for CeO2NP.134 The impact of AgNP in a forest soil increased the C
N ratio of the microbial biomass (CNmic), yet in that study fungi made up less than 20% of the total microbial biomass. An increased CNmic upon exposure to Fe3O4NP and SnO2NP in one study might suggest a community shift towards mycorrhizal fungi, but this was not found for CeO2NP.134 The impact of AgNP in a forest soil increased the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio from 14.9 to up to 17.9 after 60 days95 (Table 3). Several MENM (perhaps also nZVI51) could thus affect both N and C turnover via multiple mechanisms, from selective toxicity to sensitive taxa (see above) to increased N emission and reduced nitrogen fixation or mineralisation (Table 3).
N ratio from 14.9 to up to 17.9 after 60 days95 (Table 3). Several MENM (perhaps also nZVI51) could thus affect both N and C turnover via multiple mechanisms, from selective toxicity to sensitive taxa (see above) to increased N emission and reduced nitrogen fixation or mineralisation (Table 3).
In summary – despite considerable variation between both MENM type and experimental conditions – both carbon and nitrogen turnover have often shown dramatic responses to MENM exposure, hinting at a net loss of carbon from the soil system and a lowered nitrogen availability for plants through reduced nitrification and N fixation, in one case also increased N emission (Table 3). Negative effects on the cycling of other elements have been reported as well, yet existing evidence is too scarce to draw any general conclusions.
Altogether, there is plenty of evidence that many MENM have negative effects on element cycling – reducing desirable ecosystem services, in particular nitrogen fixation and mineralisation, and increasing greenhouse gas emissions. If these effects are direct or indirect – via impact of MENM on plant roots or soil animals – is often not clear. How important these interactions are for the understanding of MENM effects we will point out later on.
Relevance of soil properties
Besides particle characteristics such as size, shape, coating or functionalisation, soil properties (in particular texture, pH value, ion concentration, redox status, clay and organic matter) have a massive impact on the fate and bioavailability of MENM.18,19,46,145–147 The chemistry of the soil solution varies not only between soils but also within one soil, due to weather, management and organism activity (see next section), and it also determines the particle corona148 and subsequent bioavailability. Thus, MENM effects on soil organisms or processes often vary with soil type or treatments, as shown in several studies reported in our review.11,16,18,47,51,64,129 Fate and physicochemical behaviour of MENM in different soils have been studied comparably well (e.g.ref. 16) whereas less is known how the variation in soil properties affects their impact on soil organisms and communities.18,51,149 Rousk et al.47 found that both ZnO (20 nm) and CuO (40–80 nm) NP were more toxic to bacterial communities in mineral than in organic soil at 0–200 mol kg−1. Corresponding salts showed higher and bulk compounds lower toxicity, but in organic soil bulk ZnO were more toxic than ZnONP.43 Pawlett et al.51 showed that clay protects microorganism communities against negative effects of nZVI. They also demonstrated that straw aggravated nZVI effects on the microbial biomass, which in turn was insensitive to soil organic matter content. On the other hand, Simonin et al.149 found a negative effect of TiO2NP only in one out of six soils with a high clay and organic matter content (Table 3). They explained this by the latter and an accordingly altered zeta potential of the particles in this soil.So what is specific for MENM? These are particularly two processes: size-dependent slow dissolution of soluble MENM18 (compared to fast for corresponding salts and very slow for bulk materials) and physical (or physico-chemical) interference. Physical interference refers to physical modifications of either the environment or the organism, not only by pristine MENM but also by (hetero-)aggregates formed by these (see ref. 19). This may affect manifold processes, for instance solute transport, bacterial sorption, function of exoenzymes, plant nutrient uptake or chemoreception. Silica clay included during synthesis of AgNP formed highly bactericidal nanohybrids, causing cell death by surface contact only.150 If heteroaggregation of pristine AgNP in soils with naturally occurring clays could lead to similar detrimental effects on beneficial bacteria urgently needs to be studied.
Generally, the colloidal nature of MENM makes predictions in a complex matrix like soil extremely difficult, if not impossible: Are they inactive due to complexation with organic matter? Where do they adsorb, at mineral or organic particles, or rather at organisms' membranes? Which conditions favour desorption? And to what extent does all this interfere with organism activity (cf.ref. 131) and temporal variation?
Variation over time
Evidently, MENM fate over time will be a function of soil properties. For instance, at drier conditions the low water holding capacity of sandy soils reduces the available time for dissolution compared to soils with a finer texture. Another example would be the quantity and quality of dead organic matter which strongly affects biomass and activity of soil organisms and thus their impact on, e.g., the degradation of organic coatings.As almost any metal compound, also MENM in soil undergo aging, i.e. both coating and core material change over time.19 Slow dissolution of metal-based MENM causes a permanent, chronic exposure. At the same time a chemical equilibrium is not achieved, which alters speciation. For instance, when silver nitrate dissolves in soils, a large share of the dissolved silver ions will form sparingly soluble precipitates with chloride or sulphide which are not bioavailable and therefore not toxic. Slowly dissolving AgNP behave different and their effects may show only after long periods of time, see.11,151 In standard tests and many study designs the target variables are measured at only one point in time, often after a very short exposure duration. For reasons of efficiency and resource restrictions this is reasonable, yet, how misleading this can be was shown in several studies reviewed here. The results by Schlich et al.11 raise even more concern, they clearly showed that dramatic effects on microbial C and N turnover intensified over time or became apparent only after 100 an more days of incubation.
The results by Vittori Antisari et al.134 (Table 3) varied both with soil conditions (upper, more organic soil M1 and lower, more mineral soil M2) and time. NP effects on the microbial C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio (CNmic) were more pronounced in M2 than in M1. After 7 days, the metabolic quotient (MQ) increased significantly for all three NP types and both concentrations in M2, which in M1 was only the case for CeO2NP. In turn, after 60 days CeO2NP and Fe3O4NP had no more effect at all whereas SnO2NP increased MQ in both soils and at both concentrations. AgNP in a forest soil showed the most drastic effects on microorganisms after 60 days: although part of the community appeared to somewhat recover after 90 days the observed community shift became more pronounced with extended incubation time95(cf.Table 3). The toxicity of both TiO2 (15–20 nm, 81% anatase, 0–2000 g kg−1) and ZnO NP (20–30 nm, 0–500 g kg−1) on extractable soil DNA drastically increased over 60 days whereas it rather decreased when looking at substrate induced respiration,137 see also next section. Many other studies reviewed here contained observations over shorter and longer periods of time, which in most cases revealed differential effects of MENM.7,9,11,16,41,46,121,143,145–147 This is by no means surprising as all ecosystems and inherent processes and interactions are highly dynamic. Processes include the physical environment (variation especially of water content, pH, salinity, redox conditions and temperature), the fate of MENM, from aggregation, sorption, dissolution to speciation of dissolved ions and all kind of biotic actions and interactions. How tightly these processes are connected to activities of soil organisms is shown in Table 4.
