Organic matter and iron oxide nanoparticles: aggregation, interactions, and reactivity
Abstract
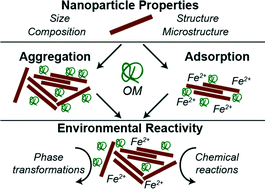
Understanding the fate and transport of engineered and naturally-occurring nanoparticles is vital to predicting their ecological and toxicological impacts. Much of the current literature details the effects of solution conditions, such as pH and ionic strength, on aggregation and reactivity. Such work has drastically improved our ability to predict how nanoparticles could impact chemistry occurring in natural waters. Recently, a focus on how organic matter (OM) impacts chemistry occurring at the solid–liquid interface has emerged. This review focuses on summarizing major findings of how OM affects iron oxide nanoparticle reactivity, with particular focus on the underlying processes. First, we review work focused on the chemical reactivity of iron oxide nanoparticles in aqueous environments. Second, the current state of knowledge regarding the adsorption of OM onto mineral surfaces and its effects on nanoparticle aggregation and ion adsorption is presented. Third, how OM impacts chemical and solid-state transformations, oxidative/reductive reactivity, and photocatalytic activity of iron oxide nanoparticles is reviewed. Finally, we provide our vision of future research directions, with particular focus on improving our ability to predict the fate, transport, and chemical behavior of nanoparticles in complex, environmental systems.

 Please wait while we load your content...
Please wait while we load your content...