Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Alkylzinc diorganophosphates: synthesis, structural diversity and unique ability to incorporate zincoxane units†
Małgorzata
Wolska-Pietkiewicz‡
a,
Adam
Świerkosz‡
a,
Iwona
Justyniak
b,
Agnieszka
Grala
b,
Kamil
Sokołowski
b and
Janusz
Lewiński
*ab
aFaculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
bInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
First published on 19th October 2016
Abstract
Equimolar reactions between ZntBu2 and diphenyl phosphate (dpphe-H) or dimethyl phosphate (dmphe-H) result in the formation of [tBuZn(O2P(OR′)2)]-type compounds which crystallize as tetranuclear aggregates, [tBuZn(dpphe)]4 (14) or [tBuZn(dmphe)]4 (24), with the phosphate ligands spanning 4-coordinate Zn centers. The utility of ZnMe2 instead of ZntBu2 dramatically changes the reaction outcome and leads to [MeZn(O2P(OR′)2)]-type moieties incorporating zincoxane units, i.e., pentanuclear [{MeZn(dpphe)}3(Me2Zn2O)(THF)2] (3) and nonanuclear [{MeZn(dmphe)}6(Me2Zn3O2)] (4) aggregates. The resulting compounds were characterized by 1H and 31P NMR spectroscopy and single-crystal X-ray diffraction analysis.
Since the first zinc-based phosphate molecular sieves related to the structure of zeolitic aluminosilicates were published in 1991,1 there has been rapid and continuous growth in the area of the synthesis and the application of metal complexes and the corresponding functional materials supported by a vast array of phosphorus oxyacids and their organic derivatives.2 Such materials revealing versatile structures from zero-dimensional molecular complexes to three-dimensional frameworks have been used mainly in catalysis, ion-exchange and materials chemistry including rational design of porous materials or gas storage.2 Moreover, molecular metal complexes of phosphorus oxyacids have also received considerable interest as potential secondary building blocks of extended structures of various dimensionalities and architectures.2–4 Within this realm, zinc phosphates occupy a prominent position. However, both the number of structurally well-characterized molecular zinc phosphates as well as the knowledge on their transformation modes from low- to high-dimensional structures remain relatively limited. This obstacle mainly arises from the fact that zinc and other metal phosphates are commonly synthesized by hydrothermal synthetic methods.5 In order to isolate molecular complexes, addition of ancillary ligands is usually required and is in line with this strategy, for example, a number of novel inorganic zinc-based molecular complexes incorporating organophosphate ligands were isolated and structurally characterized.6 An alternative to this inorganic route may potentially be an organometallic approach. However, the corresponding organozinc compounds still represent a highly unexplored field. Strikingly, only a few organometallic zinc compounds bearing a characteristic structural motif [RZn(OxPR′y)] (wherein R and R′ = alkyl or aryl, x = 2–4, y = 1–2) have been crystallographically characterized to date. For example, in the course of extensive investigations on metal phosphonates,2a,7 Roesky and co-workers described the reaction of tert-butylphosphonic acid with zinc dialkyls in different molar ratios, which resulted in the formation of structurally varied organozinc phosphonates: tetranuclear [{(ZnMe)4(THF)2}{tBuPO3}2] and hexanuclear [{(ZnEt)3(Zn(THF))3}{tBuPO3}4{μ3-OEt}] complexes, and a dodecanuclear aggregate [{Zn2(THF)2(ZnEt)6Zn4(μ4-O)}{(tBuPO3)8}] with a [Zn4(μ4-O)]6+ core, respectively.8 To the best of our knowledge, heretofore only one structurally characterized organozinc phosphate, namely a dimeric iodomethylzinc phosphate [ICH2Zn(dpphe)]2 (where, dpphe – diphenyl phosphate monoanion), was reported. This compound was derived from the reaction of dpphe-H with 1 equiv. of ZnEt2 along with the consecutive addition of 1 equiv. of CH2I2 and then was used as an efficient reagent for the cyclopropanation of alkenes.9
As part of our ongoing interest in both new molecular organozinc building blocks for inorganic–organic functional materials10 and well-defined precursors of organic ligand-coated ZnO nanocrystals,11 we turn our attention to molecular complexes incorporating organophosphate ligands. Herein we report the preliminary experiments involving the reactions of ZnR2 (R = tBu and Me) with organophosphate diesters. To gain insights into the factors determining reaction outcomes and structures of the resulting alkylzinc phosphates, except various substituents at zinc, two different organophosphates were selected as proligands, i.e., diphenyl phosphate (dpphe-H) and dimethyl phosphate (dmphe-H) (Scheme 1a).