N ratio (CNmic) were more pronounced in M2 than in M1. After 7 days, the metabolic quotient (MQ) increased significantly for all three NP types and both concentrations in M2, which in M1 was only the case for CeO2NP. In turn, after 60 days CeO2NP and Fe3O4NP had no more effect at all whereas SnO2NP increased MQ in both soils and at both concentrations. AgNP in a forest soil showed the most drastic effects on microorganisms after 60 days: although part of the community appeared to somewhat recover after 90 days the observed community shift became more pronounced with extended incubation time95(cf.Table 3). The toxicity of both TiO2 (15–20 nm, 81% anatase, 0–2000 g kg−1) and ZnO NP (20–30 nm, 0–500 g kg−1) on extractable soil DNA drastically increased over 60 days whereas it rather decreased when looking at substrate induced respiration,137 see also next section. Many other studies reviewed here contained observations over shorter and longer periods of time, which in most cases revealed differential effects of MENM.7,9,11,16,41,46,121,143,145–147 This is by no means surprising as all ecosystems and inherent processes and interactions are highly dynamic. Processes include the physical environment (variation especially of water content, pH, salinity, redox conditions and temperature), the fate of MENM, from aggregation, sorption, dissolution to speciation of dissolved ions and all kind of biotic actions and interactions. How tightly these processes are connected to activities of soil organisms is shown in Table 4.
| Biotic process | Impact, specifications | Effects on MENM in soil | Ref. |
|---|---|---|---|
| Production | C input (mainly carbohydrates): food source for microorganisms, herbivores and decomposers | Sorption to biological surfaces (membranes, EPS etc.): bioconcentration;90,111,161 retardation of ENM transport by bacterial biofilms19 | Horst et al. 2012,161 Priester et al. 2012,90 Yeo et al. 2013,111 Cornelis et al. 2014,19 |
| Respiration | C output, acidification via dissolved CO2, dissolution of calcareous minerals | Altered zeta potential and ionic composition of SPW | |
| Ingestion | N input by symbiotic and free-living bacteria; (selective) uptake of nutrients, organisms, bulk minerals; altered community structure, population size and activity | Altered ionic strength and composition;19 enlarged surface of organic matter by comminution ≥ (de-)stabilization, sorption or dispersion; bioaccumulation11 | Cornelis et al. 2014,19 Schlich et al. 2013,11 |
| Metabolisation | Chemical degradation; altered chemistry, physics and biology inside cell/gut | Degradation of coating; modification via extracellular enzymes/excreta or inside organism/cell19,50 | Cornelis et al. 2014,19 Gupta et al. 201550 |
| Excretion | Root exudates: sugars, organic acids etc. microorganisms and animals: extracellular enzymes, polysaccharides, toxins, minerals, greenhouse gases, infochemicals etc. | Dissolution via reduced pH, chelation;19 inactivation by EPS;130 excretion of NP by fungi;50 Reduction of ions, e.g. Ag+? | Dinesh et al. 2012,130 Cornelis et al. 2014,19 Gupta et al. 201550 |
| Egestion | e.g. nitrite → nitrate; dead leaf → faeces | Altered solute chemistry; aggregates with physically and chemically modified OM60,116 | Lavelle et al. 2006,60 Maaß et al. 2015116 |
| Growth | Growth of individuals and populations | De-aggregation;161 dilution or concentration within growing individual | Horst et al. 2012161 |
| Reproduction | Clonal growth, release of spermatophores, eggs, spores etc.; genetic modification | New sorption surfaces; transfer to next generation;66 potential resistance | Meyer et al. 201066 |
| Movement | Formation of soil pores, aggregation at OM hot spots, dispersal of OM and microbial propagules in soil | Aeration, altered spatial distribution, altered solute transport and redox status60 | Lavelle et al. 200660 |
| Communication | Quorum sensing, pheromones, perception, avoidance behaviour | Preference (?) and avoidance by animals;79 distribution of animal surfaces via aggregation behaviour; biofilm formation161 | Amorim & Scott-Fordsmand 2012,79 Horst et al. 2012161 |
| Predation | Impact on activity and composition of prey or parasite host | Bioaccumulation;17 biomagnification?; indirect effects on SPW and aggregate properties | Hou et al. 201517 |
| Facilitation | Mycorrhiza, legume symbiosis, “fungus gardens” of ants or termites, provision of secondary food sources (e.g. carbohydrates, faeces or nitrite) | Increased plant uptake via mycorrhizal hyphae? Dispersal and accumulation by invertebrates? |
Temporal variation of contaminated soils has been studied intensively (e.g.ref. 25 and 154), and many of the observed processes also apply to MENM. Based on our long-term expertise with various kinds of pollutants10,122,155–158 in soil we suggest long-term changes in the soil community upon MENM exposure. Features that might specifically emerge from MENM will be shown in the last chapter.
Methodological remarks
Multiple techniques are being applied in soil community analysis, from standardized reproduction tests and simple enzyme assays to high-resolution sequencing. For instance, culture methods dramatically underestimate the number of microbial operational taxonomic units (OTU) compared to those revealed by DNA-based methods (e.g.ref. 124). Yet also these differ substantially: profiles in banding patterns revealed by, for instance DGGE, are way less variable than those from deep sequencing techniques. For merely statistical reasons, high resolution methods will detect differences upon MENM exposure much more frequently than methods that render only comparably few OTU. Not too surprising, the latter appear more sensitive to detect effects caused by MENM, as shown by the studies where various methods were used in parallel (e.g.ref. 133 and 152). Also for the estimation of microbial biomass various methods exist. Most common are substrate induced respiration (SIR) and extraction of C and N after chloroform fumigation (CFE). More recently, total extracts of DNA or phospholipid fatty acids (PLFA) have increasingly been used. The drawback of DNA extraction or enzyme assays is that they also render extracellular compounds (and inactive stages) whereas the other methods refer to living microorganisms. Functional parameters such as respiration or enzyme activity integrate over the entire community and are therefore less dynamic and less sensitive than structural parameters. Whenever two methods are being used in parallel, the substantial differences become apparent (see e.g.ref. 137, Table 3). When interpreting results or comparing effective concentrations from various authors, this must be kept in mind.Due to the opaque and crystalline nature of soil, direct imaging of ENM in soil is a difficult task. TEM images can be used if the ENM under study can be clearly distinguished from soil minerals by their optical density, size or shape (e.g.ref. 82). If this is not the case, TEM-EDX is necessary to characterize the elemental composition. However, the core components of several frequently used ENM, especially silica and iron oxides, belong to the most abundant soil minerals at all. In such cases more advanced techniques are required such as specifying the detailed crystalline structure of the ENM or labelling them with fluorescent markers. In the latter case, however, it has to be kept in mind that most of such coatings and functionalisations will be decomposed sooner or later.
Toxicity tests with nematodes and plants sometimes include information on MENM concentrations in liquid medium rather than in soil. This makes the comparison with other studies and test systems difficult and does not reflect environmental behaviour in soil, which might be considered a drawback in the use for risk assessment.
During the literature search and review it became apparent that explicit information on coating or dispersant of MENM is often missing. This was found in various papers in which the particles used were otherwise well characterized and thoroughly described (e.g.ref. 61, 71, 73 and 90, for details refer to the ESI†). We therefore call attention to the importance of this information because coatings can have strong impacts of the behaviour, fate and toxicity of MENM.18,159,160
Finally we stress that any attempt for generalizing the findings summarized by us must only be made when supported by multiple evidence, as for instance in case of frequently observed effects on nitrifiers or shifts in the fungi-bacteria ratio. In all other cases readers must keep in mind the extreme variation in particle characteristics (size, shape, coating etc.), soil properties and any details of the respective methods used.
