Open Access Article
Open Access ArticleRational designs and engineering of hollow micro-/nanostructures as sulfur hosts for advanced lithium–sulfur batteries
Zhen
Li
a,
Hao Bin
Wu
a and
Xiong Wen
(David) Lou
*ab
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459, Singapore. E-mail: xwlou@ntu.edu.sg; davidlou88@gmail.com; Web: http://www.ntu.edu.sg/home/xwlou/
bState Key Laboratory of Silicon Materials, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027, P. R. China
First published on 8th September 2016
Abstract
Lithium–sulfur (Li–S) batteries have attracted much attention in the field of electrochemical energy storage and conversion. As a vital part of the cathode electrode, the host materials of sulfur usually have a strong impact on the capacity, energy density, cycle life and Coulombic efficiency of Li–S batteries. With their unique physical and chemical properties, the rationally designed hollow nanostructures show conspicuous advantages as sulfur hosts, and have significantly improved the overall performance of Li–S cells. The scope of this review considers the unique structural advantages of hollow host materials for high-performance Li–S batteries, together with a summary of recent advances in the design and synthesis of various hollow micro-/nanostructures with controlled shapes, tailored shell structures and designed chemical compositions. Finally, we propose some emerging requirements of sulfur hosts which we hope will shed some light on the future development trend of hollow structures for advanced Li–S batteries.
Broader contextAfter two decades of development, lithium-ion batteries (LIBs) based on intercalation compounds are approaching their energy density limit, and unable to further satisfy the ever-growing demands of mobile electronic devices with increased power consumption, and electric vehicles with an extended driving range. New battery systems with higher energy densities are urgently required for the rapidly evolving markets. In recent years, lithium–sulfur (Li–S) batteries, which are able to gain up to 2–3 times higher practical energy density than those of commercial LIBs, have been considered as very promising candidates for the next-generation of rechargeable batteries. As a vital part of the sulfur-based electrode, the host materials usually have a strong impact on the capacity, energy density, cycle life and Coulombic efficiency of the Li–S batteries. With their unique physical and chemical properties, some rationally designed hollow nanostructures show conspicuous advantages as sulfur hosts, and have significantly improved the comprehensive performance of Li–S batteries. The scope of this Review considers the unique structural advantages of hollow host materials for high-performance Li–S batteries, together with a summary of recent research achievements in the design and synthesis of various hollow micro-/nanostructures with controlled shapes, tailored shell structures and selected chemical compositions. |
Introduction
In the field of rechargeable batteries, high energy density electrochemical systems have been a research focus over the past decades.1,2 After two decades of development, lithium-ion batteries (LIBs) based on intercalation compounds are approaching their energy density limit, and hence are unable to further satisfy the ever-growing demands of mobile electronic devices with increased power consumption, or electric vehicles with an extended driving range.3 New systems with higher energy densities are urgently required for the rapidly evolving markets.4–6 In recent years, lithium–sulfur (Li–S) batteries have been considered as very promising candidates for the next-generation of rechargeable batteries. By coupling a sulfur cathode (with a theoretical capacity of 1675 mA h g−1) and a lithium anode (with a theoretical capacity of 3840 mA h g−1), the Li–S battery affords an average voltage of about 2.2 V and a high theoretical energy density of 2600 W h kg−1, which is able to gain up to 2–3 times higher practical energy density than the state-of-the-art commercial LIBs.7,8 In addition, the natural abundance and non-toxicity of sulfur enable Li–S batteries with a more attractive cost advantage and better environmental friendliness compared to LIBs.9 Despite the overwhelming advantages, the Li–S battery also has several significant technological obstacles. First, the insulating nature of sulfur and its discharge products leads to a low specific capacity. Second, the dissolution of polysulfides and their shuttle effect result in the loss of active materials, poor cycling stability, as well as low Coulombic efficiency. In addition, high volumetric expansion in the lithiation process and lithium anode degradation during cycling are also serious issues of the Li–S system.10The reduction from S to the end product of Li2S is accompanied by a series of intermediate Li2Sx (2 ≤ x ≤ 8). Among them, the long-chain lithium polysulfides (LiPSs) are typically soluble in the ether-based electrolytes, and it is very difficult to control the complicated dissolution–precipitation transitions of the sulfur electrode.11 Therefore, the host materials of sulfur are extremely important for the development of high-performance sulfur cathodes. In recent years, extensive research efforts have been devoted to the design and engineering of micro-/nanostructured hosts, which significantly enhance the conductivity and stability of the sulfur cathodes, and greatly improve the electrochemical performance of Li–S batteries.7,12–15
Among various sulfur hosts, hollow structured materials generally show the following unique advantages. Firstly, the large internal void space of the hollow structures allows relatively high loading of sulfur, while nanoparticles and nanosheets can only load sulfur on their exposed surfaces. Besides, there would also be enough space in the hollow structured materials to accommodate the large volumetric expansion during the lithiation process. Secondly, the integrated shells can promise more efficient confinement of soluble LiPSs. Different from the simple adsorption of LiPSs on the surfaces of particle-/sheet-type hosts, the shell of hollow structures can act as the gate. Once the active sulfur materials are filled into the inner space, it is difficult for them to come out again due to the physical/chemical obstruction provided by the integrated shells. Thirdly, the multitudinous morphologies and chemical components of the hollow structures promise high flexibility for the design and synthesis of materials. Taking advantage of the great advances in the design and synthesis of hollow micro-/nanostructures,16–18 researchers in the field of Li–S batteries can easily design and validate the appropriate hollow structured sulfur host for the electrochemical systems of Li–S batteries.
