Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A highly-robust solid oxide fuel cell (SOFC): simultaneous greenhouse gas treatment and clean energy generation†
T.
Li
a,
M. F.
Rabuni
a,
L.
Kleiminger
a,
B.
Wang
a,
G. H.
Kelsall
a,
U. W.
Hartley
b and
K.
Li
 *a
*a
aDepartment of Chemical Engineering, Imperial College London, London, SW7 2AZ, UK. E-mail: Kang.li@imperial.ac.uk
bChemical Process Engineering, The Sirindhorn International Thai-German Graduate School of Engineering, King Mongkut's University of Technology North Bangkok, Bangkok 10800, Thailand
First published on 4th November 2016
Abstract
Herein, results of combined greenhouse gas treatment with clean energy conversion is reported for the first time. Multi-channel tubular SOFCs were operated with N2O instead of air as the oxidant leading to a 50% increase in power density. Techno-economic evaluation suggested the feasibility of the combined approach eliminating the cost penalty for N2O abatement.
Broader contextN2O has been identified as one of the most detrimental ozone-depletion substances, with a global warming potential (GWP) that is ca. 298 times greater than that of CO2. However, conventional industrial abatement processes (thermal or catalytic decomposition) require either high working temperatures (>1000 °C) or expensive catalysts with limited lifespans. Solid oxide fuel cells (SOFCs) have been well established as one of the most promising technologies for clean electrical energy generation in the future. In this study, it was reported for the first time that SOFCs can be operated using N2O as the oxidant. A considerable improvement in cell performances of approximately 50% was observed when using N2O compared to conventionally used air. Such enhanced power output, together with N2O decomposition at intermediate temperatures (750 °C), indicates successful integration of clean energy conversion and greenhouse treatment. The techno-economic analysis suggested that this novel conceptual design may well eliminate the cost penalty for industrial N2O abatement. |
With a global warming potential 298 times greater than that of CO2, N2O is one of the greenhouse gases contributing to ozone depletion and climate change.1–5 N2O is released by both natural, e.g. microbial transformation of nitrogen in soil, and anthropogenic sources.6–8 The main anthropogenic emission of the chemical industry comes from the manufacturing of adipic acid (C6H10O4) and nitric acid (HNO3), used as the feedstock in fertilizer and synthetic fiber production, in which N2O is generated as a by-product.9,10
To date, several technologies have been proposed to control the release of N2O from agricultural and industrial processes. For the former, the mitigation approaches often involve the use of soil microbes via biological processes.11,12 Control measures in the production of adipic or nitric acid involve thermal/catalytic decomposition processes. The catalytic route can be selective or non-selective, i.e. the reaction with a fuel. The drawbacks of these are either the requirement of a high working temperature (>1000 °C) to facilitate decomposition, i.e. a very energy intense process, or expensive catalysts with limited lifespans.13,14
Solid oxide fuel cells (SOFCs) can convert chemical to electrical energy cleanly and with higher efficiencies than conventional power plants.15,16 The high operating temperatures (>600 °C) provide enhanced kinetics and fuel flexibility.17–19 The geometry of micro-tubular SOFCs has shown improved thermal shock resistance, simplified sealing and increased volumetric power densities.20,21 However, this geometric design has yet to be commercialized, inter alia because of low mechanical strength,22 as the long, slim micro-tubes are vulnerable to fracture by forces applied vertically on a longitudinal direction, which makes the micro-tubular SOFC stacks quite fragile to external vibrations. Therefore, the enhancement in mechanical strength is crucial before it can be applied in practical applications, such as an auxiliary power unit (APU) and a combined heat and power (CHP) system.
We report herein a new concept: operating SOFCs with nitrous oxide as an oxidant instead of conventionally used air. This combines the treatment of N2O as a greenhouse gas with clean electrical energy generation. A novel multi-channel tubular reactor design was utilized, providing enhanced mechanical strength. Results for fuel cell operation with air or nitrous oxide as the oxidant are presented, and the techno-economic aspects of the combined systems are discussed.