Synthesis
We have briefly sketched the soil ecosystem, its organisms, their activities, interactions and associated ecosystem processes. Although the current knowledge is limited and sometimes also positive effects were reported, many studies have shown that any trophic or functional group, any ecosystem process in the soil can be adversely affected by MENM – in particular by those based on soluble toxic metals and the anatase form of TiO2. Mind that also mechanical effects may occur, for instance by blocking of receptors or inhibiting gas exchange or water uptake of the organisms via surface adsorption. A number of studies have shown nano-specific effects, yet the mechanisms behind in many cases remain unclear. We propose that these can often be related to the complexity of organisms and processes in the soil, and to indirect effects and interactions that are not taken into consideration, particularly in soil animals. Another important point is that most studies do not take into account that not only MENM affect organisms but there is also a reverse side, namely the impact of organisms on MENM.Impact of soil organisms on MENM properties
Table 4 shows the main processes and interactions performed by soil organisms and how these affect the physical–chemical soil environment, with corresponding effects on MENM. Besides modification inside the organism, any biotic process in soil affects the chemistry of the soil pore water, often also the quality and physical arrangement of its solid constituents and the spatial distribution of biota. As much of this was discussed earlier, we elaborate here only on three examples from Table 4: TiO2NP (75–80% anatase) preferably sorbed to the cell membrane of Pseudomonas aeruginosa (diameter increase by >30%), which in turn caused dispersion of about 70% of large NP agglomerates through bacterial growth.161 Soil microorganisms can exploit almost any organic compound, and lots of inorganic ones as well. MENM coatings, but also sometimes the core materials as well, will thus sooner or later be modified or completely degraded by them. Loss of organic coatings can increase bactericidal activity of MENM, yet overall the chemical stability of coatings in the environment is poorly understood.46 Note that respiration, water uptake and egestion of faeces also substantially influence the redox potential within the single microhabitats (aggregates, pore water, dead organic matter etc.). Finally, several soil-living fungi and bacteria can produce highly toxic metal nanoparticles themselves from dissolved ions.49,50Cascading and potentially catalytic effects in soil food webs
Many studies found no effects of MENM at low, often not even at very high concentrations. There are four main explanations for this: (1) the particles are not toxic: fine; (2) NP interact with the soil solution, which causes aggregation, precipitation to sparingly soluble salts such as AgCl, or chelation by organic molecules – all conditions which make them less reactive and/or bioavailable, at least temporarily;18 (3) soil conditions determine MENM fate and bioavailability – so no effect in one particular soil does not preclude effects in other soils;149 (4) the test duration was too short as many effects manifest only after a longer period of time11 – worst case. We conclude with a hypothetical worst-case scenario that might explain the reported strong effects of low MENM concentrations after longer exposure and at often very realistic conditions, compiling evidence from several studies performed mainly, but not exclusively, with AgNP.8,11,20,66 We consider this mixture justified as there is much research into mixed (e.g. CdSe QD) and doped MENM such as Ag@TiO2 (ref. 157) – and because several types of MENM will co-occur in the real environment.Immediate toxic effects on single species, groups, or processes have extensively been described above; here we focus on how interactions might explain and/or predict long-term effects of MENM, starting with some relevant general phenomena. Only part of the community will be compromised, and often dominant organisms are affected to a larger extent, simply because of the higher probability of abundant species to come in contact with the toxic compounds.155 This releases resources, so that any other competing species will profit and increase in abundance or biomass. An excess of resources can be exploited best by r strategists with high growth rates, which then will dominate162 – yet also other species may take advantage of this, perhaps immigrate or switch from dormant to active status. Each step will be accompanied by cascading effects on all interaction partners, from mutualists to prey, predators and parasites. Therefore often community structure changes (e.g.ref. 21). Finally, any consumption of other organisms may result in bioaccumulation and trophic transfer (see above).
Now we dig into the soil community (bold numbers in the following refer to Fig. 1). Let us start with the community at the rhizosphere (1). Studies with hydroponic cultures have shown a strong enrichment of MENM at the root surface.93,163 Although in soil this process will be considerably reduced,19 substantial uptake from a sandy loam into corn plants164 shows high mobility of ZnONP. Thus it is likely that a large percentage of MENM will concentrate at surfaces related to water and nutrient uptake of plants and mycorrhizal fungi.55,98 This will also expose associated consumers, namely root herbivores (2), bacterial (3) and fungal grazers (4). Soil bacteria exploit root exudates and are thus mostly found in the rhizosphere.35 Above we have shown that their majority appears to be very sensitive towards a range of MENM Bacteria often grow as biofilms (5), largely protected by exopolysaccharides (EPS).130,144 If MENM slowly penetrate this EPS layer they will first kill the uppermost layer of bacteria. This releases nutrients which will readily be used by the surviving part of the population, causing de-aggregation of the MENM and thus higher reactivity161 – killing more bacteria. As MENM can interfere with biofilm formation,161 this process will be inhibited, in the long term also affecting soil aggregate stability. Typical bacterial consumers are protozoa97 and nematodes.35 Nematodes will first thrive as they may grow even better with killed than with living bacteria66 – yet this study lasted only three days, and of course then food shortage will start, on top of direct effects such as growth reduction.62 Apparently, smaller AgNP are easier adsorbed and internalised by nematodes than larger AgNP, and even transferred to the next generation66 (6). Although adult invertebrates often remain unaffected, offspring may suffer as reproduction is generally much more sensitive than mortality.66,74 Effects of contaminants may be more pronounced or even occur for the first time in subsequent generations.154,165,166 When tomato plants were treated with CeO2NP, second generation seedlings were weaker and smaller than control plants (Wang et al. 2013, cited in ref. 16). The reduction of nematode populations61,65 and of protozoan bacterial grazers97 will dramatically reduce the NH4+ excreted by both groups,35 with cascading effects on nitrifying bacteria, plant nutrition and nematode predators (mainly microarthropods and certain fungi). As also microarthropods largely increase the availability of plant nutrients,167 plant nutrition will further deteriorate, resulting in reduced growth and thus lower carbon input in the soil, which will further reduce microbial populations.
This is related to the labile carbon pool in the soil food web which mainly refers to root exudates, bacteria and their grazers.35 More recalcitrant plant litter and dead wood will rather foster fungi (7), arthropods and long-term carbon input, often associated with lower fertility121,168 and microbial pathogens (8). Pathogens will be suppressed by antimicrobial MENM, yet also their natural enemies such as nematodes or collembola167 and beneficial microorganisms (9). The most efficient use of nutrients with associated positive effects on plant growth is provided by a balanced interplay of fungi, bacteria and soil animals.35 Although resistant bacteria exist and negative effects of MENM on fungi have been reported as well,56 fungi as eukaryotes are generally better able to cope with metal stress than most bacteria.49,50 Even new MENM can be formed by interactions between plants and endomycorrhizal fungi, as shown by Manceau et al.169 for copper. Once MENM are chemically (e.g. complexation, precipitation) or biologically (uptake, ingestion) removed from the system, fast-growing microorganisms will recover – on the cost of slow-growing ones such as free-living nitrogen fixers (10). Thus we postulate a shift of the microbial community towards fungi and fast-growing bacteria upon MENM exposure.170 However, any tolerance to metal exposure requires additional energy, e.g. for the activity of metal transporters or the synthesis of metallothioneins, resulting in increased respiration (11). Many of the studies in Table 3 have shown an increased metabolic quotient upon MENM exposure, meaning a higher loss of carbon – the main limiting factor for the microbial soil community.35 Consequently, its biomass will decrease over time.
Microorganisms are also the main energy source for larger animals such as earthworms, millipedes, ants or termites (12). Together with the direct toxic effects on burrowing invertebrates reported above, the microbial decrease will on the long term reduce invertebrate populations and consequently all beneficial ecosystem services provided by them (13); (see also Table 4). On top of that comes a tricky aspect, namely behaviour: the study by Schlich et al.74 clearly shows higher bioaccumulation of AgNP in earthworms at lower concentrations, most likely due to reduced avoidance behaviour. Although the BAF was below 1, that study was too short to assess the final bioaccumulation as a) life expectancy of earthworms is much longer (up to several years) than the duration of an OECD reproduction test (56 days) and b) earthworms repeatedly ingest soil. This means that BAF over time might well exceed 1, resulting in accumulation in earthworms. As these are prey to numerous larger invertebrates and vertebrates (14), the potential of AgNP biomagnification in top predators (birds of prey, foxes etc.) cannot be excluded. Behaviour in soil largely relies on chemical communication, which is well known, for instance, in social insects such as ants (15). To our knowledge no studies in this direction exist, yet the fact that MENM interfere with chemical communication and aggregation behaviour in bacteria161 requires further research into soil animal communication.
