NanoEHS – defining fundamental science needs: no easy feat when the simple itself is complex†
Vicki H.
Grassian
*a,
Amanda J.
Haes
*a,
Imali A.
Mudunkotuwa
a,
Philip
Demokritou
b,
Agnes B.
Kane
c,
Catherine J.
Murphy
d,
James E.
Hutchison
e,
Jacqueline A.
Isaacs
f,
Young-Shin
Jun
g,
Barbara
Karn
h,
Saiful I.
Khondaker
i,
Sarah C.
Larsen
a,
Boris L. T.
Lau
j,
John M.
Pettibone
k,
Omowunmi A.
Sadik
hl,
Navid B.
Saleh
m and
Clayton
Teague
n
aDepartment of Chemistry, University of Iowa, Iowa City, IA 52242, USA. E-mail: vicki-grassian@uiowa.edu; amanda-haes@uiowa.edu
bHarvard T. Chan School of Public Health, Harvard University, Boston, MA 02115, USA
cDepartment of Pathology and Laboratory Medicine, Brown University, Providence, RI 02912, USA
dDepartment of Chemistry, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA
eDepartment of Chemistry and Biochemistry, University of Oregon, Eugene, OR 97403, USA
fDepartment of Mechanical and Industrial Engineering, Northeastern University, Boston, MA 02115, USA
gDepartment of Energy, Environmental & Chemical Engineering, Washington University in Saint Louis, St. Louis, MO 63130, USA
hSustainable Nanotechnology Organization, 2020 Pennsylvania Ave. NW Washington, DC 20006, USA
iNanoscience Technology Center, University of Central Florida, Orlando, FL 32826, USA
jDepartment of Civil and Environmental Engineering, University of Massachusetts, Amherst, MA 01003, USA
kNational Institute of Standards and Technology, Material Measurement Laboratory, Gaithersburg, MD 20899, USA
lDepartment of Chemistry, State University of New York at Binghamton, Binghamton, NY 13902-6000, USA
mCivil, Architectural and Environmental Engineering, University of Texas, Austin, TX 78712, USA
nPixelligent Technologies, Baltimore, MD 21224, USA
First published on 1st September 2015
Abstract
Nanotechnology is no longer in its infancy and has made significant advances since the implementation of the National Nanotechnology Initiative (NNI) in 2000. Incorporation of nanotechnology in many fields including information technology, medicine, materials, energy, catalysis and cosmetics has led to an increase in engineered nanomaterial (ENM) production, and consequently, increased nanomaterial use. In comparison, the generation of concrete and consistent evidence related to the environmental health and safety of nanomaterials (NanoEHS) is lacking. The main factors contributing to the slower progress in NanoEHS versus conventional EHS are related to the complexity, property transformations, life cycles and behavior of nanomaterials even in carefully controlled environments. Therefore, new systematic, integrated research approaches in NanoEHS are needed for overcoming this complexity and bridging current knowledge gaps. A workshop on “NanoEHS: Fundamental Science Needs” brought together scientists and engineers to identify current fundamental science challenges and opportunities within NanoEHS. Detailed discussions were conducted on identifying the fundamental properties that are critical in NanoEHS, differentiating between conventional and NanoEHS studies as well as understanding, the effect of dynamic transformations on nanometrology, role of dosimetry and mechanistic data gaps in nanotoxicology. An important realization that even simple nanoscale materials can be complex when considering NanoEHS implications was noted several times during the workshop. Despite this fact, a number of fundamental research areas to further the scientific foundation to address NanoEHS needs are suggested.
Nano impactSlower progress in NanoEHS versus conventional EHS arises from the complexities encountered with nanomaterials even when carefully controlled model systems are used. This calls for a systematic approach towards fundamental research with the objective of de-convoluting these complex phenomena to establish structure–function relationships. As a step towards achieving this objective, this perspective identifies current challenges and fundamental research needs in NanoEHS. |
Introduction
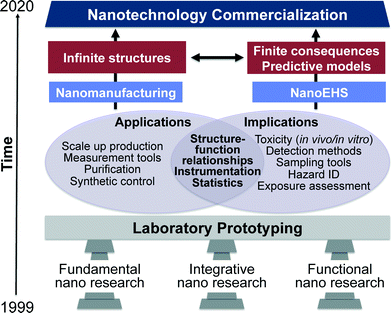
When considering length scales, nanomaterials represent the transition region between molecular entities and bulk materials and thus exhibit distinctly different properties from both, which manifest in many interesting chemical and physical phenomena.1,2 Nanoscale phenomena have led to numerous scientific discoveries and innovations over the past few decades and are impacting many areas such as information technology, materials, medical diagnostics, catalysis, energy and environmental applications.3–7The World Technology Evaluation Center (WTEC) reported on the vision, direction and progress from 2000–2020 extensively in their “nano1 (1999)” and “nano2 (2010)” reports.8–10 The timeline put forth in these reports aspires to achieve nanotechnology commercialization, mass production and use by 2020. According to the progress evaluation in “nano2 (2010)”, key nanotechnology indicators including science citation index (SCI), patent applications, final products in the market, research and development funding, venture capital and primary workforce in the nanotech industry show an average growth rate of 25% during the years 2000–2008. Four overlapping generations of products were identified in this progression: passive nanostructures (1st generation), active nanostructures (2nd), nanosystems (3rd) and heterogeneous molecular nanosystems (4th). A partial list of passive nanostructures includes coatings, nanoparticle dispersions, metals, polymers and ceramics. Actuators, amplifiers, transistors, targeted drugs and chemicals, and adaptive structures illustrate some active nanostructures. Examples of nanosystems include artificial tissues, photonic devices, nanoscale electromechanical systems (NEMS) and scalable plasmonic devices. Heterogeneous molecular nanosystems are the most advanced and include biomimetic systems with capabilities including, among many others, quantum control, selective and efficient catalysis, controlled interactions between light and matter and subcellular interventions. The current state of nanomanufacturing has successfully resulted in third generation nanostructures i.e., nanosystems. Therefore, the complexity, dynamics and transdisciplinarity of these materials have increased substantially. Consequently, this has resulted in a great deal of interest in understanding any risks associated with the environmental health and safety of nanomaterials.
To date, no known human diseases or serious environmental impacts have been reported that are specific to engineered nanomaterials. This is partly a result of their currently limited usage, a lack of epidemiological research, the vast variety of materials, and lack of extensive EHS research. Lessons from the past suggest that environmental and health consequences may take some time to manifest themselves. For example, by the time adverse effects of chlorofluorocarbons (CFCs) were discovered, the ozone hole had developed.11 Likewise, the adverse health implications of asbestos were found after many lung cancer cases, specifically after mesothelioma was diagnosed. Use of dichlorodiphenyltrichloroethane (DDT) for the control of malaria and typhus, as well as its use as an agricultural insecticide endangered wildlife and was later determined to be a human carcinogen.12 These unforeseen consequences ultimately resulted in phasing out the usage of these chemicals under international treaties such as the Montreal Protocol and Stockholm Convention and national environmental regulations. In order to prevent a similar fate and ensure sustainability of nanotechnology industry and maximum societal benefits, there is a need to develop and validate, robust scientific platforms for hazard identification, exposure monitoring and risk assessment along with predictive toxicological approaches for ENMs. Most importantly, NanoEHS must be an integral aspect of nanomaterial development.