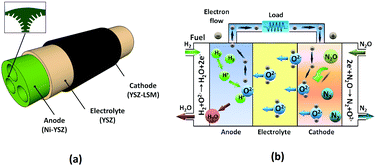
A schematic diagram of the multi-channel SOFC (for experimental fabrication process, see the ESI†) and the operating principle of the SOFC using N2O as an oxidant are depicted in Fig. 1a and b, respectively.
Nitrous oxide was fed to the cathode side, which has a bi-layer structure with a lanthanum strontium manganite (LSM)–yttria-stabilized zirconia (YSZ) inner layer (LSM/YSZ = 50/50 by weight) and a pure LSM outer layer for current collection. N2O was then decomposed catalytically into nitrogen and oxygen, the latter being reduced to oxide ions (O2−) and transported through the YSZ electrolyte.
Fig. 2 shows SEM images of the multi-channel anode and electrolyte (see the ESI† for images with higher magnifications showing the details of the micro-channels and sponge-like region). Uniform channels of the anode are produced using a specially designed spinneret (extruder) as shown in Fig. 2a. As the internal coagulant was split into three streams, several phase inversion processes occurred simultaneously, leading to the formation of a hierarchical asymmetric structure composed of micro-channels and sponge-like regions.23 A well-tailored micro-structure can improve cell performances considerably.24 The presence of micro-channels has been proven to facilitate mass transport rates, whereas the sponge-like region increases the densities of triple-phase boundaries (TPBs) where fuel oxidation occurs.23 The dip-coated YSZ electrolyte was approximately 10 μm thick (Fig. 2b) and well densified after sintering at 1450 °C for 6 hours (Fig. 2c).
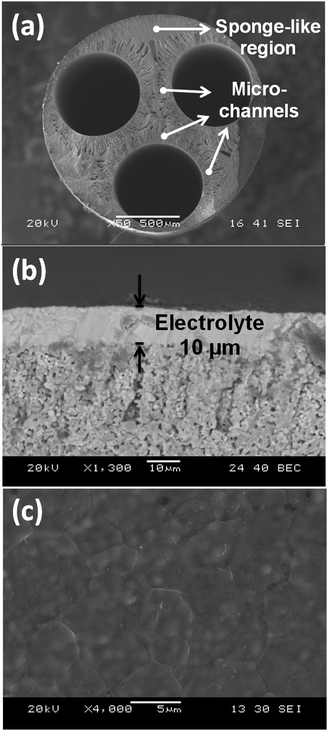 | ||
| Fig. 2 SEM images of: (a) overview of anode cross-section; (b) close-up of the electrolyte cross-section; (c) close-up of the electrolyte outer surface. | ||
The nitrogen permeance at room temperature was determined to be of the order 1 × 10−11 mol m−2 s−1 Pa−1, implying suitable gas-tightness had been obtained for the YSZ electrolyte.25 The fracture robustness of a 3-channel anode was investigated using three-point bending tests (for experimental details see the ESI†); the fracture load was measured as approximately 24 N, which is more than 3–4 times higher than that of single-channel counterparts fabricated under similar conditions.26 Such marked enhancement in mechanical robustness could effectively facilitate stack assembly and improve the resistance towards external impact. Hence, this novel multi-channel design could be a promising solution to the long-existing problem of low mechanical strength that has hindered the micro-tubular SOFC design from wider applications.