Over longer exposure, additional resistance (16) will evolve, for instance via overproduction of EPS in bacteria131 or by various mechanisms of metal tolerance in other organisms.107,143,147 The bacterium Pseudomonas stutzeri, isolated from silver mines, forms AgNP by itself, just as many resistant fungal species do.49,50 Thus, even if part of the AgNP had first dissolved, their presence in the system is sustained – resulting in a catalytic effect. Qiu et al.171 showed that four types of NP (TiO2, SiO2, Fe2O3, Al2O3) at low concentrations (e.g. 5 mmol L−1 for Al2O3) promoted plasmid transfer between various bacterial species (even between Gram-positive and -negative ones) 20 to 200 fold. This does not only imply fast evolution of MENM resistance but raises additional concern with respect to the development of multidrug-resistant strains. Luckily, thus far increased antibiotics resistance due to MENM exposure has only been shown in the laboratory but not in the field at environmentally relevant conditions.42,49,171 Resistant organisms thrive fairly well in an environment with few competitors (e.g.ref. 49 and 154). If these are challenged by the same or a similar contaminant later on, this advantage becomes apparent, yet upon exposure to other contaminants these organisms are often more affected than communities from the control site. Filser et al.172 and Wakelin et al.143 have shown this for Cu pollution, the latter authors also for CuNP as secondary stressors. In their study, the microbial communities previously exposed to varied levels of total Cu were much more tolerant to CuNP than to Cu2+.
Conclusions and outlook
We have summarized manifold examples of negative effects of MENM on soil organisms and processes, albeit often at concentrations far exceeding those that can be reasonably expected in the natural environment. Only some tests were conducted at MENM concentrations predicted in nature. The minority of published evidence reported no or (desirable) positive effects on soils. Despite their doubtlessly useful application for the remediation of contaminated soils, not even iron-based MENM can be considered generally harmless. Ten studies showed strong effects at concentrations of MENM below 1 mg kg−1 and/or temporal variation and long delay of partly dramatic effects on the microbial community, element turnover and greenhouse gas emissions.8,11,40,44,49,50,54,61,89,153 These are environmentally relevant concentrations and therefore raises concern for risk regulation. The present REACH standard of relying on only very few short-term aquatic tests at low production volumes (as they are typical for many MENM) bears a very high risk for soils – in particular when keeping in mind that most MENM are persistent and rather immobile in soil; i.e. their concentration will increase over time.There is a strong need of studies assessing not only hazards to single species but also to the entire soil community. As shown here, there are various interactions between organisms within the soil community that could potentially be hampered by exposure to MENM and these might influence ecosystem services and functions. Another point relevant here is that the sensitivity of standardized microbiological tests is much lower than state-of-the art techniques (see methodological remarks): finding no effect in, e.g., the soil respiration test does by no means imply that no dramatic changes occurred in the microbial community – with potential consequences for biogeochemical cycling. Moreover, MENM may enrich in soil beyond direct application with biosolids, namely via plant litter, animal faeces,93,108 carcasses or exuvia.116 Concern for negative effects on critical ecosystem services has been raised repeatedly (e.g.ref. 20 and 40), and our respective chapter has emphasized this. From a precautionary point of view (based on experience with other contaminants marketed at comparably low concentrations, e.g. diclofenac173) such a potential should be taken into account in risk regulation of MENM. To date the potential toxic effects of MENM on bacteria and plants have received more attention than soil animals and there remains a large knowledge gap regarding the trophic transfer of MENM and its effects on interactions within the soil community. If bioaccumulation and -magnification of MENM will be of concern cannot be predicted at present. This means that over time the impact of MENM on soil organisms, and in particular predators, might increase. Understanding the consequences that the emission of MENM has at the ecosystem level is therefore of major importance and further research in this area is undoubtedly necessary.
Acknowledgements
We thank Stephan Hackmann for helpful comments on an earlier version of the manuscript.References
- F. Piccinno, F. Gottschalk, S. Seeger and B. Nowack, J. Nanopart. Res., 2012, 14 Search PubMed.
- G. Lövestam, H. Rauscher, G. Roebben, B. S. Klüttgen, N. Gibson, J.-P. Putaud and H. Stamm, JRC Ref. Reports, European Commission Joint Research Centre, 2010, 24403 Search PubMed.
- G. Oberdörster, E. Oberdörster and J. Oberdörster, Environ. Health Perspect., 2005, 113, 823–839 CrossRef.
- A. Kahru, H. C. Dubourguier, I. Blinova, A. Ivask and K. Kasemets, Sensors, 2008, 8, 5153–5170 CrossRef CAS.
- S. F. Hansen and A. Baun, Nat. Nanotechnol., 2012, 7, 409–411 CrossRef CAS PubMed.
- B. Pan and B. Xing, Eur. J. Soil Sci., 2012, 63, 437–456 CrossRef CAS.
- V. Shah and I. Belozerova, Water, Air, Soil Pollut., 2008, 197, 143–148 CrossRef.
- M. Hänsch, C. Emmerling and J. Plant Nutr, Soil Sci., 2010, 173, 554–558 Search PubMed.
- A. García, L. Delgado, J. A. Torà, E. Casals, E. González, V. Puntes, X. Font, J. Carrera and A. Sánchez, J. Hazard. Mater., 2012, 199–200, 64–72 CrossRef PubMed.
- J. Filser, D. Arndt, J. Baumann, M. Geppert, S. Hackmann, E. M. Luther, C. Pade, K. Prenzel, H. Wigger, J. Arning, M. C. Hohnholt, J. Köser, A. Kück, E. Lesnikov, J. Neumann, S. Schütrumpf, J. Warrelmann, M. Bäumer, R. Dringen, A. von Gleich, P. Swiderek and J. Thöming, Nanoscale, 2013, 5, 1034–1046 RSC.
- K. Schlich, T. Klawonn, K. Terytze and K. Hund-Rinke, Environ. Sci. Eur., 2013, 25 Search PubMed.
- V. Ghormade, M. V. Deshpande and K. M. Paknikar, Biotechnol. Adv., 2011, 29, 792–803 CrossRef CAS PubMed.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- S. Mishra and H. B. Singh, Appl. Microbiol. Biotechnol., 2015, 99, 1097–1107 CrossRef CAS PubMed.
- M. Kah and T. Hofmann, Environ. Int., 2014, 63, 224–235 CrossRef CAS PubMed.
- C. O. Dimkpa, J. Basic Microbiol., 2014, 54, 889–904 CrossRef CAS PubMed.
- W.-C. Hou, P. Westerhoff and J. D. Posner, Environ. Sci.: Processes Impacts, 2013, 15, 103–122 CAS.
- P. S. Tourinho, C. A. M. van Gestel, S. Lofts, C. Svendsen, A. M. V. M. Soares and S. Loureiro, Environ. Toxicol. Chem., 2012, 31, 1679–1692 CrossRef CAS PubMed.
- G. Cornelis, K. Hund-Rinke, T. Kuhlbusch, N. Van den Brink and C. Nickel, Crit. Rev. Environ. Sci. Technol, 2014, 44, 2720–2764 CrossRef CAS.
- J. D. Judy, D. H. McNear, C. Chen, R. W. Lewis, O. V. Tsyusko, P. M. Bertsch, W. Rao, J. P. Stegemeier, G. V. Lowry, S. P. McGrath, M. Durenkamp and J. M. Unrine, Environ. Sci. Technol., 2015, 49, 8751–8758 CrossRef CAS PubMed.