Over the past decade, the perspective that “nano is dangerous” shifted to “nano can be made safe” by employing engineering principles to their safe design, which should positively impacted nanomanufacturing and NanoEHS.10,13 This has been a multidisciplinary effort with fundamental, mechanistic, functional and computational study designs. However, given the rapid advancements in nanomanufacturing potentially generating an infinite number of nanostructures, the sustainable nanotechnology community is faced with concerns about increasing knowledge gaps between new material designs and their safety.14 Despite efforts to assess the toxicological impacts of nanomaterials, uncertainty remains. At a recent workshop sponsored by the International Agency for Research on Cancer (IARC) in October 2014 to evaluate carcinogenicity of inhaled fibrous nanomaterials for humans, the working group was able to evaluate only one type of carbon nanotube as possibly carcinogenic to humans based on a limited number of studies showing carcinogenicity in rodents.15 Furthermore, no established predictive models for adverse environmental and health impacts of ENMs have been developed as envisioned by WTEC in the year 2000.
In the “nano2 (2010)” report, three pillars of nanotechnology are classified as fundamental nano, evolutionary nano and revolutionary nano.10 In this current perspective these three pillars have been reclassified to apply to both nanomanufacturing as well as NanoEHS and are classified as fundamental, integrative and functional nano research (Fig. 1). From laboratory prototyping to commercialization of nanotechnology, the development in all three pillars must continue at equal paces. Fundamental studies facilitate fabricating materials with increasing complexity, novel, properties and predictable structure–function relationships. This enables conceptual developments leading to materials classification, extending molecular concepts to nanostructures such as valency, and moving beyond the controlled and simple designs to anisotropic structures and complex matrices encountered in integrative and functional nano research. These fundamental discoveries are critical for the advancements in both nanomanufacturing as well as NanoEHS; therefore, the workshop on “NanoEHS: Fundamental Science Needs” that took place in November 2014 focused on identifying the current fundamental science challenges, opportunities, and needs of the broad area of NanoEHS to move the field forward so that the next level of challenges and complexity can be addressed. An important realization of the workshop was that “simplifying complexity is no easy feat when the simple itself is complicated”.
There are many literature examples that show even single component systems of nanoscale materials can exhibit complex behavior that are sensitive to even the smallest change in the surrounding environment and capable of triggering changes to the surrounding environment. An extensive study conducted using surface enhanced Raman scattering for the detection and quantification of 2-naphthalenethiol in a carefully controlled aqueous medium with 12 nm gold nanoparticles showed that the measurement signals are subjected to temporal fluctuations resulting in inconsistent and irreproducible nanomaterial interactions and signals.16 The study attributed these fluctuations to changes in the aggregation state of the nanoparticles that varies with molecular surface coverage as well as nanoparticle concentration. Another study with 4 nm titanium dioxide nanoparticles in an aqueous medium showed dramatically different aggregation behavior depending on the presence of citric acid ligands as well as the medium pH.17 Both pH and ligand adsorption resulted in surface charge changes on these nanoparticles. Calculations of longitudinal surface plasmon polariton resonances of gold nanorods showed that the generated electric fields are not uniformly distributed on the metal surfaces but concentrated at the two ends.18 From these examples, it is clear that additional fundamental work is warranted to develop an improved understanding of how these materials behave as these simple examples are complex and are discussed below in detail.
Highlights of the workshop on NanoEHS fundamental science needs
The proceedings of this workshop are summarized in this perspective under five main categories: (i) Size and Shape Dependent Properties Important in NanoEHS; (ii) Understanding Nanomaterial – Molecule Systems; (iii) Dynamic Transformations of Nanoparticles; (iv) Dosimetry in NanoEHS; and (v) Mechanistic Data Gaps in Toxicity of Nanomaterials. These categories were based upon the four presentations given by the speakers at the workshop. The first section introduces the fundamental properties of nanomaterials while linking their functional forms to NanoEHS and some important but often overlooked considerations. The objective of the second section is to convey subtle and obvious differences between conventional molecule–molecule systems and nanomaterial–molecule systems. Given the first and second sections provide an overall understanding of the unique nature of ENMs, the third section aims to describe the complexity of nanomaterial systems and how to tackle this complexity using fundamentals concepts and techniques. The fourth section focuses on the often overlooked use and importance of dosimetry and how this might relate to inconsistencies and irreproducibility in nanotoxicology. The final section highlights some of the missing links from mechanistic studies to nano-bio systems and the importance of having high throughput in vitro assessment models without limiting in vivo studies. Overall, these sections aim to synthesize viewpoints from a wide group of interdisciplinary researchers to identify the challenges facing NanoEHS as well as research needs to further the use of nanotechnology for the benefit of society in a sustainable way.Size and shape dependent properties important in NanoEHS
The total free energy of any material is given in eqn (1),| Gtotal = Gbulk + Gsurface | (1) |
Dimensionality categorization allows for classification of nanostructures based on their morphology. Zero dimensional nanostructures are nanoparticles where all dimensions are confined to the nanoscale. Nanorods and nanowires are some examples of one dimensional nanostructures as one dimension of the materials can extend beyond the nanoscale. Thin films with nanoscale thickness are considered as two dimensional nanostructures. Therefore, the physical – chemical properties of these nanoscale materials become both size and shape dependent, which depend on their dimensionality and are often unique from their bulk counterparts.
The most prominent size and shape dependent properties that are critical for NanoEHS are summarized in the following section. Although these are well-known concepts, the current perspective links the fundamental properties and nanomaterial functional forms to NanoEHS investigations for a broad audience. In addition, the critically important but often overlooked role of impurities, dopants, defects and sample heterogeneity is discussed as it relates to NanoEHS.
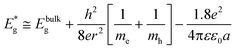 | (2) |
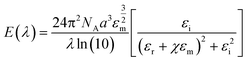 | (3) |
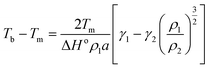 | (4) |
Understanding nanomaterial – molecule systems
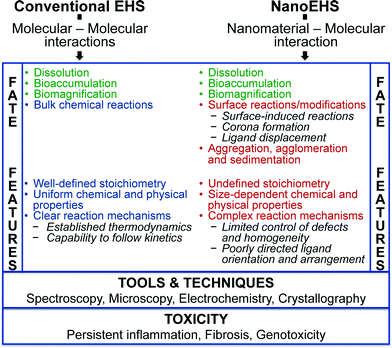
There are fundamental similarities and differences between conventional EHS and NanoEHS studies. The most critical similarity between these is the size dependence of biological barrier crossing and motivates concern towards nanotechnology given toxicological impacts. Critical differences between conventional EHS and NanoEHS studies originate at a fundamental level where molecule–molecule and nanomaterial–molecule interactions are considered, respectively (Fig. 2). Molecule–molecule interactions are established and described in terms of interaction energies, stoichiometry, thermodynamics and kinetics. Molecule–molecule fate and transformations are well-studied. Although these investigations are ongoing, a satisfactory understanding has been achieved and used to set up regulatory controls and engineering measures. The same, however, cannot be said about nanomaterial–molecular systems including interactions between nanomaterials and small molecules as well as large macromolecules with similar nanoscale dimensions (e.g., proteins). The following are some unique features of nanomaterial–molecular/macromolecular systems:• The thermodynamics of nanomaterial systems depend on surface energy. Molecules adsorb onto nanoparticle surfaces to reduce the surface energy but do not follow conventional EHS molecular–molecular interactions. Instead, a non-stoichiometric distribution of molecules to nanoparticles is observed.