In recent years, with a better understanding of the Li–S system, tremendous efforts have been devoted to the development of advanced hollow structured sulfur hosts to improve the electrochemical performance of Li–S batteries. In the early years, since conductive carbon can effectively improve the conductivity of the sulfur composites, enhance the reaction activity of sulfur and increase the specific capacity, it was widely believed that carbon materials would work best for the sulfur cathodes,12,19 and various hollow carbon nanostructures have been developed.20,21 But it was soon realized that the nonpolar C–C bonds are not able to provide sufficient chemical binding energy to the polar LiPSs.14 Accordingly, heteroatoms-doped hollow carbon materials were developed as sulfur hosts to control the diffusion of LiPSs.22,23 Most recently, polar transition metal compounds were found to be capable of providing a much stronger adsorption capability for LiPSs.24–28 Inspired by such surface-chemistry, several types of hollow structured metal oxides/sulfides have been fabricated and applied in Li–S batteries to minimize the loss of LiPSs.29–32 In this Review, we focus mainly on recent research progresses in the design and synthesis of hollow structured sulfur hosts with controlled shapes, tailored shell structures and desired chemical compositions. After that, we also discuss some emerging requirements for advanced sulfur hosts, which we hope will shed some light on the future development of hollow structures for advanced Li–S batteries.
Hollow carbon nanostructures as the sulfur host
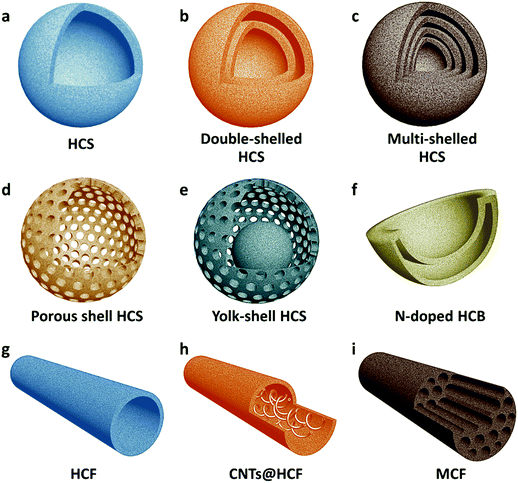
Because of their good conductivity and affinity to sulfur, carbonaceous materials are the most popular auxiliary materials employed in Li–S batteries. In 2011, Archer's group developed highly graphitized hollow carbon spheres (HCS) as the sulfur host (Fig. 1a).20 By repeatedly exposing HCS to sulfur vapor three times, a high content of sulfur (70 wt%) can be successfully loaded into the carbon shells. The HCS/S composite greatly improves the utilization and stability of sulfur, delivering a high specific capacity of 1100 mA h g−1 with stable cycling up to 100 cycles. It is also noticeable that the HCS/S cathode exhibits a stable Coulombic efficiency of >90% without adding LiNO3 in the electrolyte, suggesting that the dissolution of LiPSs is well controlled by the carbon shells. Later on, a series of HCSs were fabricated as sulfur hosts via different strategies to improve the performance of the Li–S batteries.33–36 To more effectively confine sulfur and restrict the dissolution of LiPSs, Lou and coworkers synthesized novel double-shelled hollow carbon nanospheres as the sulfur host (Fig. 1b).21 Sulfur can be easily loaded within the complex shell structures using the facile melting-diffusion method. Moreover, the flexible double shells could effectively mitigate the outward diffusion of LiPSs and withstand volume variation of active materials. This work opens a new avenue of tailoring the shell structures of hollow carbon hosts to further improve the performance of sulfur cathodes. Similar double-shelled HCSs developed by other groups also show their structural superiorities in Li–S batteries.37,38 Furthermore, multi-shelled hollow carbon nanospheres have also been synthesized using an aqueous emulsion approach (Fig. 1c), and further demonstrate the advantages of using carbon shells to restrict LiPSs and enhance the utilization of sulfur.39 Besides increasing the number of carbon shells, Nazar's group focused on tailoring the porous structure of HCS (Fig. 1d).40 It is revealed that by deliberately creating porosity on the shells and utilizing the interior void volume of HCS, it is possible to load up to 70 wt% of sulfur and still maintain good electrochemical reactivity.40 Xiao's group revealed that by carefully controlling the pore size of HCS, sulfur impregnation could be further improved.22 When the pore size of carbon shells is 2.8 nm, a high content of sulfur (85 wt%) can diffuse into the internal void of the hollow carbon shells, and could be well confined during the electrochemical measurements.22 To more easily introduce sulfur into the void space of HCS, a core–shell interlinked hollow carbon structure was designed as the sulfur host (Fig. 1e).41 Such a core–shell structured host could not only facilitate diffusion of sulfur into the inner void space, but also provide a better electronically connecting matrix for the active sulfur species.41 To increase the tap density, which is a critical parameter affecting the volumetric energy density of the electrode, bowl-like hollow carbon nanostructures were developed by Zheng's group (Fig. 1f).23 The hollow carbon bowls (HCB) design apparently improves the tap density of the carbon/sulfur composite without sacrificing the sulfur loading capability.Compared with HCSs, one-dimensional (1D) hollow carbon fibers (HCFs) with a high aspect ratio can better construct an electrical conducting network in the electrode (Fig. 1g), thus improving the reaction kinetics and rate capability of the sulfur cathode. Several research groups reported the fabrication of various HCFs by using the anodized aluminum oxide (AAO) membrane as the hard template.42–44 After loading with sulfur, all of these HCF/S composites delivered improved capacity and extended cycle life. To further increase the utilization