- C. Xu, C. Peng, L. Sun, S. Zhang, H. Huang, Y. Chen and J. Shi, Soil Biol. Biochem., 2015, 86, 24–33 CrossRef CAS.
- B. Scholz-Starke, A. Nikolakis, T. Leicher, C. Lechelt-Kunze, F. Heimbach, B. Theißen, A. Toschki, H. T. Ratte, A. Schäffer and M. Roß-Nickoll, Ecotoxicology, 2011, 20, 1932–1948 CrossRef CAS PubMed.
- S. Chelinho, X. Domene, P. Andrés, T. Natal-da-Luz, C. Norte, C. Rufino, I. Lopes, A. Cachada, E. Espíndola, R. Ribeiro, A. C. Duarte and J. P. Sousa, Appl. Soil Ecol., 2014, 83, 200–209 CrossRef.
- J. Filser, S. Wiegmann and B. Schröder, Appl. Soil Ecol., 2014, 83, 193–199 CrossRef.
- C. Vaj, C. A. M. Van Gestel and M. Vighi, Ecotoxicology, 2014, 2009, 898–913 Search PubMed.
- D. U. Hooper, E. C. Adair, B. J. Cardinale, J. E. K. Byrnes, B. A. Hungate, K. L. Matulich, A. Gonzalez, J. E. Duffy, L. Gamfeldt and M. I. O'Connor, Nature, 2012, 486, 105–108 CAS.
- C. Wagg, S. F. Bender, F. Widmer and M. G. A. van der Heijden, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 5266–5270 CrossRef CAS PubMed.
- E. Navarro, A. Baun, R. Behra, N. B. Hartmann, J. Filser, A.-J. Miao, A. Quigg, P. H. Santschi and L. Sigg, Ecotoxicology, 2008, 17, 372–386 CrossRef CAS PubMed.
- A. A. Keller and A. Lazareva, Environ. Sci. Technol. Lett., 2014, 1, 65–70 CrossRef CAS.
- F. Gottschalk, C. Lassen, J. Kjoelholt, F. Christensen and B. Nowack, Int. J. Environ. Res. Public Health, 2015, 12, 5581–5602 CrossRef CAS PubMed.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- A. Massarsky, V. L. Trudeau and T. W. Moon, Environ. Toxicol. Pharmacol., 2014, 38, 861–873 CrossRef CAS PubMed.
- C. H. Walker, R. M. Sibly, S. P. Hopkin and D. B. Peakall, Princples of Ecotoxicology, CRC Press Taylor & Francis Group, London, 4th edn, 2012 Search PubMed.
- M. H. Beare, D. C. Coleman, D. A. J. Crossley, P. F. Hendrix and E. P. Odum, in The Significance and Regulation of Soil Biodiversity, 1995, pp. 5–22 Search PubMed.
- M. Bonkowski, New Phytol., 2004, 162, 617–631 CrossRef.
- A. Gogos, K. Knauer, T. D. Bucheli and J. Agric, Food Chem., 2012, 60, 9781–9792 CrossRef CAS PubMed.
- J. L. Gardea-Torresdey, C. M. Rico and J. C. White, Environ. Sci. Technol., 2014, 48, 2526–2540 CrossRef CAS PubMed.
- T. N. V. K. V. Prasad, P. Sudhakar, Y. Sreenivasulu, P. Latha, V. Munaswamy, K. R. Reddy, T. S. Sreeprasad, P. R. Sajanlal, T. Pradeep, P. Sudhakar, Y. Sreenivasulu, P. Latha, Y. Sreenivasulu and R. Sajanlal, J. Plant Nutr., 2012, 35, 905–927 CrossRef CAS.
- D. J. Burke, S. Zhu, M. P. Pablico-Lansigan, C. R. Hewins and A. C. S. Samia, Biol. Fertil. Soils, 2014, 50, 1169–1173 CrossRef CAS.
- B. P. Colman, C. L. Arnaout, S. Anciaux, C. K. Gunsch, M. F. Hochella, B. Kim, G. V. Lowry, B. M. McGill, B. C. Reinsch, C. J. Richardson, J. M. Unrine, J. P. Wright, L. Yin and E. S. Bernhardt, PLoS One, 2013, 8(2), e57189 CAS.
- J. Filser, Pedobiologia, 2002, 46, 234–245 Search PubMed.
- P. Hartemann, P. Hoet, A. Proykova, T. Fernandes, A. Baun, W. De Jong, J. Filser, A. Hensten, C. Kneuer, J.-Y. Maillard, H. Norppa, M. Scheringer and S. Wijnhoven, Mater. Today, 2015, 18, 122–123 CrossRef.
- M. Simonin and A. Richaume, Environ. Sci. Pollut. Res., 2015, 22(18), 13710–13723 CrossRef CAS PubMed.
- A. P. Ingle, N. Duran and M. Rai, Appl. Microbiol. Biotechnol., 2014, 98, 1001–1009 CrossRef CAS PubMed.
- O. Choi and Z. Hu, Environ. Sci. Technol., 2008, 42, 4583–4588 CrossRef CAS PubMed.
- A. K. Suresh, D. A. Pelletier and M. J. Doktycz, Nanoscale, 2013, 5, 463–474 RSC.
- J. Rousk, K. Ackermann, S. F. Curling and D. L. Jones, PLoS One, 2012, 7, e34197 CAS.
- C. O. Dimkpa, J. E. Mclean, D. W. Britt and A. J. Anderson, Nanotoxicology, 2011, 6, 635–642 CrossRef PubMed.
- M. J. Sweet and I. Singleton, in Advances in Applied Microbiology, 2011, pp. 115–133 Search PubMed.
- I. R. Gupta, A. J. Anderson and M. Rai, J. Hazard. Mater., 2015, 286, 48–54 CrossRef CAS PubMed.
- M. Pawlett, K. Ritz, R. A. Dorey, S. Rocks, J. Ramsden and J. A. Harris, Environ. Sci. Pollut. Res., 2013, 20, 1041–1049 CrossRef CAS PubMed.
- M. Premanathan, K. Karthikeyan, K. Jeyasubramanian and G. Manivannan, Nanomedicine, 2011, 7, 184–192 CAS.
- C. Mora, D. P. Tittensor, S. Adl, A. G. B. Simpson and B. Worm, PLoS Biol., 2011, 9(8), e1001127 CAS.
- Y. Feng, X. Cui, S. He, G. Dong, M. Chen, J. Wang and X. Lin, Environ. Sci. Technol., 2013, 47, 9496–9504 CrossRef CAS PubMed.
- M. Gryndler, H. Hršelová, L. Soukupová and J. Borovička, BioMetals, 2012, 25, 987–993 CrossRef CAS PubMed.
- Z. Zabrieski, E. Morrell, J. Hortin, C. Dimkpa, J. Mclean, D. Britt and A. Anderson, Ecotoxicology, 2015, 24, 1305–1314 CrossRef CAS PubMed.
- M. Vijver, The ins and outs of bioaccumulation, Metal Bioaccumulation Kinetics in Soil Invertebrates in Relation to Availability and Animal Physiology, 2005 Search PubMed.
- T. Jager, R. H. L. J. Fleuren, E. A. Hogendoorn and G. De Korte, Environ. Sci. Technol., 2003, 37, 3399–3404 CrossRef CAS PubMed.
- T. Culliney, Agriculture, 2013, 3, 629–659 CrossRef.
- P. Lavelle, T. Decaëns, M. Aubert, S. Barot, M. Blouin, F. Bureau, P. Margerie, P. Mora and J. P. Rossi, Eur. J. Soil Biol., 2006, 42(Suppl. 1), S3–S15 CrossRef.