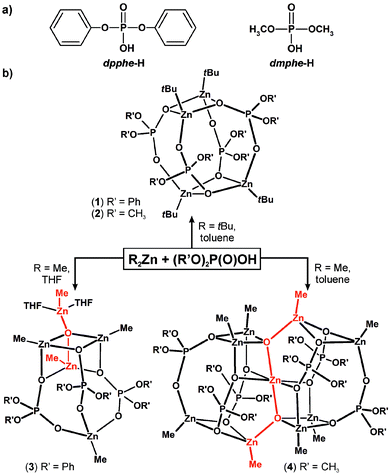 | ||
| Scheme 1 (a) Selected proligands: diphenyl phosphate and dimethyl phosphate, and (b) syntheses of alkylzinc diorganophosphates. | ||
All the reactions between the selected phosphate proligands and ZnR2 (R = tBu, Me) were carried out according to Scheme 1b. An equimolar reaction of ZntBu2 with dpphe-H or dmphe-H in toluene at −78 °C lead to the formation of [tBuZn(O2P(OR′)2)]-type compounds which crystallize as tetranuclear aggregates [tBuZn(dpphe)]4 (14), and [tBuZn(dmphe)]4 (24) (for details, see the ESI†). In both cases colourless rectangular crystals suitable for X-ray diffraction analyses were almost quantitatively isolated from the postreaction mixtures. Conversely, the utility of ZnMe2 instead of ZntBu2 dramatically changes the reaction outcome and afforded two methylzinc(oxo)phosphate complexes, which exhibited solvent-dependent aggregation degrees. The reaction between ZnMe2 and 1 equiv. of dpphe-H in a toluene solution leads to an insoluble product. Dissolution of the resulting solid in THF followed by crystallization at low temperature afforded a novel organozinc oxo cluster supported by the diphenyl phosphate ligands with the formula [{MeZn(dpphe)}3(Me2Zn2O)(THF)2] (3). However, in a similar reaction system involving dmphe-H, a nonanuclear cluster [{MeZn(dmphe)}6(Me2Zn3O2)] (4) was isolated in high yield. The observed formation of zinc oxo species is an intriguing issue. We note that the reported reactions were performed using the standard Schlenk technique with the same batches of solvents. Because of the observed relatively high reaction yields, the presence of the oxo-zinc species cannot be attributed to adventitious moisture or traces of dioxygen. In our opinion, the origin of zincoxane units may likely be associated with a condensation of organophosphate molecules mediated by ZnMe2 and the subsequent formation of an organopyrophosphate moiety related to the processes observed in biochemistry (cf. ATP biosynthesis); the presence of tetraphenyl pyrophosphate and products of its decomposition were confirmed by EI-MS in the postreaction mixtures (for details, see the ESI†).
The resulting compounds 1–4 were fully characterized using spectroscopic techniques and single crystal X-ray diffraction studies. The IR spectra are devoid of any characteristic absorption in the region 2700–2500 cm−1 and ∼3600 cm−1, indicating a complete reaction of all P–O–H groups with ZnR2. Moreover, for 1–4 the characteristic vibrational P–O–Zn stretches appear as bands of strong intensities at 1054 cm−1, 1032 cm−1, 1097 cm−1 and 1025 cm−1, respectively. All 1H NMR spectra are consistent with the structures found in the solid state. The 1H NMR spectra of 1–4 contain well-resolved signals characteristic for the alkyl groups bonded to zinc centres and the sets of signals for the corresponding phosphate ligands (Fig. S1a–S4a†). The 31P NMR spectra of 1, 2 and 3 show a single resonance at −16.79 ppm, −1.35 ppm and −9.67 ppm, respectively, (Fig. S1b–S3b†). However, the 31P NMR of 4 comprises two signals at 1.37 ppm and 5.91 ppm which is consistent with the presence of two nonequivalent dmphe ligands in the solid state structure (Fig. S4b†).
Compounds 14 and 24 crystallize in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group as tetrameric structures with four-coordinate zinc centres. The molecular structures of 1 and 2 are shown in Fig. 1 and S6 (see the ESI†), respectively. In both cases the association of four [tBuZn(O2P(OR′)2)] moieties results in a quasi-cube-shaped central core, which is made up of two 8-membered heterocycles Zn2P2O4 joined by two 4-membered Zn2(μ2-O)2 rings. Each of these eight-membered rings adopts a pseudo-boat conformation. The periphery of the central Zn4P4O8 polyhedron is surrounded by hydrophobic tert-butyl and –P(OR′)2 groups (where R′ = –Ph (14), –CH3 (24)), which is consistent with the high solubility of 14 and 24 in common organic solvents. The bridging phosphate groups between three zinc centres display a μ3–μ2:η1 coordination mode. The Zn–C and Zn–O bonds fall within a narrow range of 1.982–1.988 Å (for 14), 1.987–1.994 Å (for 24) and 1.972–2.151 Å (14), 1.977–1.216 Å (24), respectively.