of the active material, carbon nanotubes (CNTs) are filled inside each HCF to construct a tube-in-tube structure (Fig. 1h).45 Starting with CNTs as a template, another type of tube-in-tube carbon structure was formed with a single CNT in each HCF.46 Both structures can accommodate high contents of sulfur (85 wt%45 and 71 wt%46) and deliver stable cycle life with high capacities. Compared with traditional template methods, the electrospinning technique is more feasible for the low-cost and mass production of 1D nanofibers with a high surface-to-volume ratio.47 Recently, a lotus root-like multichannel carbon fiber (MCF) has been developed as the sulfur host by Lou's group using the electrospinning method (Fig. 2c).48 Analogous to parallel-assembled HCFs, MCF provides a large void space for sulfur accommodation, and allows close contact between sulfur and the conductive host in the parallel channels. In addition, the 3D interconnected conductive framework constructed using MCFs greatly reduces the resistance for electron and ion transport. After wrapping with a thin layer of amino-functionalized graphene, the pie-like electrodes could deliver high areal capacities of 3.8, 7.2 and 10.7 mA h cm−2 with either a single, two or three layers of the free-standing MCF/S electrode film, respectively, and show good capacity retention.48
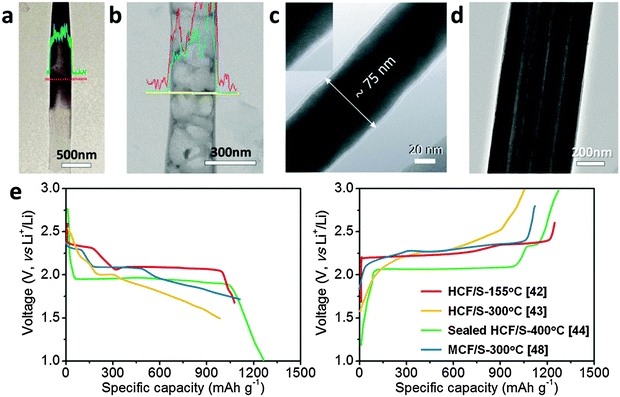 | ||
| Fig. 2 TEM images of (a) HCF/S after being heated at 155 °C for 12 h, (b) HCF/S after being heated at 300 °C for 2 h, (c) sealed HCF/S after being heated at 400 °C for 2 h, and (d) MCF/S after being heated at 300 °C for 12 h. (e) Typical discharge–charge voltage profiles of the samples in panel (a–d) with various sulfur composing methods. Panel (a) reproduced with permission.42 Copyright 2011, American Chemical Society. Panel (b) reproduced with permission.43 Copyright 2011, American Chemical Society. Panel (c) reproduced with permission.44 Copyright 2013, Wiley. Panel (d) reproduced with permission.48 Copyright 2015, Nature Publishing Group. Panel (e) reproduced with permission.42–44,48 | ||
It is noteworthy to mention that the sulfur loading strategies would greatly affect the electrochemical behaviors of the derived HCF/S cathodes. If sulfur is loaded into HCF using the common melting-diffusing method at 155 °C (Fig. 2a), the HCF/S composite shows the typical two-plateau behavior of the conventional sulfur cathodes, corresponding to the formation of long-chain polysulfides (Li2Sx, 4 ≤ x ≤ 8) at 2.3 V and short-chain Li2S2/Li2S at 2.1 V (Fig. 2e).42 In Guo's work, after treating the HCF/S composite at 300 °C in vacuum (Fig. 2b), the sulfur cathode exhibits improved cycling stability but a very different voltage profile, with an additional slope-shaped discharge plateau in the voltage range of 2.0–1.5 V (Fig. 2e).43 Such an additional plateau is likely related to the smaller S6–2 molecules with strong C–S bonds in the defects and graphite layers in the HCFs, which are formed by breaking the S8 molecules at a high temperature.43 In another work reported by Moon et al.,44 when the opening ends of the HCF/S are sealed by a Pt layer and heated at 400 °C for 2 h (Fig. 2c), a large proportion of sulfur is converted into an uncommon monoclinic phase inside HCF, showing an extraordinary single plateau at ∼1.97 V during discharging (Fig. 2e). In view of the smaller channel diameter (∼60 nm) of the above mentioned lotus root-like MCFs compared with that of AAO-derived HCFs (usually 200–300 nm), a different strategy is employed to incorporate sulfur into the MCFs. After being heated at 300 °C for 12 h in a stainless steel vessel under an argon atmosphere, the derived MCF/S composite (Fig. 2d) shows a complex voltage profile with both characteristics of normal S8 and smaller S6–2 molecules (Fig. 2e). It is highly possible that some of the sulfur molecules infuse into the defects or small pores of the amorphous carbon during the high temperature treatment, and form smaller sulfur molecules or recrystallize into some other uncommon phases (e.g., monoclinic phase). Although the exact mechanisms of these unusual electrochemical behaviors need further investigation, these studies reveal that both the existing forms of sulfur (e.g., molecular size and crystal phases) and the local environment/interaction with carbon hosts might play important roles in addressing the critical LiPSs-related issues of Li–S batteries, which are worthy of further research efforts in the future.
Hollow transition metal compound nanostructures as the sulfur host
For carbonaceous hollow structured hosts, the nonpolar carbon shells could only provide physical confinement of LiPSs. When part of the intermediate LiPSs dissolves into the electrolyte, the precipitation of the final products (Li2S2/Li2S) could not be easily controlled, and they may deposit as particles or thick films outside of the carbon hosts. Recently, it was found that host materials with polar surfaces can efficiently tackle the polysulfide-shuttle issues in virtue of their much stronger interactions with the polar LiPSs compared to a conventional nonpolar carbon surface. Over the years, many inorganic polar adsorbents have been developed as sulfur hosts in Li–S batteries, including MXene (Ti2C),49 SiO2,50 TiO2,51 Ti4O7,52,53 indium tin oxide,54 MnO2,24,32 TiS2,55 CoS2,56 V2O525 and Co9S8.57 Most of these materials are applied in the forms of either