• In the place of well-defined chemical bonding and weaker interactions, nanomaterial–molecule systems depend on nanoparticle shape and size dependent adsorption, surface modification, corona formation, displacement and surface induced reactions. Variations in molecular orientation, conformation and structure on nanomaterial surfaces can subsequently influence reactivity. For instance, a case study conducted with enzyme-functionalized Au nanorods demonstrated that the exact chemical attachment method used to immobilize the enzyme on the nanorod surfaces affected biological activity.48
• Nanomaterial–molecule system stability becomes multi-dimensional and complex from physical, chemical and biological perspectives, but minimal information related to the thermodynamics and kinetics of these complex systems is known.
• Inter-particle interactions often lead to aggregation and agglomeration. Aggregation in real environmental systems can be either homogeneous or heterogeneous. Aggregation also decreases the surface area and surface energy of the nanomaterials and impacts the overall thermodynamic and kinetic stability of the system in addition to the properties of the nanomaterial itself.
Two key questions raised in a recent perspective article on the surface-ligand chemistry of nanocrystals are (1)what is the nature of the surface interaction and (2) how do these interactions influence the frontier orbital structure and the fate of excited charge carriers.49 An additional question to add to the list is whether these interactions control the “toxicity” of nanoparticles. These questions originate from (but are not limited to) the observations made in changes to the electronic structure of nanocrystals upon surface ligand coordination. They not only encapsulate all of the above highlighted unique features of nanomaterial–molecule systems but also emphasize the need of fundamental research to understand surface interactions in great molecular detail. Given that in all NanoEHS related research, nanomaterial–molecular/macromolecular systems can influence nanoparticle behavior and implications, answering these questions are important. Although mathematical equations that link shape and size dependent chemical-physical properties are known, similar expressions relating biological responses (e.g. ROS production) to extrinsic and intrinsic nanomaterial properties are still needed.
A common set of tools and techniques available to address these NanoEHS concerns involve detecting, monitoring and analyzing nanomaterial systems. These techniques, including atomic force microscopy (AFM), secondary ion mass spectrometry (SIMS), surface enhanced Raman spectroscopy (SERS), single particle inductively coupled plasma mass spectrometry (SP-ICP-MS), X-ray photoelectron spectroscopy (XPS) and infrared spectroscopy (IR), provide some sense of proximity of chemical signatures relative to a nanoparticle surface but suffer from several limitations thus failing to provide absolute molecular spatial arrangement. For instance, vibrational spectroscopy is limited by laser spot size, which can be as large as several microns. AFM, on the other hand, can only distinguish molecules with significantly different molecular heights. The sensitivity in the z-direction is excellent (sub-nanometer resolution), but spatial resolution in the x–y plane is poor. Measurements with nanoparticles are especially difficult due to many contributing factors, which should be better recognized. For instance, all of these techniques make assumptions based on flat surfaces; therefore, surface curvature effects are not considered. Super-resolution spectroscopy, on the other hand, holds great potential at overcoming this limitation but currently is restricted to matter with large optical cross-sections and suffers from high cost and low sample throughput. Furthermore, surface adsorbed ligands can exhibit different dielectric properties from non-adsorbed ligands. This difference can induce changes in the dielectric environment near the nanoparticle surface, which can influence several of these surface analytical measurements in unexpected ways.
In terms of characterization, current techniques involve mostly dry characterization and to a lesser extent, wet characterization. Although dry characterization provides a basis to build on for NanoEHS studies, work is needed to translate these measurements into structure–function relationships. Given the dynamic nature of nanomaterials, initial dry characterization conducted with primary nanoparticles might not be sufficient. With the exception of visible absorption spectroscopy, fluorescence, dynamic light scattering, nanoparticle tracking analysis and some vibrational spectroscopic techniques, many of these characterization techniques are not suitable for understanding the biological, chemical, and physical properties of nanoparticles in dynamic environments. Therefore, a pressing NanoEHS need is the development of methods capable of in situ characterization of nanomaterials in complex systems. In this aspect and instead of a single technique, hyphenated techniques are promising for multiplexed measurements in the same environment.
Dynamic transformations of nanoparticles
The main underlying factor contributing to challenges encountered in NanoEHS studies within complex matrices is related to the lack of understanding of the dynamic nature of nanomaterials. Nanomaterials interact with the surrounding system and undergo dynamic transformations at rapid time scales in a system dependent manner. For instance, the dynamic transformations of metal or metal oxide nanoparticles were shown to contribute to the overall interactions of these nanomaterials with cells and the environment.50,51 Often, these are monitored using various analytical measurements as well as predicted using theoretical models52 but are limited by the detectable properties of various particle populations as discussed earlier. These interactions and transformations can be experimentally evaluated as a change in or loss of signal over time; however, these signal variations often lack reproducibility and contrast theoretical predictions. Lack of consistent and reproducible analytical measurements of nanoparticle systems is a challenge in NanoEHS and can hinder progress in the field. These dynamic interactions and transformations, however, occur in a complex yet predictable manner.53 Thus, identification of parameters that affect nanomaterial dynamics is the first step towards achieving control.Parameters that can affect nanomaterial measurements include time, molecular concentration, nanoparticle concentration, sample heterogeneity, solvent composition as well as surface roughness, curvature, charge, functionality and hydrophobicity. These contribute to dynamic nanomaterial transformations by changing their solution phase states and dimensionality as discussed in the previous section.17,50,51,54 A combination of several transformations including ion dissolution, chemical transformations (e.g., oxidation, sulfidation, etc.) and protein corona fingerprinting are likely important in these complex systems and could lead to new and/or unexpected interactions in complex environments. These transformations, in turn, can change the biochemical, biophysical and physicochemical properties of the materials that are observed.55 An underlying challenge, however, is the reproducibility of these measurements and nanomaterial products both of which are crucial in both applications and NanoEHS implications.56 One approach to tackle this system complexity and track dynamics is an in-depth investigation of fate descriptors with the aid of reference materials with homogenous surfaces. These include Coulomb forces, London dispersion (hydrophobicity), acidity, basicity, polarity and polarizability, and lone pairs.57–59 By investigating how these properties are affected as a function of time, molecular concentration, solvent composition and nanoparticle concentration, surface roughness, curvature, charge, surface functionality, surface ligand conformation and orientation, density, charge, and hydrophobicity,53 a systematic understanding of nanomaterial transformations can be made. In fact, these numerical descriptors could potentially be used in NanoEHS regulations if accurate estimates are obtained. This requires the development of standards and reference materials for validation.