- J.-Y. Roh, Y.-K. Park, K. Park and J. Choi, Environ. Toxicol. Pharmacol., 2010, 29, 167–172 CrossRef CAS PubMed.
- H. Wang, R. L. Wick and B. Xing, Environ. Pollut., 2009, 157, 1171–1177 CrossRef CAS PubMed.
- C. Ratnasekhar, M. Sonane, A. Satish and M. K. R. Mudiam, Nanotoxicology, 2015, 9, 994–1004 CrossRef PubMed.
- M. L. Saccà, C. Fajardo, G. Costa, C. Lobo, M. Nande and M. Martin, Chemosphere, 2014, 104, 184–189 CrossRef PubMed.
- C. Fajardo, M. Gil-Díaz, G. Costa, J. Alonso, A. M. Guerrero, M. Nande, M. C. Lobo and M. Martín, Sci. Total Environ., 2015, 535, 6–11 CrossRef PubMed.
- J. N. Meyer, C. A. Lord, X. Y. Yang, E. A. Turner, A. R. Badireddy, S. M. Marinakos, A. Chilkoti, M. R. Wiesner and M. Auffan, Aquat. Toxicol., 2010, 100, 140–150 CrossRef CAS PubMed.
- J. I. Kwak and Y.-J. An, Hum. Ecol. Risk Assess., 2015, 21, 1566–1575 CrossRef CAS.
- L.-H. Heckmann, M. B. Hovgaard, D. S. Sutherland, H. Autrup, F. Besenbacher and J. J. Scott-Fordsmand, Ecotoxicology, 2011, 20, 226–233 CrossRef CAS PubMed.
- W. A. Shoults-Wilson, O. I. Zhurbich, D. H. McNear, O. V. Tsyusko, P. M. Bertsch and J. M. Unrine, Ecotoxicology, 2011, 20, 385–396 CrossRef CAS PubMed.
- M. Novo, E. Lahive, M. Díez-Ortiz, M. Matzke, A. J. Morgan, D. J. Spurgeon, C. Svendsen and P. Kille, Environ. Pollut., 2015, 205, 385–393 CrossRef CAS PubMed.
- J. E. Cañas, B. Qi, S. Li, J. D. Maul, S. B. Cox, S. Das and M. J. Green, J. Environ. Monit., 2011, 13, 3351–3357 RSC.
- C. W. Hu, M. Li, Y. B. Cui, D. S. Li, J. Chen and L. Y. Yang, Soil Biol. Biochem., 2010, 42, 586–591 CrossRef CAS.
- J. G. Coleman, D. R. Johnson, J. K. Stanley, A. J. Bednar, C. A. Weiss, R. E. Boyd and J. A. Steevens, Environ. Toxicol. Chem., 2010, 29, 1575–1580 CrossRef CAS PubMed.
- K. Schlich, T. Klawonn, K. Terytze and K. Hund-Rinke, Environ. Toxicol. Chem., 2013, 32, 181–188 CrossRef CAS PubMed.
- H. McShane, M. Sarrazin, J. K. Whalen, W. H. Hendershot and G. I. Sunahara, Environ. Toxicol. Chem., 2012, 31, 184–193 CrossRef CAS PubMed.
- M. J. C. van der Ploeg, R. D. Handy, P. L. Waalewijn-Kool, J. H. J. van den Berg, Z. E. Herrera Rivera, J. Bovenschen, B. Molleman, J. M. Baveco, P. Tromp, R. J. B. Peters, G. F. Koopmans, I. M. C. M. Rietjens and N. W. van den Brink, Environ. Toxicol. Chem., 2014, 33, 743–752 CrossRef CAS PubMed.
- E. Lapied, J. Y. Nahmani, E. Moudilou, P. Chaurand, J. Labille, J. Rose, J.-M. Exbrayat, D. H. Oughton and E. J. Joner, Environ. Int., 2011, 37, 1105–1110 CrossRef CAS PubMed.
- S. I. L. Gomes, A. M. V. M. Soares, J. J. Scott-Fordsmand and M. J. B. Amorim, J. Hazard. Mater., 2013, 254–255, 336–344 CrossRef CAS PubMed.
- M. J. B. Amorim and J. J. Scott-Fordsmand, Environ. Pollut., 2012, 164, 164–168 CrossRef CAS PubMed.
- P. L. Kool, M. D. Ortiz and C. A. M. van Gestel, Environ. Pollut., 2011, 159, 2713–2719 CrossRef CAS PubMed.
- S. Manzo, A. Rocco, R. Carotenuto, F. D. L. Picione, M. L. Miglietta, G. Rametta and G. Di Francia, Environ. Sci. Pollut. Res., 2011, 18, 756–763 CrossRef CAS PubMed.
- P. S. Tourinho, C. A. M. Van Gestel, K. Jurkschat, A. M. V. M. Soares and S. Loureiro, Environ. Pollut., 2015, 205, 170–177 CrossRef CAS PubMed.
- P. S. Tourinho, P. L. Waalewijn-Kool, I. Zantkuijl, K. Jurkschat, C. Svendsen, A. M. V. M. Soares, S. Loureiro and C. A. M. van Gestel, Ecotoxicol. Environ. Saf., 2015, 113, 201–206 CrossRef CAS PubMed.
- A. Lang, Oecologia, 2003, 134, 144–153 CrossRef PubMed.
- D. E. Salt, R. D. Smith and I. Raskin, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1998, 49, 643–668 CrossRef CAS PubMed.
- K. Haselwandter, M. Berreck and P. Brunner, Trans. Br. Mycol. Soc., 1988, 90, 171–174 CrossRef CAS.
- P. Kalač, Food Chem., 2010, 122, 2–15 CrossRef.
- A. Heikens, W. J. Peijnenburg and A. J. Hendriks, Environ. Pollut., 2001, 113, 385–393 CrossRef CAS PubMed.
- C. Larue, H. Castillo-Michel, S. Sobanska, L. Cécillon, S. Bureau, V. Barthès, L. Ouerdane, M. Carrière and G. Sarret, J. Hazard. Mater., 2014, 264, 98–106 CrossRef CAS PubMed.
- J. H. Priester, Y. Ge, R. E. Mielke, A. M. Horst, S. C. Moritz, K. Espinosa, J. Gelb, S. L. Walker, R. M. Nisbet, Y.-J. An, J. P. Schimel, R. G. Palmer, J. A. Hernandez-Viezcas, L. Zhao, J. L. Gardea-Torresdey and P. A. Holden, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, E2451–E2456 CrossRef CAS PubMed.
- S. Arora, P. Sharma, S. Kumar, R. Nayan, P. K. Khanna and M. G. H. Zaidi, Plant Growth Regul., 2012, 66, 303–310 CrossRef CAS.
- D. L. Jacob, J. D. Borchardt, L. Navaratnam, M. L. Otte and A. N. Bezbaruah, Int. J. Phytorem., 2013, 15, 142–153 CrossRef CAS PubMed.
- Y. Koo, J. Wang, Q. Zhang, H. Zhu, E. W. Chehab, V. L. Colvin, P. J. J. Alvarez and J. Braam, Environ. Sci. Technol., 2015, 49, 626–632 CrossRef CAS PubMed.
- C. O. Dimkpa, J. E. McLean, D. W. Britt and A. J. Anderson, Ecotoxicology, 2015, 24, 119–129 CrossRef CAS PubMed.
- S. Carbone, L. Vittori Antisari, F. Gaggia, L. Baffoni, D. Di Gioia, G. Vianello and P. Nannipieri, J. Hazard. Mater., 2014, 280, 89–96 CrossRef CAS PubMed.