nanosheets or nanoparticles, aiming to provide a large exposed surface for adsorbing LiPSs but without well-defined porous/hollow structures for effective physical confinement. Since the interaction between polar materials and LiPSs in these cases is mostly based on monolayered chemical adsorption,27 only a very little amount of LiPSs can be directly adsorbed (Fig. 3a). Even with a large exposed surface area and high efficiency for LiPSs adsorption/deposition, immobilizing all the active species relying solely on surface interactions appears to be impossible due to the huge volume difference between LiPSs and the host materials (considering the different density and high sulfur content, e.g., typically ≥70 wt%) (Fig. 3b). Based on these considerations, hollow polar micro-/nanostructures, which can provide both physical and chemical entrapments of LiPSs, would be more effective as sulfur hosts. Despite the high content of LiPSs encapsulated in the hollow host, the polar shell can chemically adsorb some of the LiPSs near the shell and physically block the outward diffusion pathways to the rest (Fig. 3c). Therefore, hollow structures with rationally designed polar shells are expected to offer much more efficient confinement of LiPSs during cycling than the carbonaceous hollow hosts, and the dissolution–precipitation processes of the sulfur electrode can be well controlled inside the void space of the hollow host materials.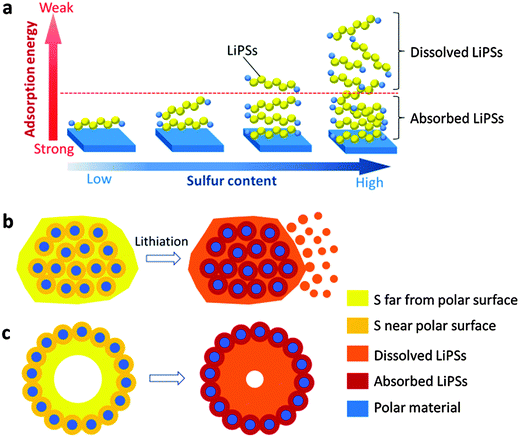 | ||
| Fig. 3 (a) Schematic of the LiPSs adsorption on a polar surface. When the content of LiPSs is higher than a certain limit, some LiPSs far from the polar substrate will not be effectively anchored. (b) When the sulfur content of the composite exceeds the limit, the polar nanoparticles are not able to restrict the diffusion of LiPSs far away from the substrate. (c) The hollow polar structure only needs to chemically adsorb some of the LiPSs near the surface, and then it naturally blocks the diffusion channels of the inner LiPSs. Panels b and c reproduced with permission.64 Copyright 2016, Nature Publishing Group. | ||
Hollow spherical MnO2 shells could in situ form on sulfur particles as the bifunctional host to provide both physical confinement and chemical adsorption of LiPSs in Li–S batteries.30,31 The S@MnO2 composite cathode exhibits a very low capacity fading rate, indicating the successful synergistic encapsulation of LiPSs. V2O5 hollow spheres have also been proved to be effective LiPS mediators.25 Compared with these simple metal oxide hollow structures, mixed metal compounds with complex hollow structures might be even better sulfur hosts.58 Very recently, double-shelled nanocages with a cobalt hydroxide inner shell and a layered double hydroxides outer shell (denoted as CH@LDH) have been successfully prepared and applied as highly efficient LiPS hosts.29 Compared with single shelled hollow hosts, double-shelled CH@LDH nanocages can provide much larger polar surfaces for chemically adsorbing LiPSs and a complex shell structure to suppress their outward diffusion. Moreover, the abundant hydrophilic/hydroxy groups and the potential electrocatalytic properties of LDH might further enhance its adsorption of soluble LiPSs and promote their conversion to short-chain PSs during discharging,59–62 making it an ideal polysulfide mediator. The synthesis of the double-shelled CH@LDH nanocages starts with using ZIF-67 polyhedral crystals as the sacrificial template (Fig. 4a and b). Hollow polyhedral LDH shell coated ZIF-67 particles (ZIF-67@LDH) are first formed by the reaction of ZIF-67 polyhedral crystals with Ni(NO3)2 in ethanol (Fig. 4c), and further converted to CH@LDH by reacting with an aqueous solution of Na2MoO4 (Fig. 4d). Finally, a high content of sulfur (75 wt%) is loaded into CH@LDH using the melt-diffusion method (Fig. 4e). When evaluated as a cathode material for Li–S batteries, the CH@LDH/S composite shows significantly improved cycling stability.29 This work also demonstrates that layered double hydroxides can be applied to be a new type of LiPS mediator for Li–S batteries.
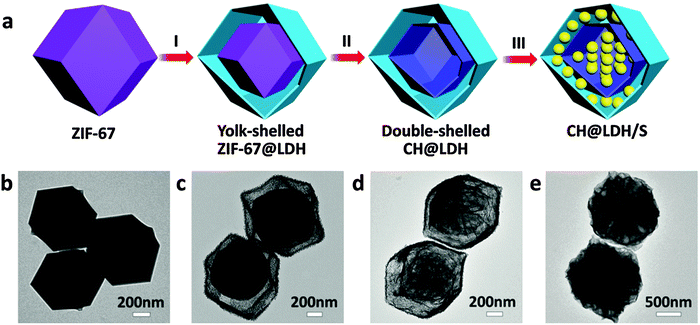 | ||
| Fig. 4 (a) Schematic illustration and (b–e) TEM images of the step-by-step synthesis of the CH@LDH/S composite: (b) ZIF-67, (c) yolk-shelled ZIF-67@LDH, (d) double-shelled CH@LDH, and (e) CH@LDH/S. Reproduced with permission.29 Copyright 2016, Wiley. | ||
Despite the fact that metal oxides/hydroxides obviously improve the cycling stability of sulfur cathodes, their insulating nature hinders the electron transport, resulting in relatively low C-rate capacities, especially with a high sulfur mass loading of >3 mg cm−2. A practical strategy is to construct hybrid structures of metal oxides/sulfides and carbon to inherit advantages of both polar surfaces and high conductivity. As a successful demonstration, a hybrid structure of 1D HCF filled with MnO2 nanosheets (MnO2@HCF) has been designed and fabricated (Fig. 5a).32 The 1D carbon nanofibers with a high aspect ratio can form a 3D conductive and porous network in the electrode, which could facilitate both ion and electron transfer during the charge–discharge process. Meanwhile, the inner MnO2 nanosheets with strong chemical adsorption capability for LiPSs effectively prevent the shuttle issues (Fig. 5b). With a high content of sulfur (71 wt%) and a high areal mass loading (3.5 mg cm−2), such a rationally designed MnO2@HCF/S composite delivers high specific capacities with a prolonged cycle life (Fig. 5c).32 Another similar design is embedding cobalt nanoparticles within N-doped HCFs, showing synergistically enhanced adsorption of LiPSs.63