Alternative to these experimental approaches for understanding nanoparticle dynamics on NanoEHS studies, semi-empirical methods can be adopted to improve realistic theoretical predictions of nanomaterial stability in different matrices. For colloidal suspensions, extended Derjaguin–Landau–Verwey–Overbeek (xDLVO) theory provides a platform to predict nanomaterial stability by calculating energetics as a function of separation distance in two-dimensions by considering several interaction parameters including: van der Waals, electrostatic, acid-base, bridging, elastic, hydration, hydrophobic, magnetic and/or osmotic contributions.59 Each of these parameters accounts for different nanoparticle attributes in the system of interest; however, application of this theory on nanoscale colloidal suspensions needs careful consideration of size and shape dependent properties as well as the surface adsorbed ligands.60,61 For example, the Hamaker constant used in calculating the attractive van der Waals interactions depends on the size-dependent dielectric properties of the material.61 Additionally, electrostatic, osmotic and elastic interactions are largely affected by the surface potential, solvent, ligand packing density and orientation at the surface, as well as surface curvature. While DLVO models and other non-DLVO models are useful in qualitatively describing nanomaterial stability, fate and transformations in complex matrices; these methods can also aid in optimizing the synthetic and storage conditions for nanomaterials. This information can potentially guide materials design and storage solution conditions used.
Another significant challenge for understanding surface dynamics is time scales. While equilibrium thermodynamics dictate the ultimate final state of the system, kinetic factors often are more important and control the state of the system. Depending on particle mobility under constantly changing environmental and biological conditions, a given system can exhibit several quasi-equilibrium states specific to a given set of conditions. Given the rapid (or not so rapid) times scales, these transformations can occur, and analytical methods used for analyzing and tracking these transformations require a dynamic range of measurements. Another approach to understanding nanomaterials is molecular dynamic (MD) simulations, which can provide unique information that is difficult to learn through experiment.62–65 Typically, MD simulations use ideal surfaces and solution conditions, which may not be representative of realistic conditions. Furthermore, MD simulations are often computationally expensive thus requiring supercomputers, long computing time, and selection of best case conditions for modeling. All in all, these predictive capabilities depend on collision frequencies and functions and are only as good as the initial model inputs.61 All in all, the fate and transformation of nanomaterials is governed by the physical, chemical and biological stability of the material. Without clear definitions or guidelines of nanomaterial stability in simple systems, reliable risk assessment and hazard identification can neither be achieved for more complex systems nor reproducible. The fundamental research conducted under well-controlled conditions is being challenged for its usability in complex scenarios. Given that nanomaterial stability affects characterization measurements made on these systems, the key to linking simple and complex samples could be through establishing clear metrics, common terminology, and systematic approaches to multi-parameter experimental design, new instrumentation, and predictive modeling that incorporate size and shape dependencies as well as validation protocols. Furthermore, quantitative predictions and identifiers are of paramount importance for determining stability and fate of nanomaterials. Because some size dependent properties become more significant at size scales less than ~10 nm, appropriate size effects in dry and wet environments need to be evaluated and explored. Indeed, from a toxicological perspective; it is important to identify the size regimes where these effects are pronounced. Theoretical physicists and chemists have a responsibility to communicate with more applied disciplines as to which nanoparticle size regimes exhibit relevant biological, chemical, and physical effects thereby minimizing the number of NanoEHS studies.
In vitro dosimetry in NanoEHS
Accurate and meaningful exposure or dose metrics are a basic yet elusive requirement for in vitro screening methods to assess potential health risks of ENMs. Dosimetry is the quantification of the exact amount of materials interacting with species/cells over time and is a key determinant in pharmacological and toxicity studies. Especially in the risk assessment paradigm for ENMs, hazard identification requires information of dose and exposure conditions in addition to nanoscale properties. Hazard characterization requires additional details of exposure concentrations, deposited doses and time data. Risk is then determined from both exposure and dose-response data. More importantly, in vitro doses need to match the same scale as in vivo doses for validation and inter-comparison purposes. It is worth noting that in vitro ENM testing has often failed to produce results consistent with those of corresponding in vivo studies.66,67 These discrepancies are attributed not only to inadequate characterization of ENMs and lack of standardized methodologies, but also as a result of miscalculation or mischaracterization of the in vitro dose or exposure as a function of time.68,69 More recently, evidence continues to grow indicating that in vitro dosimetry may alter the hazard ranking of low aspect ratio ENMs.70Until recently, most in vitro studies have reported dose in terms of either an initial administered mass concentration or a total administered mass. Consequently, the biological response can be related to particle surface area, particle number or mass concentration. In each case, the dose metrics assumed that sedimentation or aggregation does not occur or is negligible. Total administered mass assumes that sedimentation is complete, with all of the suspended material instantly transported to the cells at the bottom of the cell culture vessel. Actual dosage, however, depends on the physical properties of the suspended ENM and culture media as well as the time course of exposure.
Therefore, emphasis has been placed on achieving a better understanding of exposures experienced by cells in vitro with accurate and meaningful dosimetry.68–71 These include several standardized methodologies for the physical characterization as well as fate and transportation modeling of both stable and agglomerated suspensions. For in vitro systems, hydrodynamic diameter (d) controls the diffusion (eqn (5)) while the effective density (ρE) is a fundamental property governing the sedimentation of colloidal materials (eqn (6)) as shown below,
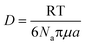 | (5) |
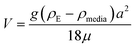 | (6) |
Measuring agglomeration for in vitro nanotoxicological studies is essential. These measurement techniques have progressed from dynamic light scattering, analytical ultracentrifugation and nanoparticle tracking analysis to volumetric centrifugation methods. Each of these has advantages and disadvantages that limit accurate characterization of agglomerates. The volumetric centrifugation method provides a fast and inexpensive alternative for determination of effective density of ENMs in suspension69 and suggest that the effective density of agglomerates are significantly different from the bulk material.