space group as tetrameric structures with four-coordinate zinc centres. The molecular structures of 1 and 2 are shown in Fig. 1 and S6 (see the ESI†), respectively. In both cases the association of four [tBuZn(O2P(OR′)2)] moieties results in a quasi-cube-shaped central core, which is made up of two 8-membered heterocycles Zn2P2O4 joined by two 4-membered Zn2(μ2-O)2 rings. Each of these eight-membered rings adopts a pseudo-boat conformation. The periphery of the central Zn4P4O8 polyhedron is surrounded by hydrophobic tert-butyl and –P(OR′)2 groups (where R′ = –Ph (14), –CH3 (24)), which is consistent with the high solubility of 14 and 24 in common organic solvents. The bridging phosphate groups between three zinc centres display a μ3–μ2:η1 coordination mode. The Zn–C and Zn–O bonds fall within a narrow range of 1.982–1.988 Å (for 14), 1.987–1.994 Å (for 24) and 1.972–2.151 Å (14), 1.977–1.216 Å (24), respectively.
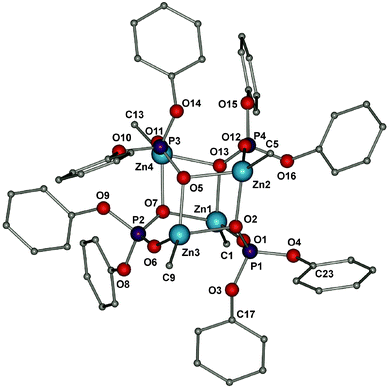 | ||
| Fig. 1 Molecular structure of 14; hydrogen atoms and methyl groups of the tert-butyl substituents are omitted for clarity. | ||
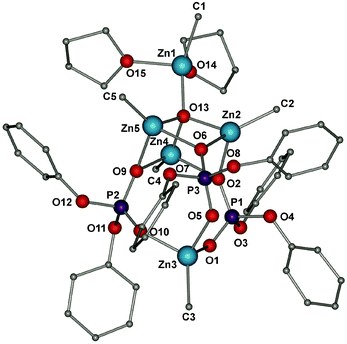
The methylzinc(oxo)phosphate 3 crystallizes in the triclinic space group as a pentanuclear complex. The molecular structure of 3 contains a Zn4O7P3 core stabilized by the three phosphate ligands (Fig. 2). Each dpphe ligand bridges three zinc centers and adopts a μ3–μ2:η1 coordination mode. A striking feature of this structure is the presence of a zincoxane unit. Hence, the revealed very unusual oxo-zinc structure of 3 can be formally described as a zincoxane (Me2Zn2O) moiety entrapped by three [MeZn(dpphe)] molecules. As a result, the molecular structure of 3 contains a total of two eight-membered Zn2P2O4 rings and three four-membered Zn2O2 rings. Moreover, one MeZn subunit of the zincoxane is anchored in the central core and the second, terminal MeZn subunit is stabilized by two η1-THF molecules. All the zinc centres are tetra-coordinate and possess a near tetrahedral geometry. Due to a poor quality of crystals of 3 for single-crystal X-ray diffraction, it was not possible to obtain a fully satisfactory refinement of the molecular structure, and this precludes also a detailed discussion of the geometrical parameters.
Compound 4 crystallizes in the centrosymmetric monoclinic space group and its molecular structure is shown in Fig. 3. The oxo-zinc structure of 4 can be formally described as two trinuclear [MeZn(dpphe)]3 subunits with an entrapped trinuclear zincoxane moiety, (Me2Zn3O2). It results in the formation of the Zn9P6O13 cage, which is made up of a combination of two eight-membered Zn2P2O4, four six-membered Zn2P1O3 and eight four-membered Zn2O2 rings. Depending on the coordination modes, the six dmphe groups can be classified into two different types and the phosphate monoanionic ligands are in two distinctive μ4–μ2:μ2 and μ3–μ2:η1 coordination modes. The Zn4(μ4-O) bond distances in 4 range from 1.834 to 2.006 Å, and the Zn–O(phosphate) distances range from 1.968 to 2.196 Å. These values are similar to the average value of those found in the reported zinc oxophosphate tetranuclear cluster [Zn4(μ4-O){O2P(OtBu)2}]6 (2.00 and 1.93 Å, respectively).12 Moreover, there are no formal P–O and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in 1–2 and 4, and the average P–O bond lengths: 1.51 Å (for 14), 1.50 (for 24), 1.50 Å (for 4) are much shorter than a formal P–O bond (1.59–1.60 Å) and considerably longer than a formal P
O bonds in 1–2 and 4, and the average P–O bond lengths: 1.51 Å (for 14), 1.50 (for 24), 1.50 Å (for 4) are much shorter than a formal P–O bond (1.59–1.60 Å) and considerably longer than a formal P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (1.45–1.46 Å).13 It is essential to note that compounds 3 and 4 bear a striking resemblance to each other. Particularly, both methylzinc(oxo)phosphates contain a pentanuclear structural motif with a zincoxane unit incorporated into methylzinc diorganophosphate moieties.