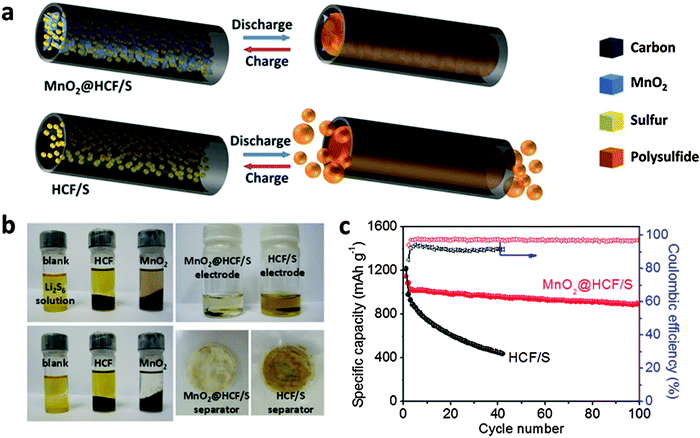 | ||
| Fig. 5 (a) Schematic illustrations of the advantage of MnO2@HCF as the sulfur host over HCF. (b) A visual observation to show the effects of MnO2 and HCF on the LiPSs adsorption. (c) Cycling properties of MnO2@HCF/S in comparison with HCF/S at 0.2C. Reproduced with permission.32 Copyright 2015, Wiley. | ||
To accelerate the redox kinetics for the reduction of directly bonded LiPSs to Li2S2/Li2S, the host materials are expected to be simultaneously polar and conductive. Besides the constructoin of composite structures as discussed above, some transition metal compounds would satisfy such requirements with inherent metallic conductivity and strong polysulfide affinity, such as Ti4O7,52,53 Ti2C49 and Co9S8.57 However, all these metallic polar host materials are reported in particle forms, which could hardly build up a conductive network in the electrode or immobilize most of the dissolved LiPSs. To maximize the advantage of such highly conductive polar materials, Lou and coworkers have designed and synthesized polar hollow nanospheres with highly conductive shells composed of titanium monoxide (TiO) nanoparticles and a thin carbon layer (TiO@C-HS) as the sulfur host (Fig. 6a). Benefiting from the highly conductive TiO and the hollow structure (Fig. 6b and c), the TiO@C-HS host maximizes the effectiveness of shutting down LiPSs diffusion and enhances the redox reaction kinetics of the sulfur species at the same time. Filled with 70 wt% of sulfur in the inner space (Fig. 6d), the TiO@C-HS/S composite could deliver a much better cycling stability and excellent C-rate capability that greatly outperform the control group based on an identical host material in a nanoparticle form.64 Even with a high sulfur loading of 4.0 mg cm−2, the TiO@C-HS electrode can still deliver high areal capacities at various current densities with a stable cycling performance (Fig. 6e). This work overcomes the major limitations associated with other polar and nonpolar sulfur hosts, and opens up a new venue for the construction of sulfur cathodes combining the advances in novel host materials and suitable nanostructure designs.
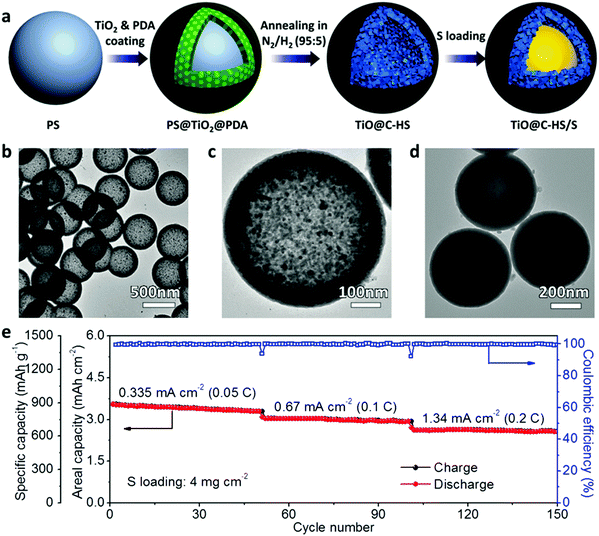 | ||
| Fig. 6 (a) Schematic illustration of the synthesis process of the TiO@C-HS/S composite. TEM images of (b and c) TiO@C-HS and (d) TiO@C-HS/S. (e) Cycling performance of the TiO@C-HS/S electrode with a sulfur mass loading of 4.0 mg cm−2. Reproduced with permission.64 Copyright 2016, Nature Publishing Group. | ||
Summary and perspectives
As a vital part of the cathode electrode, the appropriate design and engineering of hollow hosts is of significant importance for the research and development of advanced Li–S batteries. Herein, we provide a summary focusing on the recent advances of hollow micro-/nanostructures utilized as sulfur hosts in Li–S batteries. These hollow sulfur host materials are broadly divided into two categories, namely carbonaceous materials and metal compounds. The design rationales and advantages of various hollow structured sulfur hosts are analyzed and discussed. We then highlight several most recently reported innovative hollow nanostructures, which are delicately designed to both physically and chemically restrict the diffusion of LiPSs. A performance comparison between some representative composite cathodes is summarized in Table 1.| Host materials | S loading method | S content (wt%) | Areal S loading (mg cm−2) | Cycle capacity (mA h g−1) | Energy densitya (W h kg−1) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Composite | Electrode | Initial | Retention | |||||
a The specific gravimetric energy density (E) of these Li–S cells is calculated based on the whole cathode electrode film and the Li anode, excluding the current collector, electrolyte, etc. using the following formula 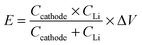 where Ccathode is the initial discharge capacity of the cathode electrode, and CLi is the theoretical capacity of Li (3860 mA h g−1), and the average voltage difference (ΔV) between the sulfur cathode and the Li anode is 2.1 V (vs. Li/Li+). However, it should be noted that, in practical applications, the mass contents of the current collector, electrolyte, packaging, casing, and the mass ratio between the cathode and the anode will greatly affect the cell's final energy density. Thus, for the practical electrode design, thick electrodes with a higher areal mass loading of S will deliver a higher energy density than those of thin electrodes. where Ccathode is the initial discharge capacity of the cathode electrode, and CLi is the theoretical capacity of Li (3860 mA h g−1), and the average voltage difference (ΔV) between the sulfur cathode and the Li anode is 2.1 V (vs. Li/Li+). However, it should be noted that, in practical applications, the mass contents of the current collector, electrolyte, packaging, casing, and the mass ratio between the cathode and the anode will greatly affect the cell's final energy density. Thus, for the practical electrode design, thick electrodes with a higher areal mass loading of S will deliver a higher energy density than those of thin electrodes.