In order to gain time-dependent dose data, in vitro sedimentation and diffusion dosimetry models and advanced multidimensional numerical models can be used to calculate nanoparticle concentration gradients within in vitro systems by considering effective density values. These models make it possible to determine the mass, particle number or surface area of material deposited or delivered to cells as a function of time.69–72 Although limitations of the models assume monodisperse agglomerates, useful approximation of the relative transport and effective dose of different ENMs are provided.69,70
These models, however, do not account for many dynamic nanomaterial transformations such as dissolution, re-precipitation and displacement reactions that are also capable of influencing in vitro dosimetry data. Current studies in nanotoxicology, which depend on comparisons with negative and positive controls, assume that the particles used in these controls have the same partico-kinetic behavior as the inherently transformed nanomaterials. In light of this, existing hazard rankings need re-evaluation by considering in vitro dosimetry results. In addition, standard practices for dispersion protocols, solvent compositions and particle concentrations need to be unified in order to facilitate study-to-study comparisons.68
Mechanistic data gaps in toxicity of nanomaterials
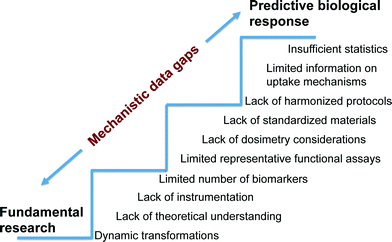
Establishing the mechanisms responsible for adverse biological impacts of nanomaterials can be an extremely challenging task because contrary to the relatively controlled systems used in fundamental research, biological systems are multi-component, multi-functional and dynamic. Thus, assumptions and ideal conditions used in fundamental studies are often not directly applicable to complex biological systems (Fig. 3). Furthermore, clear identification of the specific chemical and physical properties that trigger toxicity are not known for the wide range of ENMs already commercialized. In addition, arguments relating the sensitivity of biological systems to materials in the scale of 1–100 nm are inconclusive as a result of limited understanding of uptake, translocation, and excretion mechanisms relative to their larger counterparts.73Nevertheless, there is a body of literature that suggests significant potential detrimental biological impacts of nanomaterials. For example, experimental studies of human epithelial cells in vitro suggest that carbon nanotubes (CNTs) induce disruption of the mitotic spindle, centrosomal damage, and aneuploidy, all of which are indicative of genotoxicity.74 Parallel in vitro and in vivo studies have shown correlations between metal catalyst residues in multiwall carbon nanotubes (MWCNTs) and lung inflammation.75 Furthermore, engineered metal and metal oxide nanomaterials composed of Cu, Zn, Ag, TiO2, ZnO, CuO, CeO2 and Fe2O3 have been shown to induce membrane permeability, cytotoxicity, DNA damage, enhanced oxidative stress, inflammation and developmental abnormalities in both in vitro and in vivo models.76–80 Investigators at the National Institute of Occupational Safety and Health (NIOSH) conducted a CNT inhalation study in mice in which lung tissue was sectioned after exposure and evaluated using fluorescence and dark-field microscopy as well as scanning electron microscopy. MWCNTs were observed on the alveolar wall, which is indicative of incomplete lung clearance mechanisms. Lung histology one year after exposure revealed an increase in alveolar wall thickness and collagen deposition. There was also evidence of translocation of 8% of the deposited dose to distant sites including the lymph nodes, liver, kidney, heart, and brain.81 Despite this important in vivo study, there are significant gaps in understanding how MWCNTs penetrate the alveolar wall, stimulate collagen deposition or scarring, and translocate to distant sites. There are many contributing factors responsible for significant data gaps in mechanisms of nanomaterial toxicity.
• There are many variations in chemical and physical properties even within the same class of materials (i.e., sample heterogeneity). A straight-forward example is that CNTs have a single CAS number (CAS 308068-56-6) yet their chemical and physical properties vary significantly over a broad range of chirality, dimensions, mechanical strengths, shapes and surface areas as well as electrical, electronic and thermal properties. This leads to challenges in generalizing and correlating biologically-relevant mechanisms.
• The inability to synthesize ENMs with narrow size distributions, single chirality and known defect densities or zero defects increases the number of possible toxicological outcomes. This calls for the development of reproducible synthesis procedures, and efficient purification processes as well as standard reference material library development.
• Mechanisms of crossing anatomic barriers and understanding how nanomaterial properties transform in biological environments and influence their toxicity are not established for most ENMs.
• Failure to consider dosimetry, poor selection of dose-metrics and the lack of tools for in situ characterization lead to conflicting results and an inability to draw general NanoEHS conclusions.
To overcome these challenges, systematic studies that identify specific chemical and physical properties that are related to toxicological outcomes, translocation across anatomic barriers, and transformation of nanomaterial properties in vivo are required. There is an increasing demand for developing cheaper, faster, and high throughput toxicity testing assays capable of predicting biology outcomes without the use of animal toxicity testing. This requires understanding of mechanistic toxicity pathways for the construction of models based on well-characterized, standardized materials.82 These in vitro toxicity assays are expected to be established via fundamental research. However, it is currently difficult to directly translate in vitro cellular toxicity assays to potential disease outcomes in humans. In addition, chronic disease impacts are difficult to predict because most in vitro toxicity assays use cells in 2-dimensional, monolayer cultures and acute (24–48 hours) exposures. Advances in tissue engineering could potentially provide a solution to this data gap as 3-dimensional cell cultures maintain their stability and differentiation for 14–21 days.83 New techniques are emerging for in situ localization and characterization of nanomaterials in cells and tissues. For example, confocal Raman microscopy is a powerful visualization tool for carbon nanomaterials that exhibits minimal interferences from background biological tissues.84 Finally, molecular dynamics simulations have the potential to predict nanomaterial–biological interactions at a fundamental level resulting in deeper insights into mechanistic toxicology of ENMs.85
Challenges and research needs for NanoEHS identified in the workshop
To make simultaneous progress with nanomanufacturing such that “nano can be made safe” and to address potential challenges arising with the increasing commercialization of nanotechnology, there are several research areas that are critical for sustainable nanotechnology to move forward. In order to address research needs, some key challenges were identified during the workshop:• There are an infinite number of nanostructures with distinct chemical and physical properties. Typically, these diverse structures are not intentionally engineered to be different. Rather, poor synthetic control, especially during mass production, gives rise to nanomaterials with inconsistent nuclei formation in the initial nucleation stages before further particle growth.
• Once released into the environment, these materials undergo rapid and dynamic transformations. Unfortunately, the limited capabilities of established tools and techniques for tracking these nanomaterials prevent nanomaterial characterization from source to sink.
• As a consequence of these dynamic transformations, currently employed analytical techniques result in inconsistent measurements leading to unreliable predictions.
• NanoEHS is related to nanomaterial–molecule systems that are significantly different from conventional EHS studies that focus on molecule–molecule systems. This difference exists at very fundamental levels, but many current NanoEHS studies correlate toxicity to bulk material properties rather than to unique nanomaterial properties.
• Molecular dynamic simulations for these systems require supercomputing power and selection of best-case scenario model inputs but ultimately depend on input parameters. These input parameters are not well-established for nanomaterials.
• There are significant inconsistencies, lack of statistics and irreproducibility among nanotoxicity data for a single class of nanomaterials. Sources of these inconsistencies have not yet been identified or are preliminary.
• Applicability of existing model studies using single nanomaterial systems are not yet tested for more complex hierarchical nanostructures such as nanohybrids. These materials encompass intrinsic material properties as well as unique dimensionalities and functional properties that can potentially deviate from the single components.
To address these challenges, there are multiple and tiered levels of research needs. Especially in fundamental chemical and physical research studies, efforts need to be translated from experiments done on model systems in cuvettes and under tightly controlled systems to complex and dynamic environmental systems. Piecing together the complex reaction network from individual reactions similar to physical chemistry approaches in other fields.14 By doing so, progress can be made for developing structure–activity relationships. Currently, the International Organization for Standardization has described a classifying system (ISO/TR 11360:2010) for a wide range of materials based on the dimensionality of different physical, chemical, magnetic and biological properties. In addition, the Organization for Economic Corporation and Development (OECD) has identified four categories for the end points: (1) state of dispersion, aggregation and agglomeration, (2) size and size distribution, (3) surface area and porosity and (4) surface reactivity. Although these classifications provide basic tools to address the challenges in NanoEHS, workshop participants identified a hierarchical set of research needs with objectives to:
• Establish an approach for nanomaterial classification that will enable reduction of the infinite number of nanostructures into a manageable set of outcomes. This requires going beyond the classification set forth by the ISO and OECD and incorporating complex properties such as material defects, dopants and sample heterogeneity into the characterization and classification.