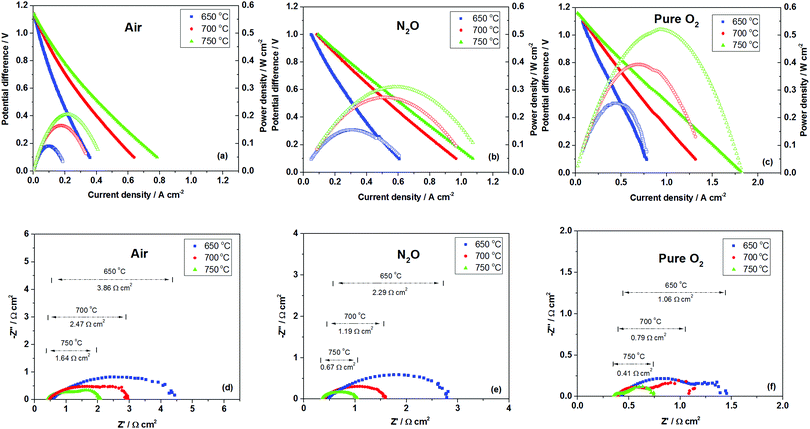
The electrochemical performance was measured by supplying 30 ml min−1 of pure hydrogen as the fuel and 50 ml min−1 of oxidant (air, N2O or pure O2). As depicted in Fig. 3a–c, open-circuit potential differences (OCPDs) for various oxidants ranged between 1.15 and 1.17 V, which are relatively close to the theoretical values, indicating gas-tightness of the YSZ electrolyte. Maximum power densities of 0.09, 0.16 and 0.20 W cm−2 were determined for air at 650, 700 and 750 °C, respectively, whereas the values were increased significantly to 0.14, 0.20, 0.31 W cm−2 when air was replaced with N2O. Such an increase of up to 50% could be attributed to the improved effective oxygen mass transport due to the catalytic activity of LSM to N2O decomposition. Like other SOFCs, here the cathode configuration used a bi-layer structure with a pure LSM outer layer and a LSM–YSZ inner layer. In the case of air, being a pure electronic conductor, the exterior LSM layer functioned mainly as a current collector to transport electrons, but did not contribute to the reduction of air and conduction of oxygen ions. Therefore, air needed to diffuse through the LSM layer, which may act as a barrier for an oxidant to reach the TPBs in the LSM–YSZ composite layer at which oxygen was reduced into oxide ions. In contrast, for the N2O case, LSM exhibited a significant catalytic activity for N2O decomposition, therefore N2O would be converted into nitrogen and oxygen in the LSM layer (as confirmed via gas chromatography analysis) and in this instance the LSM functioned as an in situ oxygen generator. Assuming N2O and air have similar transport characteristics in the porous LSM layer, it can be estimated that after decomposition of N2O, the local oxygen partial pressure in the LSM layer of the N2O case would be up to 238% of the air case, resulting in much more oxygen transport from the LSM layer to the reactive LSM–YSZ layer, and hence, significantly enhancing the reaction in the cathode and the overall performance.
When pure oxygen was investigated as an oxidant, although the LSM layer may also show the resistance for oxygen to reach the TPBs, the pure oxygen contents in the LSM–YSZ composite layer led to a much pronounced effect, resulting in an enhanced performance compared to air and N2O as shown in Fig. 3c. Such observation is in good agreement with previous studies in which pure oxygen was tested as an oxidant in comparison to air and exhibited a markedly improved power density and reduced over potential.27,28 The above results are further supported by the electrochemical impedance spectra which were recorded for temperatures in the range of 650–750 °C under open-circuit conditions, as shown in Fig. 3d–f. As can be seen, with N2O as an oxidant, the area specific resistances (ASRs) were measured as 2.29, 1.19 and 0.67 Ω cm2 at 650, 700 and 750 °C, respectively, which are significantly lower than the electrode polarization of air, but higher than that of pure oxygen, indicating the importance of local oxygen concentration on electrode polarization and hence cell performances.