|
||||||||
| HCS | Exposing at S vapor/3 times | 70 | 64.8 | N/A | 1071 | 974 (100 cycles at 0.5C) | 1235 | 20 |
| N-Doped HCS | 160 °C/10 h in sealed tubes | 85 | 72.3 | 0.5–0.7 | 1113 | 980 (100 cycles at 0.2C) | 1398 | 22 |
| Double shelled HCS | 400 °C/12 h in a sealed autoclave | 64 | 44.8 | N/A | 1020 | 690 (100 cycles at 0.1C) | 858 | 21 |
| Multi shelled HCS | 155 °C/12 h in flow Ar | 86 | 68.8 | N/A | 1350 | 1250 (200 cycles at 0.1C) | 1572 | 39 |
| Porous-shell HCS | Heated at 155 °C | 70 | 56 | 1.4–1.8 | 1015 | 880 (100 cycles at 0.2C) | 1040 | 40 |
| Yolk–shell HCS | 155 °C/12 h in a vacuum tube | 70 | 52.5 | 1 | 1100 | 960 (200 cycles at 0.5C) | 1055 | 41 |
| N-Doped HCB | 155 °C/6 h in a sealed glass bottle | 70 | 49 | 1.1–1.5 | 1192 | 706 (400 cycles at 1C) | 1065 | 23 |
| HCF | 155 °C/12 h | 75 | 75 | 1 | 1380 | 730 (150 cycles at 0.2C) | 1714 | 42 |
| Sealed HCF | 400 °C/2 h in Ar | N/A | N/A | 1 | 1139 | 863 (1000 cycles at 5C) | N/A | 44 |
| CNTs@CNF | 155 °C/2 h in N2 | 85.2 | 68 | 2 | 1633 | 1193 (100 cycles at 0.1C) | 1811 | 45 |
| CNT@CNF | 155 °C/24 h | 71 | 56 | N/A | 1274 | 918 (50 cycles at 0.5 A g−1) | 1265 | 46 |
| MCF | 300 °C/12 h in a sealed vessel | 72 | 72 | 3.6 | 1215 | 950 (200 cycles at 0.2C) | 1498 | 48 |
| CH@LDH | 155 °C/12 h | 75 | 52.5 | 3 | 1014 | 653 (100 cycles at 0.1C) | 982 | 29 |
| MnO2@HCF | 155 °C/12 h | 71 | 49.7 | 3.5–3.9 | 1147 | 662 (300 cycles at 0.5C) | 1043 | 32 |
| TiO@C-HS | 300 °C/4 h in a sealed glass vessel | 70 | 56 | 1.5 | 1285 | 750 (500 cycles at 0.2C) | 1274 | 64 |
| 4 | 886 | 821 (50 cycles at 0.05C) | 923 | |||||
Although much progress has been achieved over the past years, challenges remain in Li–S batteries, and a high-performance sulfur cathode that meets the criteria for commercialization is still on the way. On the basis of the working principles of sulfur cathodes, the future development of hollow micro-/nanostructures as sulfur hosts might focus on the following aspects. Firstly, the choice of host material with an ability to immobilize LiPSs is one of the important considerations. Since a higher binding energy is not always advantageous for retaining LiPSs,26 the interactions between the host surface and LiPSs should be suitably controlled. Furthermore, beyond the surface affinity interactions between the host materials and LiPSs, some new mechanisms are proposed, such as the thiosulfate–polythionate conversion generated on the interface of δ-MnO2 and LiPSs during discharging.24 Benefiting from the redox reaction between the host materials and LiPSs, these host materials can provide better interfaces for controllable LiPSs diffusion and Li2S2/Li2S deposition. When these advanced host materials are designed into hollow structured hosts, they will promise a more enhanced electrochemical performance. Secondly, good electronic conductivity is indispensable for high electrochemical activity. Searching for host materials with inherent high conductivity could be one option, whereas the construction of composites based on carbonaceous supports and strong LiPS binding compounds would be a more versatile approach. Moreover, it would be much easier to manipulate the hollow structure in the presence of carbonaceous components. Thirdly, since sulfur hosts do not directly contribute to the energy storage, the content of sulfur hosts should be minimized without degrading the performance. This requires a reasonably designed hollow interior to accommodate a large amount of active species and sufficient surface to block LiPSs from shuttling and facilitate redox reactions. Fourthly, infusing sulfur into a desirable location of the hollow hosts remains another technical challenge. Tailoring the structure of the hollow hosts and optimizing the sulfur infusion method might be the solutions to this problem. Finally, a better understanding of how the hollow micro-/nanostructures accommodate and interact with an active sulfur species would be very helpful for the future design of advanced sulfur cathodes.
From a practical application point of view, a high areal/volumetric capacity of the sulfur cathode and a low amount of electrolyte required for successful operation are primary requirements to allow high energy/power densities of real Li–S batteries. In this regard, the porosity of the sulfur cathode, including both the interior voids in the hollow hosts and the outer space when packed in the electrode film, should be optimized. The former could be improved by tuning the porosity and sulfur content in the hollow micro-/nanostructure hosts, while the latter is largely related to the shape of the hollow hosts and how they are packed in the electrode. A balance should be achieved through the elimination of the excess empty space with minimal compromise on the performance, which would be a challenging and important research topic in this field. Moreover, enhancing the robustness of hollow micro-/nanostructures, for example by incorporating a flexible carbon shell, would be necessary to maintain their integrity and functionality after rolling press during the conventional electrode fabrication process. Last but not least, cost-effective and scalable fabrication techniques are yet to be developed for practical applications of these hollow micro-/nanostructures for use in Li–S batteries.