• Define and determine the physical, chemical and biological stabilities of nanomaterials such that the correct endpoints for measurements can be standardized for correct classification.
• Achieve a better theoretical understanding of the size dependent physicochemical properties such as band gap energies, redox potentials, phase transitions, surface relaxation, reconstruction and defects and subsequent implications of these nanomaterial properties.
• Understand the mechanistic aspects in nanomaterial synthesis and fabrication to better control polydispersity and nucleation.
• Identify the chemical and physical properties that might significantly induce and influence nanotoxicity. With carefully controlled experiments based on these properties, a finite set of data can be collected so that stability identifiers can be taken into consideration and reported in a time dependent manner (short, medium and long terms). The fate and transformation of nanoparticles in the environment throughout their life cycle should be characterized in terms of their overall properties, which should be related to step-wise changes in size, shape and surface chemistry.
• Elucidate the impact of thermodynamic and kinetic stability through detailed investigation of nanomaterial homo- and hetero-aggregation under varying conditions.
• Incorporate accurate in vitro and in vivo dosimetry in all NanoEHS investigations.
• Establish standardized toxicity assessment protocols and develop high purity reference materials to overcome the inconsistencies and irreproducibility of nanotoxicological studies.
• Develop functional assays that link laboratory based-fundamental research to more complex environmental systems. However, a strong foundation using fundamental studies to develop predictive models prior to these ensemble measurements is imperative.
• Translate the outcomes of functional assays to understand, model and predict the fate of nanomaterials in biological and environmental systems.
• Develop numerical descriptors that incorporate all relevant factors such as toxicity and mobility for numerical nanomaterial hazard potential.
• Use these findings as inputs in molecular dynamic simulations to improve the resulting outputs and widen model applicability.
• Develop tools for the in situ characterization of nanomaterials and nanomaterial surfaces.
Although there have been great strides on some issues related to NanoEHS in the last decade, further consideration and new approaches are necessary for additional progress. Thus, the development of new approaches and tools are necessary for successfully addressing the outlined NanoEHS challenges and research needs. Additionally, nanomaterial synthesis has advanced from primary particles to also include conjugated, complex, and adaptive nanostructures.86 Such complex nanohybrids modulate both the inherent physicochemical properties of the materials as well as novel emergent properties, (i.e., unique dimensionality, stiffness, and multifunctionality).87–89 There are only few systematic studies assessing the NanoEHS concerns for these hybrids given the previously discussed fundamental knowledge gaps for primary nanoparticles. Finally, human epidemiological studies that investigate the long-term effects of ENMs such as Ag nanoparticles, which are directly consumed as a medication, are lacking. Taking everything into consideration that has been discussed here, it is clear that funding agencies need to support NanoEHS at an appropriate level.
Acknowledgements
The workshop was supported by the National Science Foundation (NSF) under grant CBET-1441457. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. The organizers also thank Omowunmi Sadik and Barbara Karn, President and Executive Director, respectively, of the Sustainable Nanotechnology Organization (SNO) and the 2014 SNO organizers Philip Demokritou and Jacqueline Isaacs for facilitating the organization of the workshop.References
- G. M. Whitesides, Small, 2005, 1, 172–179 CrossRef CAS PubMed.
- M. C. Roco, Nanoscale Science, Engineering and Technology Subcommittee, U.S. National Science and Technology Council, AIChE J., 2004, 50, 890–897 CrossRef CAS.
- B. Radisavljevic, M. B. Whitwick and A. Kis, ACS Nano, 2011, 5, 9934–9938 CrossRef CAS PubMed.
- M. G. Panthani, J. M. Kurley, R. W. Crisp, T. C. Dietz, T. Ezzyat, J. M. Luther and D. V. Talapin, Nano Lett., 2013, 14, 670–675 CrossRef PubMed.
- M. A. Mahmoud, R. Narayanan and M. A. El-Sayed, Acc. Chem. Res., 2013, 46, 1795–1805 CrossRef CAS PubMed.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS PubMed.
- G. Pyrgiotakis, J. McDevitt, A. Bordini, E. Diaz, R. Molina, C. Watson, G. Deloid, S. Lenard, N. Fix, Y. Mizuyama, T. Yamauchi, J. Brain and P. Demokritou, Environ. Sci.: Nano, 2014, 1, 15–26 RSC.
- M. C. Roco, R. S. Williams and P. Alivisatos, Nanotechnology Research Directions, U.S. National Science Foundation and Technology Council, Washington DC (also published in 2000 by Kluwer Academic Publishers, Boston), 1999 Search PubMed.
- M. C. Roco, C. A. Mirkin and M. C. Hersam, J. Nanopart. Res., 2011, 13, 897–919 CrossRef.
- M. C. Roco, C. A. Mirkin and M. C. Hersam, Nanotechnology Research Directions for Societal Needs in 2020: Retrospective and Outlook, 2011 Search PubMed.
- E. A. Parson, Protecting the Ozone Layer : Science and Strategy, Oxford University Press, New York, 2003 Search PubMed.
- V. Turusov, V. Rakitsky and L. Tomatis, Environ. Health Perspect., 2002, 110, 125–128 CrossRef CAS PubMed.
- J. E. Hutchison, ACS Nano, 2008, 2, 395–402 CrossRef CAS PubMed.
- V. H. Grassian and R. J. Hamers, Nanomaterials and the Environment-The Chemistry and Materials Perspective : A report from a NSF supported workshop, 2011 Search PubMed.
- Y. Grosse, D. Loomis, K. Z. Guyton, B. Lauby-Secretan, F. El Ghissassi, V. Bouvard, L. Benbrahim-Tallaa, N. Guha, C. Scoccianti, H. Mattock and K. Straif, Lancet Oncol., 2014, 15, 1427–1428 CrossRef CAS PubMed.
- M. C. S. Pierre, P. M. Mackie, M. Roca and A. J. Haes, J. Phys. Chem. C, 2011, 115, 18511–18517 CAS.
- I. A. Mudunkotuwa and V. H. Grassian, J. Am. Chem. Soc., 2010, 132, 14986–14994 CrossRef CAS PubMed.
- S. T. Sivapalan, J. H. Vella, T. K. Yang, M. J. Dalton, R. N. Swiger, J. E. Haley, T. M. Cooper, A. M. Urbas, L.-S. Tan and C. J. Murphy, Langmuir, 2012, 28, 9147–9154 CrossRef CAS PubMed.
- F. D. Fischer, T. Waitz, D. Vollath and N. K. Simha, Prog. Mater. Sci., 2008, 53, 481–527 CrossRef CAS.
- K. E. Andersen, C. Y. Fong and W. E. Pickett, J. Non-Cryst. Solids, 2002, 299–302(Part 2), 1105–1110 CrossRef CAS.
- K.-F. Lin, H.-M. Cheng, H.-C. Hsu, L.-J. Lin and W.-F. Hsieh, Chem. Phys. Lett., 2005, 409, 208–211 CrossRef CAS.
- L. E. Brus, J. Chem. Phys., 1984, 80, 4403–4409 CrossRef CAS.
- Z.-C. Zhang, B. Xu and X. Wang, Chem. Soc. Rev., 2014, 43, 7870–7886 RSC.
- Z. Yin, L. Lin and D. Ma, Catal. Sci. Technol., 2014, 4, 4116–4128 CAS.