From the high frequency intercepts on the real impedance (x) axis ohmic potential losses were 0.40–0.47 Ω cm2 when N2O was used as the oxidant, whereas the corresponding values for air were 0.47–0.57 Ω cm2. This difference could have been due to the local temperature increase of over 30 °C at the cathode (see the ESI† for the experimental results of the temperature change between air/N2O, i.e. Fig. S5) as a result of the heat released from the exothermic N2O decomposition reaction (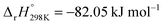 ) and subsequently increased conductivity.5
) and subsequently increased conductivity.5
Considering that pure oxygen is not often used in SOFCs due to it having a much higher cost than air, it is justifiable that air should be the counterpart for techno-economic and performance comparisons with the N2O case. Fig. 4 shows the effects of temperature (650–750 °C) on maximum power densities for N2O and air, as well as N2O conversion data; (neat) N2O as the oxidant increased the power densities by up to 50%. Table 1 compares the electrochemical performance results with data reported in the literature for similar materials and operating conditions. The maximum power density for air as the oxidant compares quite well with data for micro-tubular SOFCs reported in the literature. The 50% improvement in N2O as the oxidant combined with the enhanced mechanical robustness suggests that this new design and operating condition would be more advantageous over the current state-of-the-art tubular SOFCs. A preliminary stability test has also been conducted at 0.7 V and 750 °C, as shown in Fig. S5 (ESI†).
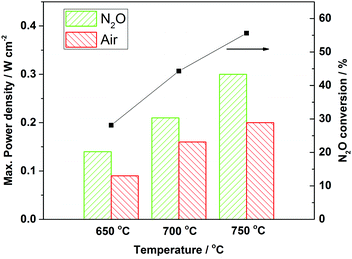 | ||
| Fig. 4 Effects of temperature and oxidant type (N2O or air) on maximum power densities, together with data for N2O conversion. | ||
The highest N2O conversion, calculated from gas chromatography data, was 55.6% at 750 °C. Although industrial catalytic or thermal decomposition can achieve >95% N2O removal, it is noteworthy that the conversion reported here resulted from an SOFC with an active cathode length of only 10 mm and a thickness of 30 μm. If SOFC stacks were to be used, to increase the cathode active area, N2O conversion could well be increased to match those achieved industrially.
Fig. 5 shows the techno-economic analysis for N2O as the oxidant in a 3-channel SOFC (for calculations and assumptions see the ESI†). The fuel chosen was methane, as renewable hydrogen is not yet economically viable with e.g. 6.64 $ kg−1 for wind-powered electrolysis compared to reforming natural gas 1.03 $ kg−1 without CO2 emission tax.35 Direct SOFC operation using methane has proven to be viable with a marginal decrease in power density.18,36,37 The minimum electricity price, at which the capital expenditure, maintenance and methane feed costs are off-set by the revenue from selling the generated electrical energy, was predicted to be ca. 0.07 $ kW h−1. Above this price electricity generation using a 3-channel micro-tubular SOFC was predicted to be profitable; with the additional benefit of simultaneously treating N2O.
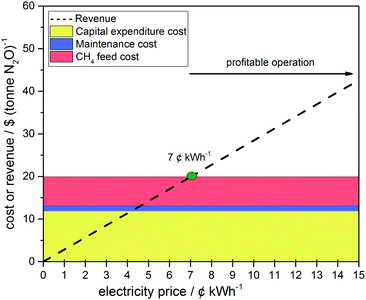 | ||
| Fig. 5 Effect of electricity price on profitability for electrical energy generation using micro-tubular SOFCs utilizing CH4 (fuel) and N2O (oxidant) operated at 750 °C and 0.31 W cm−2. | ||
Conventional catalytic decomposition of N2O requires high temperatures and catalysts.38 The SOFC fulfils both these requirements with operating temperatures >600 °C and incorporating a LSM catalyst, while at the same time generating electrical energy. No modification of the SOFC is required for N2O operation compared to air. Moreover, as shown in Fig. 3, the N2O actually enhances the performance of the SOFC compared to O2. Similarly, N2O decomposition should benefit, as the product oxygen is removed. Thus, N2O treatment at no effective cost penalty can be achieved.