Based on the above discussions, one could confidently conclude that using hollow micro-/nanostructures as sulfur hosts is one of the very few promising approaches available at this stage to achieve truly advantageous Li–S batteries. Together with the rapid progress in Li anodes, electrolytes and membrane separators, one can optimistically expect that Li–S batteries will surpass current LIBs in the near future.
Acknowledgements
X. W. L. is grateful to the Ministry of Education (Singapore) for financial support through the AcRF Tier 1 funding (Grant RG12/14; M4011258).References
- J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 16013 CrossRef
.
- M.-S. Balogun, W. Qiu, Y. Luo, H. Meng, W. Mai, A. Onasanya, T. K. Olaniyi and Y. Tong, Nano Res., 2016 DOI:10.1007/s12274-016-1171-1
.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J. M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed
.
- Z. Cui, C. Zu, W. Zhou, A. Manthiram and J. B. Goodenough, Adv. Mater., 2016, 28, 6926–6931 CrossRef CAS PubMed
.
- M.-S. Balogun, W. Qiu, W. Wang, P. Fang, X. Lu and Y. Tong, J. Mater. Chem. A, 2015, 3, 1364–1387 CAS
.
- Y. Luo, M. S. Balogun, W. Qiu, R. Zhao, P. Liu and Y. Tong, Chem. Commun., 2015, 51, 13016–13019 RSC
.
- M. Wild, L. O'Neill, T. Zhang, R. Purkayastha, G. Minton, M. Marinescu and G. J. Offer, Energy Environ. Sci., 2015, 8, 3477–3494 CAS
.
- X. L. Ji and L. F. Nazar, J. Mater. Chem., 2010, 20, 9821–9826 RSC
.
- A. Manthiram, Y. Fu, S. H. Chung, C. Zu and Y. S. Su, Chem. Rev., 2014, 114, 11751–11787 CrossRef CAS PubMed
.
- Y. X. Yin, S. Xin, Y. G. Guo and L. J. Wan, Angew. Chem., Int. Ed., 2013, 52, 13186–13200 CrossRef CAS PubMed
.
- S. Evers and L. F. Nazar, Acc. Chem. Res., 2013, 46, 1135–1143 CrossRef CAS PubMed
.
- Z. Li, Y. Huang, L. Yuan, Z. Hao and Y. Huang, Carbon, 2015, 92, 41–63 CrossRef CAS
.
- A. Manthiram, S. H. Chung and C. Zu, Adv. Mater., 2015, 27, 1980–2006 CrossRef CAS PubMed
.
- Q. Pang, X. Liang, C. Y. Kwok and L. F. Nazar, J. Electrochem. Soc., 2015, 162, A2567–A2576 CrossRef CAS
.
- L. Ma, K. E. Hendrickson, S. Wei and L. A. Archer, Nano Today, 2015, 10, 315–338 CrossRef CAS
.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS
.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016 DOI:10.1021/acs.chemrev.5b00731
.
- Z. Wang, L. Zhou and X. W. Lou, Adv. Mater., 2012, 24, 1903–1911 CrossRef CAS PubMed
.
- J. Liang, Z.-H. Sun, F. Li and H.-M. Cheng, Energy Storage Mater., 2016, 2, 76–106 CrossRef
.
- N. Jayaprakash, J. Shen, S. S. Moganty, A. Corona and L. A. Archer, Angew. Chem., Int. Ed., 2011, 50, 5904–5908 CrossRef CAS PubMed
.
- C. Zhang, H. B. Wu, C. Yuan, Z. Guo and X. W. Lou, Angew. Chem., Int. Ed., 2012, 51, 9592–9595 CrossRef CAS PubMed
.
- W. Zhou, C. Wang, Q. Zhang, H. D. Abruña, Y. He, J. Wang, S. X. Mao and X. Xiao, Adv. Energy Mater., 2015, 5, 1401752 CrossRef
.
- F. Pei, T. An, J. Zang, X. Zhao, X. Fang, M. Zheng, Q. Dong and N. Zheng, Adv. Energy Mater., 2016, 6, 1502539 CrossRef
.
- X. Liang, C. Hart, Q. Pang, A. Garsuch, T. Weiss and L. F. Nazar, Nat. Commun., 2015, 6, 5682 CrossRef PubMed
.
- X. Liang, C. Y. Kwok, F. Lodi-Marzano, Q. Pang, M. Cuisinier, H. Huang, C. J. Hart, D. Houtarde, K. Kaup, H. Sommer, T. Brezesinski, J. Janek and L. F. Nazar, Adv. Energy Mater., 2016, 6, 1501636 CrossRef
.
- Q. Zhang, Y. Wang, Z. W. Seh, Z. Fu, R. Zhang and Y. Cui, Nano Lett., 2015, 15, 3780–3786 CrossRef CAS PubMed
.
- X. Tao, J. Wang, C. Liu, H. Wang, H. Yao, G. Zheng, Z. W. Seh, Q. Cai, W. Li, G. Zhou, C. Zu and Y. Cui, Nat. Commun., 2016, 7, 11203 CrossRef CAS PubMed
.
- H. J. Peng and Q. Zhang, Angew. Chem., Int. Ed., 2015, 54, 11018–11020 CrossRef CAS PubMed
.
- J. Zhang, H. Hu, Z. Li and X. W. Lou, Angew. Chem., Int. Ed., 2016, 55, 3982–3986 CrossRef CAS PubMed
.
- X. Liang and L. F. Nazar, ACS Nano, 2016, 10, 4192–4198 CrossRef CAS PubMed
.
- X. Wang, G. Li, J. Li, Y. Zhang, A. Wook, A. Yu and Z. Chen, Energy Environ. Sci., 2016, 9, 2533–2538 CAS
.
- Z. Li, J. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 12886–12890 CrossRef CAS PubMed
.