- F. Shi, Q. Zhang, Y. Ma, Y. He and Y. Deng, J. Am. Chem. Soc., 2005, 127, 4182–4183 CrossRef CAS PubMed.
- C. Kliewer and G. Somorjai, Catal. Lett., 2010, 137, 118–122 CrossRef CAS.
- S. H. Joo, J. Y. Park, J. R. Renzas, D. R. Butcher, W. Huang and G. A. Somorjai, Nano Lett., 2010, 10, 2709–2713 CrossRef CAS PubMed.
- F. Tao, M. E. Grass, Y. Zhang, D. R. Butcher, F. Aksoy, S. Aloni, V. Altoe, S. Alayoglu, J. R. Renzas, C.-K. Tsung, Z. Zhu, Z. Liu, M. Salmeron and G. A. Somorjai, J. Am. Chem. Soc., 2010, 132, 8697–8703 CrossRef CAS PubMed.
- C. J. Kliewer, C. Aliaga, M. Bieri, W. Huang, C.-K. Tsung, J. B. Wood, K. Komvopoulos and G. A. Somorjai, J. Am. Chem. Soc., 2010, 132, 13088–13095 CrossRef CAS PubMed.
- J. C. White and P. K. Dutta, J. Phys. Chem. C, 2011, 115, 2938–2947 CAS.
- A. J. Haes, C. L. Haynes, A. D. McFarland, G. C. Schatz, R. P. Van Duyne and S. Zou, MRS Bull., 2005, 30, 368–375 CrossRef CAS.
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115–2120 CrossRef CAS PubMed.
- D. P. O'Neal, L. R. Hirsch, N. J. Halas, J. D. Payne and J. L. West, Cancer Lett., 2004, 209, 171–176 CrossRef PubMed.
- A. Navrotsky, ChemPhysChem, 2011, 12, 2207–2215 CrossRef CAS PubMed.
- S. H. Tolbert and A. P. Alivisatos, Science, 1994, 265, 373–376 CAS.
- J. B. Rivest, L.-K. Fong, P. K. Jain, M. F. Toney and A. P. Alivisatos, J. Phys. Chem. Lett., 2011, 2, 2402–2406 CrossRef CAS.
- A. N. Goldstein, C. M. Echer and A. P. Alivisatos, Science, 1992, 256, 1425–1427 CAS.
- A. Kelly and K. M. Knowles, Crystallography and Crystal Defects, John Wiley and Sons, Ltd, United Kingdom, 2nd edn, 2012, ch. 7–10, pp. 197–331 Search PubMed.
- F. Liu, Y. H. Leung, A. B. Djurišić, A. M. C. Ng and W. K. Chan, J. Phys. Chem. C, 2013, 117, 12218–12228 CAS.
- R. K. Dutta, B. P. Nenavathu and S. Talukdar, Colloids Surf., B, 2014, 114, 218–224 CrossRef CAS PubMed.
- R. G. Rayavarapu, C. Ungureanu, P. Krystek, T. G. van Leeuwen and S. Manohar, Langmuir, 2010, 26, 5050–5055 CrossRef CAS PubMed.
- D. K. Smith and B. A. Korgel, Langmuir, 2008, 24, 644–649 CrossRef CAS PubMed.
- T. G. Schaaff and R. L. Whetten, J. Phys. Chem. B, 1999, 103, 9394–9396 CrossRef CAS.
- L. M. Gilbertson, J. B. Zimmerman, D. L. Plata, J. E. Hutchison and P. T. Anastas, Chem. Soc. Rev., 2015, 44, 5758–5777 RSC.
- R. Buonsanti and D. J. Milliron, Chem. Mater., 2013, 25, 1305–1317 CrossRef CAS.
- S. V. Bhat and F. Deepak, Solid State Commun., 2005, 135, 345–347 CrossRef CAS.
- N. M. Flores, U. Pal, R. Galeazzi and A. Sandoval, RSC Adv., 2014, 4, 41099–41110 RSC.
- A. Gole and C. J. Murphy, Langmuir, 2008, 24, 266–272 CrossRef CAS PubMed.
- J. Owen, Science, 2015, 347, 615–616 CrossRef CAS PubMed.
- C. D. Walkey, J. B. Olsen, F. Song, R. Liu, H. Guo, D. W. H. Olsen, Y. Cohen, A. Emili and W. C. W. Chan, ACS Nano, 2014, 8, 2439–2455 CrossRef CAS PubMed.
- C. Levard, E. M. Hotze, G. V. Lowry and G. E. Brown, Environ. Sci. Technol., 2012, 46, 6900–6914 CrossRef CAS PubMed.
- A. R. Petosa, D. P. Jaisi, I. R. Quevedo, M. Elimelech and N. Tufenkji, Environ. Sci. Technol., 2010, 44, 6532–6549 CrossRef CAS PubMed.
- C. Gunawan, M. Lim, C. P. Marquis and R. Amal, J. Mater. Chem. B, 2014, 2, 2060–2083 RSC.
- X. Liu, J. R. Ray, C. W. Neil, Q. Li and Y.-S. Jun, Environ. Sci. Technol., 2015, 49, 5476–5483 CrossRef CAS PubMed.
- N. C. Anderson, M. P. Hendricks, J. J. Choi and J. S. Owen, J. Am. Chem. Soc., 2013, 135, 18536–18548 CrossRef CAS PubMed.
- E. K. Richman and J. E. Hutchison, ACS Nano, 2009, 3, 2441–2446 CrossRef CAS PubMed.
- X.-R. Xia, N. A. Monteiro-Riviere and J. E. Riviere, Nat. Nanotechnol, 2010, 5, 671–675 CrossRef CAS PubMed.
- X. R. Xia, N. A. Monteiro-Riviere, S. Mathur, X. Song, L. Xiao, S. J. Oldenberg, B. Fadeel and J. E. Riviere, ACS Nano, 2011, 5, 9074–9081 CrossRef CAS PubMed.
- L. V. Stebounova, E. Guio and V. H. Grassian, J. Nanopart. Res., 2011, 13, 233–244 CrossRef CAS.
- A. O. Pinchuk, J. Phys. Chem. C, 2012, 116, 20099–20102 CAS.
- L. A. Wijenayaka, M. R. Ivanov, C. M. Cheatum and A. J. Haes, J. Phys. Chem. C, 2015, 119, 10064–10075 CAS.
- M. Hoefling, F. Iori, S. Corni and K.-E. Gottschalk, Langmuir, 2010, 26, 8347–8351 CrossRef CAS PubMed.
- H. Zhang and J. F. Banfield, Nano Lett., 2004, 4, 713–718 CrossRef CAS.
- D. Xu, Y. He and E. S. Yeung, Angew. Chem., 2014, 126, 7071–7075 CrossRef.
- M. J. Penna, M. Mijajlovic and M. J. Biggs, J. Am. Chem. Soc., 2014, 136, 5323–5331 CrossRef CAS PubMed.