Conclusions
In summary, this proof-of-concept study demonstrates the feasibility of applying SOFC technology to generate clean electrical energy while simultaneously reducing N2O emissions. The novel multi-channel geometric design improved the mechanical strength by 3–4 times that of conventional micro-tubes, which could address the long-existing weakness of single micro-tubular SOFCs and enhance the system resistance towards external impact. Maximum power densities were increased by up to 50% when (neat) N2O was used instead of air as the oxidant. The highest power density of 0.31 W cm−2 was obtained at 750 °C using N2O as the oxidant. Electrochemical impedance spectra suggested that the performance improvement was mainly due to decreased polarization as N2O decomposition may lead to a more oxygen-rich atmosphere near the cathode surface. The techno-economic evaluation results suggested that this novel conceptual design may well eliminate the cost penalty for industrial N2O abatement, provided methane is used as the fuel.Acknowledgements
We acknowledge the research funding provided by the UK EPSRC Grant no. EP/M014045/1 and EP/M01486X/1.Notes and references
- J. C. Kramlich and W. P. Linak, Prog. Energy Combust. Sci., 1994, 20, 149–202 CrossRef CAS.
- M. Chipperfield, Nat. Geosci., 2009, 2, 742–743 CrossRef CAS.
- A. R. Ravishankara, J. S. Daniel and R. W. Portmann, Science, 2009, 326, 123–125 CrossRef CAS PubMed.
- P. Forster, V. Ramaswamy, P. Artaxo, T. Berntsen, R. Betts, D. W. Fahey, J. Haywood, J. Lean, D. C. Lowe, G. Myhre, J. Nganga, R. Prinn, G. Raga, M. Schulz and R. Van Dorland, Changes in Atmospheric Constituents and in Radiative Forcing. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2007 Search PubMed.
- M. Konsolakis, ACS Catal., 2015, 5, 6397–6421 CrossRef CAS.
- S. J. Hall and P. A. Matson, Nature, 1999, 400, 152–155 CrossRef CAS.
- K. A. Smith and F. Conen, Soil Use Manage., 2004, 20, 255–263 CrossRef.
- O. Oenema, N. Wrage, G. L. Velthof, W. J. van Groenigen, J. Dolfing and P. J. Kuikman, Nutr. Cycling Agroecosyst., 2005, 72, 51–65 CrossRef CAS.
- A. Shimizu, K. Tanaka and M. Fujimori, Chemosphere: Global Change Sci., 2000, 2, 425–434 CrossRef CAS.
- F. Kapteijn, J. Rodriguez-Mirasol and J. A. Moulijn, Appl. Catal., B, 1996, 9, 25–64 CrossRef CAS.
- M. Itakura, Y. Uchida, H. Akiyama, Y. T. Hoshino, Y. Shimomura, S. Morimoto, K. Tago, Y. Wang, C. Hayakawa, Y. Uetake, C. Sanchez, S. Eda, M. Hayatsu and K. Minamisawa, Nat. Clim. Change, 2013, 3, 208–212 CrossRef CAS.
- N. Gao, W. Shen, H. Kakuta, N. Tanaka, T. Fujiwara, T. Nishizawa, N. Takaya, T. Nagamine, K. Isobe, S. Otsuka and K. Senoo, Soil Biol. Biochem., 2016, 97, 83–91 CrossRef CAS.
- A. Shimizu, K. Tanaka and M. Fujimori, Chemosphere: Global Change Sci., 2000, 2, 425–434 CrossRef CAS.
- N. Russo, D. Mescia, D. Fino, G. Saracco and V. Specchia, Ind. Eng. Chem. Res., 2007, 46, 4226–4231 CrossRef CAS.
- S. C. Singhal, K. Kendall and W. Winkler, in High Temperature Solid Oxide Fuel Cells: Fundamentals, Designs and Applications, ed. S. C. Singhal and K. Kendall, Elsevier Oxford, UK, 2003 Search PubMed.