- N. Brun, K. Sakaushi, L. Yu, L. Giebeler, J. Eckert and M. M. Titirici, Phys. Chem. Chem. Phys., 2013, 15, 6080–6087 RSC
.
- F. Bottger-Hiller, P. Kempe, G. Cox, A. Panchenko, N. Janssen, A. Petzold, T. Thurn-Albrecht, L. Borchardt, M. Rose, S. Kaskel, C. Georgi, H. Lang and S. Spange, Angew. Chem., Int. Ed., 2013, 52, 6088–6091 CrossRef PubMed
.
- Y. Qu, Z. Zhang, X. Wang, Y. Lai, Y. Liu and J. Li, J. Mater. Chem. A, 2013, 1, 14306 CAS
.
- Z. Wang, S. Zhang, L. Zhang, R. Lin, X. Wu, H. Fang and Y. Ren, J. Power Sources, 2014, 248, 337–342 CrossRef CAS
.
- J. Zang, T. An, Y. Dong, X. Fang, M. Zheng, Q. Dong and N. Zheng, Nano Res., 2015, 8, 2663–2675 CrossRef CAS
.
- G. M. Zhou, Y. B. Zhao and A. Manthiram, Adv. Energy Mater., 2015, 5, 1402263 CrossRef
.
- S. Chen, X. Huang, B. Sun, J. Zhang, H. Liu and G. Wang, J. Mater. Chem. A, 2014, 2, 16199–16207 CAS
.
- G. He, S. Evers, X. Liang, M. Cuisinier, A. Garsuch and L. F. Nazar, ACS Nano, 2013, 7, 10920–10930 CrossRef CAS PubMed
.
- Q. Sun, B. He, X. Q. Zhang and A. H. Lu, ACS Nano, 2015, 9, 8504–8513 CrossRef CAS PubMed
.
- G. Y. Zheng, Y. Yang, J. J. Cha, S. S. Hong and Y. Cui, Nano Lett., 2011, 11, 4462–4467 CrossRef CAS PubMed
.
- J. C. Guo, Y. H. Xu and C. S. Wang, Nano Lett., 2011, 11, 4288–4294 CrossRef CAS PubMed
.
- S. Moon, Y. H. Jung, W. K. Jung, D. S. Jung, J. W. Choi and D. K. Kim, Adv. Mater., 2013, 25, 6547–6553 CrossRef CAS PubMed
.
- F. Jin, S. Xiao, L. Lu and Y. Wang, Nano Lett., 2016, 16, 440–447 CrossRef CAS PubMed
.
- Y. Zhao, W. Wu, J. Li, Z. Xu and L. Guan, Adv. Mater., 2014, 26, 5113–5118 CrossRef CAS PubMed
.
- J. Wu, N. Wang, Y. Zhao and L. Jiang, J. Mater. Chem. A, 2013, 1, 7290 CAS
.
- Z. Li, J. T. Zhang, Y. M. Chen, J. Li and X. W. Lou, Nat. Commun., 2015, 6, 8850 CrossRef CAS PubMed
.
- X. Liang, A. Garsuch and L. F. Nazar, Angew. Chem., Int. Ed., 2015, 54, 3907–3911 CrossRef CAS PubMed
.
- X. Ji, S. Evers, R. Black and L. F. Nazar, Nat. Commun., 2011, 2, 325 CrossRef PubMed
.
- Z. W. Seh, W. Li, J. J. Cha, G. Zheng, Y. Yang, M. T. McDowell, P. C. Hsu and Y. Cui, Nat. Commun., 2013, 4, 1331 CrossRef PubMed
.
- Q. Pang, D. Kundu, M. Cuisinier and L. F. Nazar, Nat. Commun., 2014, 5, 4759 CrossRef CAS PubMed
.
- X. Tao, J. Wang, Z. Ying, Q. Cai, G. Zheng, Y. Gan, H. Huang, Y. Xia, C. Liang, W. Zhang and Y. Cui, Nano Lett., 2014, 14, 5288–5294 CrossRef CAS PubMed
.
- H. Yao, G. Zheng, P. C. Hsu, D. Kong, J. J. Cha, W. Li, Z. W. Seh, M. T. McDowell, K. Yan, Z. Liang, V. K. Narasimhan and Y. Cui, Nat. Commun., 2014, 5, 3943 CAS
.
- Z. W. Seh, J. H. Yu, W. Li, P. C. Hsu, H. Wang, Y. Sun, H. Yao, Q. Zhang and Y. Cui, Nat. Commun., 2014, 5, 5017 CrossRef CAS PubMed
.
- Z. Yuan, H. J. Peng, T. Z. Hou, J. Q. Huang, C. M. Chen, D. W. Wang, X. B. Cheng, F. Wei and Q. Zhang, Nano Lett., 2016, 16, 519–527 CrossRef CAS PubMed
.
- Q. Pang, D. Kundu and L. F. Nazar, Mater. Horiz., 2016, 3, 130–136 RSC
.
- Q. Fan, W. Liu, Z. Weng, Y. Sun and H. Wang, J. Am. Chem. Soc., 2015, 137, 12946–12953 CrossRef CAS PubMed
.
- C. Zu and A. Manthiram, Adv. Energy Mater., 2013, 3, 1008–1012 CrossRef CAS
.
- J. Jiang, J. Zhu, W. Ai, X. Wang, Y. Wang, C. Zou, W. Huang and T. Yu, Nat. Commun., 2015, 6, 8622 CrossRef CAS PubMed
.
- Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS PubMed
.
- H. Al Salem, G. Babu, C. V. Rao and L. M. Arava, J. Am. Chem. Soc., 2015, 137, 11542–11545 CrossRef CAS PubMed
.
- M. Zhang, C. Yu, C. Zhao, X. Song, X. Han, S. Liu, C. Hao and J. Qiu, Energy Storage Mater., 2016, 5, 223–229 CrossRef
.
- Z. Li, J. Zhang, B. Guan, D. Wang, L.-M. Liu and X. W. Lou, Nat. Commun., 2016 DOI:10.1038/ncomms13065
.
| This journal is © The Royal Society of Chemistry 2016 |