- D. Krewski, D. Acosta, M. Andersen, H. Anderson, J. C. Bailar, K. Boekelheide, R. Brent, G. Charnley, V. G. Cheung, S. Green, K. T. Kelsey, N. I. Kerkvliet, A. A. Li, L. McCray, O. Meyer, R. D. Patterson, W. Pennie, R. A. Scala, G. M. Solomon, M. Stephens, J. Yager and L. Zeise, T. Staff of Committee on Toxicity and A. Assessment of Environmental, J. Toxicol. Environ. Health, Part B, 2010, 13, 51–138 CAS.
- P. Demokritou, S. Gass, G. Pyrgiotakis, J. M. Cohen, W. Goldsmith, W. McKinney, D. Frazer, J. Ma, D. Schwegler-Berry, J. Brain and V. Castranova, Nanotoxicology, 2013, 7, 1338–1350 CrossRef CAS PubMed.
- J. Cohen, G. DeLoid, G. Pyrgiotakis and P. Demokritou, Nanotoxicology, 2012, 7, 417–431 CrossRef PubMed.
- G. DeLoid, J. M. Cohen, T. Darrah, R. Derk, L. Rojanasakul, G. Pyrgiotakis, W. Wohlleben and P. Demokritou, Nat. Commun., 2014, 5 Search PubMed.
- A. K. Pal, D. Bello, J. Cohen and P. Demokritou, Nanotoxicology, 2015, 1–15, DOI:10.3109/17435390.2014.986670.
- J. G. Teeguarden, P. M. Hinderliter, G. Orr, B. D. Thrall and J. G. Pounds, Toxicol. Sci., 2007, 95, 300–312 CrossRef CAS PubMed.
- P. M. Hinderliter, K. R. Minard, G. Orr, W. B. Chrisler, B. D. Thrall, J. G. Pounds and J. G. Teeguarden, Part. Fibre Toxicol., 2010, 7, 36 CrossRef CAS PubMed.
- H. Kettiger, A. Schipanski, P. Wick and J. Huwyler, Int. J. Nanomed., 2013, 8, 3255–3269 Search PubMed.
- K. Siegrist, S. Reynolds, M. Kashon, D. Lowry, C. Dong, A. Hubbs, S.-H. Young, J. Salisbury, D. Porter, S. Benkovic, M. McCawley, M. Keane, J. Mastovich, K. Bunker, L. Cena, M. Sparrow, J. Sturgeon, C. Dinu and L. Sargent, Part. Fibre Toxicol., 2014, 11, 6 CrossRef PubMed.
- R. F. Hamilton, Jr., M. Buford, C. Xiang, N. Wu and A. Holian, Inhalation Toxicol., 2012, 24, 995–1008 CrossRef PubMed.
- A. Sarkar, M. Ghosh and P. C. Sil, J. Nanosci. Nanotechnol., 2014, 14, 730–743 CrossRef CAS PubMed.
- G. Bhabra, A. Sood, B. Fisher, L. Cartwright, M. Saunders, W. H. Evans, A. Surprenant, G. Lopez-Castejon, S. Mann, S. A. Davis, L. A. Hails, E. Ingham, P. Verkade, J. Lane, K. Heesom, R. Newson and C. P. Case, Nat. Nanotechnol., 2009, 4, 876–883 CrossRef CAS PubMed.
- H. Zhang, Z. Ji, T. Xia, H. Meng, C. Low-Kam, R. Liu, S. Pokhrel, S. Lin, X. Wang, Y. P. Liao, M. Wang, L. Li, R. Rallo, R. Damoiseaux, D. Telesca, L. Madler, Y. Cohen, J. I. Zink and A. E. Nel, ACS Nano, 2012, 6, 4349–4368 CrossRef CAS PubMed.
- S. Lin, Y. Zhao, T. Xia, H. Meng, Z. Ji, R. Liu, S. George, S. Xiong, X. Wang, H. Zhang, S. Pokhrel, L. Madler, R. Damoiseaux, S. Lin and A. E. Nel, ACS Nano, 2011, 5, 7284–7295 CrossRef CAS PubMed.
- C. McCracken, A. Zane, D. A. Knight, P. K. Dutta and W. J. Waldman, Chem. Res. Toxicol., 2013, 26, 1514–1525 CrossRef CAS PubMed.
- R. R. Mercer, J. F. Scabilloni, A. F. Hubbs, L. A. Battelli, W. McKinney, S. Friend, M. G. Wolfarth, M. Andrew, V. Castranova and D. W. Porter, Part. Fibre Toxicol., 2013, 10, 33 CrossRef CAS PubMed.
- A. Nel, T. Xia, H. Meng, X. Wang, S. Lin, Z. Ji and H. Zhang, Acc. Chem. Res., 2013, 46, 607–621 CrossRef CAS PubMed.
- T. M. Achilli, J. Meyer and J. R. Morgan, Expert Opin. Biol. Ther., 2012, 12, 1347–1360 CrossRef CAS PubMed.
- X. Wang, T. Xia, S. A. Ntim, Z. Ji, S. Lin, H. Meng, C. H. Chung, S. George, H. Zhang, M. Wang, N. Li, Y. Yang, V. Castranova, S. Mitra, J. C. Bonner and A. E. Nel, ACS Nano, 2011, 5, 9772–9787 CrossRef CAS PubMed.
- X. Shi, A. von dem Bussche, R. H. Hurt, A. B. Kane and H. Gao, Nat. Nanotechnol., 2011, 6, 714–719 CrossRef CAS PubMed.
- J. Plazas-Tuttle, L. Rowles, H. Chen, J. Bisesi, T. Sabo-Attwood and N. Saleh, Nanomaterials, 2015, 5, 1102 CrossRef CAS.
- N. B. Saleh, N. Aich, J. Lead, J. Plazas-Tuttle and G. V. Lowry, Environ. Sci.: Nano, 2015, 2, 11–18 RSC.
- N. Aich, J. Plazas-Tuttle, J. R. Lead and N. B. Saleh, Environ. Chem., 2014, 11, 609–623 CrossRef CAS.
- N. Saleh, A. Afrooz, J. Bisesi, N. Aich, J. Plazas-Tuttle and T. Sabo-Attwood, Nanomaterials, 2014, 4, 372–407 CrossRef.
Footnote |
| † Vicki Grassian – workshop co-organizer, workshop moderator and perspective writer and primary editor. Amanda Haes – workshop co-organizer, workshop speaker on Nanomaterials are not just small – fate from biological, chemical, and physical stability perspectives and workshop moderator and perspectives article writer and primary editor. Imali Mudunkotuwa – workshop scribe and perspectives article major writer. Philip Demokritou workshop speaker on Dosimetry for in vitro nanotoxicology – too complicated to consider, too important to ignore. Agnes Kane workshop speaker on Nano-bio interactions: Mechanistic data gaps using carbon nanotubes as a case study. Catherine Murphy workshop speaker on Nanomaterials are not just small – fundamentals of size dependent behavior/properties. All other co-authors (James Hutchison, Jacqueline Isaacs, Young-Shin Jun, Barbara Karn, Saiful Khondaker, Sarah Larsen, Boris Lau, John Pettibone, Omowunmi Sadik, Navid Saleh and Clayton Teague) were contributing workshop attendees and provided extensive dialog and input during the workshop that was used to formulate this perspective article and provided additional input on the final written manuscript. |
| This journal is © The Royal Society of Chemistry 2016 |