- J. H. Priester, P. K. Stoimenov, R. E. Mielke, S. M. Webb, C. Ehrhardt, J. P. Zhang, G. D. Stucky and P. A. Holden, Environ. Sci. Technol., 2009, 43, 2589–2594 CrossRef CAS PubMed.
- R. Werlin, J. H. Priester, R. E. Mielke, S. Krämer, S. Jackson, P. K. Stoimenov, G. D. Stucky, G. N. Cherr, E. Orias and P. A. Holden, Nat. Nanotechnol., 2011, 6, 65–71 CrossRef CAS PubMed.
- M. D. Whiteside, K. K. Treseder and P. R. Atsatt, Ecology, 2009, 90, 100–108 CrossRef PubMed.
- G. Tyler, Chemosphere, 1982, 11, 114–1146 CrossRef.
- P. L. Waalewijn-Kool, K. Klein, R. M. Forniés and C. A. M. van Gestel, Ecotoxicology, 2014, 23, 1629–1637 CrossRef CAS PubMed.
- Ž. Pipan-Tkalec, D. Drobne, A. Jemec, T. Romih, P. Zidar and M. Bele, Toxicology, 2010, 269, 198–203 CrossRef PubMed.
- P. S. Tourinho, C. A. M. van Gestel, A. J. Morgan, P. Kille, C. Svendsen, K. Jurkschat, J. F. W. Mosselmans, A. M. V. M. Soares and S. Loureiro, Ecotoxicology, 2016, 25, 267–278 CrossRef CAS PubMed.
- M. Golobič, A. Jemec, D. Drobne, T. Romih, K. Kasemets and A. Kahru, Environ. Sci. Technol., 2012, 46, 12112–12119 CrossRef PubMed.
- J. M. Unrine, W. A. Shoults-Wilson, O. Zhurbich, P. M. Bertsch and O. V. Tsyusko, Environ. Sci. Technol., 2012, 46, 9753–9760 CrossRef CAS PubMed.
- J. Hawthorne, R. De, T. Roche, B. Xing, L. A. Newman, X. Ma, S. Majumdar, J. Gardea-Torresdey and J. C. White, Environ. Sci. Technol., 2014, 48, 13102–13109 CrossRef CAS PubMed.
- R. De la Torre Roche, A. Servin, J. Hawthorne, B. Xing, L. A. Newman, X. Ma, G. Chen and J. C. White, Environ. Sci. Technol., 2015, 49, 11866–11874 CrossRef CAS PubMed.
- C. Ma, J. C. White, O. P. Dhankher and B. Xing, Environ. Sci. Technol., 2015, 49, 7109–7122 CrossRef CAS PubMed.
- J. Yasur and P. U. Rani, Chemosphere, 2015, 124, 92–102 CrossRef CAS PubMed.
- J. D. Judy, J. M. Unrine and P. M. Bertsch, Environ. Sci. Technol., 2011, 45, 776–781 CrossRef CAS PubMed.
- J. D. Judy, J. M. Unrine, W. Rao and P. M. Bertsch, Environ. Sci. Technol., 2012, 46, 12672–12678 CrossRef CAS PubMed.
- M. K. Yeo and D. H. Nam, Environ. Pollut., 2013, 178, 166–172 CrossRef CAS PubMed.
- C. O. Dimkpa, T. Hansen, J. Stewart, J. E. McLean, D. W. Britt and A. J. Anderson, Nanotoxicology, 2014, 9(3), 271–278 CrossRef PubMed.
- R. Fan, Y. C. Huang, M. A. Grusak, C. P. Huang and D. J. Sherrier, Sci. Total Environ., 2014, 466–467, 503–512 CrossRef CAS PubMed.
- Y. C. Huang, R. Fan, M. A. Grusak, J. D. Sherrier and C. P. Huang, Sci. Total Environ., 2014, 497–498, 78–90 CrossRef CAS PubMed.
- L. Yin, B. P. Colman, B. M. McGill, J. P. Wright and E. S. Bernhardt, PLoS One, 2012, 7(10), e47674 CAS.
- S. Maaß, T. Caruso and M. C. Rillig, Pedobiologia, 2015, 58, 59–63 CrossRef.
- H. F. Krug, Angew. Chem., Int. Ed., 2014, 53, 12304–12319 CAS.
- Y. Hayashi and P. Engelmann, Ivertebrate Surviv. J., 2013, 10, 69–76 Search PubMed.
- F. Marano, S. Hussain, F. Rodrigues-Lima, A. Baeza-Squiban and S. Boland, Arch. Toxicol., 2011, 85, 733–741 CrossRef CAS PubMed.
- B. R. Singh, B. N. Singh, A. Singh, W. Khan, A. H. Naqvi and H. B. Singh, Sci. Rep., 2015, 5, 13719 CrossRef PubMed.
- D. A. Wardle, R. D. Bardgett, J. N. Klironomos, H. Setälä, W. H. van der Putten and D. H. Wall, Science, 2004, 304, 1629–1633 CrossRef CAS PubMed.
- M. Schaefer, S. O. Petersen and J. Filser, Soil Biol. Biochem., 2005, 37, 2065–2076 CrossRef CAS.
- Y. Yang, Q. Chen, J. D. Wall and Z. Hu, Water Res., 2012, 46, 1176–1184 CrossRef CAS PubMed.
- A. Gitipour, A. El Badawy, M. Arambewela, B. Miller, K. G. Scheckel, W. Thiel, M. Elk, H. Ryu, V. Gomez-Alvarez, J. W. S. Domingo and T. Tolaymat, Environ. Sci. Technol., 2013, 47, 14385–14393 CrossRef CAS PubMed.
- V. Nogueira, I. Lopes, T. Rocha-Santos, A. L. Santos, G. M. Rasteiro, F. Antunes, F. Gonçalves, A. M. V. M. Soares, A. Cunha, A. Almeida, N. C. M. Gomes, N. N. C. M. Gomes and R. Pereira, Sci. Total Environ., 2012, 424, 344–350 CrossRef CAS PubMed.
- X. Zheng, Y. Su and Y. Chen, Environ. Sci. Technol., 2012, 46, 7182–7188 CrossRef CAS PubMed.
- S. He, Y. Feng, H. Ren, Y. Zhang, N. Gu and X. Lin, J. Soils Sediments, 2011, 11, 1408–1417 CrossRef CAS.
- C. Chen, J. M. Unrine, J. D. Judy, R. W. Lewis, J. Guo, D. H. McNear and O. V. Tsyusko, Environ. Sci. Technol., 2015, 49, 8759–8768 CrossRef CAS PubMed.
- D. S. Read, M. Matzke, H. S. Gweon, L. K. Newbold, L. Heggelund, M. D. Ortiz, E. Lahive, D. Spurgeon and C. Svendsen, Environ. Sci. Pollut. Res., 2015, 23(5), 4120–4128 CrossRef PubMed.
- R. Dinesh, M. Anandaraj, V. Srinivasan and S. Hamza, Geoderma, 2012, 173–174, 19–27 CrossRef CAS.
- N. Joshi, B. T. Ngwenya and C. E. French, J. Hazard. Mater., 2012, 241–242, 363–370 CrossRef CAS PubMed.
- J. I. Kwak and Y.-J. An, Hum. Ecol. Risk Assess., 2015, 21, 1566–1575 CrossRef CAS.
- D. F. Rodrigues, D. P. Jaisi and M. Elimelech, Environ. Sci. Technol., 2013, 47, 625–633 CrossRef CAS PubMed.
- L. Vittori Antisari, S. Carbone, A. Gatti, G. Vianello and P. Nannipieri, Soil Biol. Biochem., 2013, 60, 87–94 CrossRef CAS.