- R. J. Gorte, Science, 2015, 349, 1290 CrossRef CAS PubMed.
- R. Gorte and S. Mclntosh, Chem. Rev., 2004, 104, 4845–4866 CrossRef.
- R. Gorte, J. Vohs and S. Park, Nature, 2000, 404, 265–267 CrossRef PubMed.
- T. Suzuki, T. Yamaguchi, K. Hamamoto, Y. Fujishiro, M. Awano and N. Sammes, Energy Environ. Sci., 2011, 4, 940–943 CAS.
- K. Kendall, Int. J. Appl. Ceram. Technol., 2010, 7, 1–9 CrossRef CAS.
- V. Lawlor, S. Griesser, G. Buchinger, A. G. Olabi, S. Cordiner and D. Meissner, J. Power Sources, 2009, 193, 387–399 CrossRef CAS.
- V. Lawlor, J. Power Sources, 2013, 240, 421–441 CrossRef CAS.
- M. H. D. Othman, N. Droushiotis, Z. Wu, G. Kelsall and K. Li, Adv. Mater., 2011, 23, 2480–2483 CrossRef CAS PubMed.
- T. Suzuki, Z. Hasan, Y. Funahashi, T. Yamaguchi, Y. Fujishiro and M. Awano, Science, 2009, 325, 852–855 CrossRef CAS PubMed.
- T. Li, Z. Wu and K. Li, J. Power Sources, 2015, 280, 446–452 CrossRef CAS.
- L. Kleiminger, T. Li, K. Li and G. H. Kelsall, RSC Adv., 2014, 4, 50003–50016 RSC.
- E. P. Murray, T. Tsai and S. A. Barnett, Solid State Ionics, 1998, 110, 235–243 CrossRef CAS.
- M. C. Tucker, G. Y. Lau, C. P. Jacobson, L. C. DeJonghe and S. J. Visco, J. Power Sources, 2007, 171, 477–482 CrossRef CAS.
- T. Suzuki, M. Awano, P. Jasinski, V. Petrovsky and H. Anderson, Solid State Ionics, 2006, 177, 2071–2074 CrossRef CAS.
- M. Kendall, A. D. Meadowcroft and K. Kendall, ECS Trans., 2013, 57, 123–131 CrossRef.
- N. Droushiotis, A. Hankin, C. Rozain and G. H. Kelsall, J. Electrochem. Soc., 2013, 161, F271–F279 CrossRef.
- C. Ren, T. Liu, Y. Mao, P. Maturavongsadit, J. A. Luckanagul, Q. Wang and F. Chen, Electrochim. Acta, 2014, 149, 159–166 CrossRef CAS.
- C. Jin, C. Yang and F. Chen, J. Membr. Sci., 2010, 363, 250–255 CrossRef CAS.
- T. Mahata, S. R. Nair, R. K. Lenka and P. K. Sinha, Int. J. Hydrogen Energy, 2012, 37, 3874–3882 CrossRef CAS.
- T. Abbasi and S. A. Abbasi, Renewable Sustainable Energy Rev., 2011, 15, 3034–3040 CrossRef.
- R. Gorte, J. Vohs, S. Park and C. Wang, Adv. Mater., 2000, 12, 1465–1469 CrossRef CAS.
- J.-H. Koh, Y.-S. Yoo, J.-W. Park and H. C. Lim, Solid State Ionics, 2002, 149, 157–166 CrossRef CAS.
- O. o. A. Q. P. a. S. Sector Policies and Programs Division, U.S. Environmental Protection Agency, Research Triangle Park, North Carolina 27711 Available and Emerging Technologies for Reducing Greenhouse Gas Emissions from the Nitric Acid Production Industry 2010.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ee02562e |
| This journal is © The Royal Society of Chemistry 2016 |