- S. Wakelin, E. Gerard, A. Black, K. Hamonts, L. Condron, T. Yuan, J. Van Nostrand, J. Zhou and M. O'Callaghan, Environ. Pollut., 2014, 190, 1–9 CrossRef CAS PubMed.
- H. Chai, J. Yao, J. Sun, C. Zhang, W. Liu, M. Zhu and B. Ceccanti, Bull. Environ. Contam. Toxicol., 2015, 94, 490–495 CrossRef CAS PubMed.
- Y. Ge, J. P. Schimel and P. A. Holden, Environ. Sci. Technol., 2011, 45, 1659–1664 CrossRef CAS PubMed.
- C. Cherchi and A. Z. Gu, Environ. Sci. Technol., 2010, 44, 8302–8307 CrossRef CAS PubMed.
- G. M. Wagner, Bot. Rev., 1997, 63, 1–26 CrossRef.
- V. C. Reyes, S. O. Opot and S. Mahendra, Environ. Toxicol. Chem., 2015, 34, 887–897 CrossRef CAS PubMed.
- Y. Yang, J. Wang, Z. Xiu and P. J. J. Alvarez, Environ. Toxicol. Chem., 2013, 32, 1488–1494 CAS.
- V. Echavarri-Bravo, L. Paterson, T. J. Aspray, J. S. Porter, M. K. Winson, B. Thornton and M. G. J. Hartl, Environ. Pollut., 2015, 201, 91–99 CrossRef CAS PubMed.
- S. Wakelin, E. Gerard, A. Black, K. Hamonts, L. Condron, T. Yuan, J. van Nostrand, J. Zhou and M. O'Callaghan, Environ. Pollut., 2014, 190, 1–9 CrossRef CAS PubMed.
- X. Zheng, Y. Chen and R. Wu, Environ. Sci. Technol., 2011, 45, 7284–7290 CrossRef CAS PubMed.
- G. Cornelis, C. D. M. Thomas, M. J. McLaughlin, J. K. Kirby, D. G. Beak and D. Chittleborough, Soil Sci. Soc. Am. J., 2012, 76, 891–902 CrossRef CAS.
- C. Coutris, E. J. Joner and D. H. Oughton, Sci. Total Environ., 2012, 420, 327–333 CrossRef CAS PubMed.
- L.-Z. Li, D.-M. Zhou, W. J. G. M. Peijnenburg, C. A. M. van Gestel, S.-Y. Jin, Y.-J. Wang and P. Wang, Environ. Int., 2011, 37, 1098–1104 CrossRef CAS PubMed.
- D. Docter, D. Westmeier, M. Markiewicz, S. Stolte and R. H. Stauber, Chem. Soc. Rev., 2015, 00, 1–27 Search PubMed.
- M. Simonin, J. P. Guyonnet, J. M. F. Martins, M. Ginot and A. Richaume, J. Hazard. Mater., 2015, 283, 529–535 CrossRef CAS PubMed.
- H. L. Su, C. C. Chou, D. J. Hung, S. H. Lin, I. C. Pao, J. H. Lin, F. L. Huang, R. X. Dong and J. J. Lin, Biomaterials, 2009, 30, 5979–5987 CrossRef CAS PubMed.
- Y. Ge, J. P. Schimel and P. A. Holden, Appl. Environ. Microbiol., 2012, 78, 6749–6758 CrossRef CAS PubMed.
- B. Shrestha, V. Acosta-Martinez, S. B. Cox, M. J. Green, S. Li and J. E. Cañas-Carrell, J. Hazard. Mater., 2013, 261, 188–197 CrossRef CAS PubMed.
- S. M. Shaheen, J. Rinklebe, H. Rupp and R. Meissner, Geoderma, 2014, 228–229, 5–13 CrossRef CAS.
- L. Posthuma and N. M. Van Straalen, Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol., 1993, 106, 11–38 CrossRef.
- J. Filser, H. Fromm, R. F. Nagel and K. Winter, Plant Soil, 1995, 170, 123–129 CrossRef CAS.
- J. Filser and G. Hölscher, Pedobiologia, 1997, 41, 173–178 Search PubMed.
- M. Engelke, J. Köser, S. Hackmann, H. Zhang, L. Mädler and J. Filser, Environ. Toxicol. Chem., 2014, 33, 1142–1147 CrossRef CAS PubMed.
- M. Matzke, S. Stolte, J. Arning, U. Uebers and J. Filser, Ecotoxicology, 2009, 18, 197–203 CrossRef CAS PubMed.
- J. R. Peralta-Videa, L. Zhao, M. L. Lopez-Moreno, G. de la Rosa, J. Hong and J. L. Gardea-Torresdey, J. Hazard. Mater., 2011, 186, 1–15 CrossRef CAS PubMed.
- C. L. Arnaout and C. K. Gunsch, Environ. Sci. Technol., 2012, 46, 5387–5395 CrossRef CAS PubMed.
- A. M. Horst, Z. Ji and P. A. Holden, J. Nanopart. Res., 2012, 14, 1014 CrossRef.
- T. Bongers and H. Ferris, Trends Ecol. Evol., 1999, 14, 224–228 CrossRef PubMed.
- D. A. Navarro, M. A. Bisson and D. S. Aga, J. Hazard. Mater., 2012, 211–212, 427–435 CrossRef CAS PubMed.
- L. Zhao, J. R. Peralta-Videa, M. Ren, A. Varela-Ramirez, C. Li, J. A. Hernandez-Viezcas, R. J. Aguilera and J. L. Gardea-Torresdey, Chem. Eng. J., 2012, 184, 1–8 CrossRef CAS.
- S. Campiche, G. L'Ambert, J. Tarradellas and K. Becker-van Slooten, Ecotoxicol. Environ. Saf., 2007, 67, 180–189 CrossRef CAS PubMed.
- M. L. Paumen, E. Steenbergen, M. H. S. Kraak, N. M. Van Straalen and C. A. M. Van Gestel, Environ. Sci. Technol., 2008, 42, 6985–6990 CrossRef PubMed.
- J. Filser, Pedobiologia, 2002, 46, 234–245 Search PubMed.
- G. B. De Deyn, J. H. C. Cornelissen and R. D. Bardgett, Ecol. Lett., 2008, 11, 516–531 CrossRef PubMed.
- A. Manceau, K. L. Nagy, M. A. Marcus, M. Lanson, N. Geoffroy, T. Jacquet and T. Kirpichtchikova, Environ. Sci. Technol., 2008, 42, 1766–1772 CrossRef CAS PubMed.
- L. Jin, Y. Son, J. L. DeForest, Y. J. Kang, W. Kim and H. Chung, Sci. Total Environ., 2014, 466–467, 533–538 CrossRef CAS PubMed.
- Z. Qiu, Y. Yu, Z. Chen, M. Jin, D. Yang, Z. Zhao, J. Wang, Z. Shen, X. Wang, D. Qian, A. Huang, B. Zhang and J.-W. Li, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 4944–4949 CrossRef CAS PubMed.
- J. Filser, H. Koehler, A. Ruf, J. Römbke, A. Prinzing and M. Schaefer, Basic Appl. Ecol., 2008, 9, 346–355 CrossRef CAS.
- G. E. Swan, R. Cuthbert, M. Quevedo, R. E. Green, D. J. Pain, P. Bartels, A. A. Cunningham, N. Duncan, A. A. Meharg, J. L. Oaks, J. Parry-Jones, S. Shultz, M. A. Taggart, G. Verdoorn and K. Wolter, Biol. Lett., 2006, 2, 279–282 CrossRef CAS PubMed.
- P. Allard, R. Darnajoux, K. Phalyvong and J. P. Bellenger, Environ. Sci. Technol., 2013, 47, 2061–2068 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6en00007j |
| This journal is © The Royal Society of Chemistry 2016 |