O bond (1.45–1.46 Å).13 It is essential to note that compounds 3 and 4 bear a striking resemblance to each other. Particularly, both methylzinc(oxo)phosphates contain a pentanuclear structural motif with a zincoxane unit incorporated into methylzinc diorganophosphate moieties.
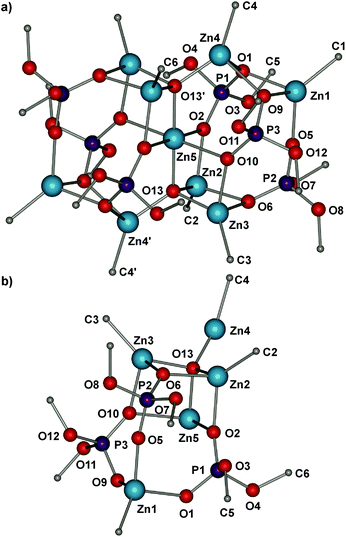 | ||
| Fig. 3 (a) Molecular structure of 4 and (b) its asymmetric unit; hydrogen atoms are omitted for clarity. | ||
Conclusions
In conclusion, we prepared and characterized novel and structurally diverse organozinc complexes stabilized by organophosphate diesters. While the reaction involving bulky ZntBu2 results in the formation of [tBuZn(O2P(OR′)2)]-type organozinc compounds, the utility of ZnMe2 dramatically changes the reaction outcome and leads to methylzinc organophosphate complexes incorporating zincoxane units. The resulting structures reproduce the salient bonding features of the phosphate zincoxanes which likely illuminate the structural changes in molecules and intermediate compounds that may exist only briefly during a transformation process of organozinc phosphates into ZnO nanocrystals. Further studies on the chemistry of organozinc complexes supported by a vast array of phosphorus oxyacids and their organic derivatives as well as their transformation into extended inorganic–organic porous materials and ZnO-based nanomaterials are currently in progress.Acknowledgements
The authors acknowledge the Foundation for Polish Science Team Program co-financed by the EU “European Regional Development Fund” TEAM/2011-7/8 and the National Science Centre, Grant Maestro DEC-2012/04/A/ST5/00595 for financial support.Notes and references
- T. E. Gier and G. D. Stucky, Nature, 1991, 349, 508 CrossRef CAS.
- (a) M. G. Walawalkar and H. W. Roesky, Acc. Chem. Res., 1999, 32, 117 CrossRef CAS; (b) R. Murugavel, A. Choudhury, M. G. Walawalkar, R. Pothiraja and C. N. R. Rao, Chem. Rev., 2008, 108, 3549 CrossRef CAS PubMed; (c) V. Chandrasekhar, T. Senapati, A. Deya and S. Hossaina, Dalton Trans., 2011, 40, 5394 RSC; (d) J. Goura and V. Chandrasekhar, Chem. Rev., 2015, 115, 6854 CrossRef CAS PubMed.
- K. J. Gagnon, H. P. Perry and A. Clearfield, Chem. Rev., 2012, 112, 1034 CrossRef CAS PubMed.
- J. A. Sheikh, H. S. Jena, A. Clearfield and S. Konar, Acc. Chem. Res., 2016, 49, 1093 CrossRef CAS PubMed.
- A. J. Norquist and D. O'Hare, J. Am. Chem. Soc., 2004, 126, 6673 CrossRef CAS PubMed.
- (a) R. Murugavel, S. Kuppuswamy, R. Boomishankar and A. Steiner, Angew. Chem., Int. Ed., 2006, 45, 5536 CrossRef CAS PubMed; (b) R. Murugavel, S. Kuppuswamy, N. Gogoi, R. Boomishankar and A. Steiner, Chem. – Eur. J., 2010, 16, 994 CrossRef CAS PubMed; (c) A. Kalita, C. Roch-Marchal and R. Murugavel, Dalton Trans., 2013, 42, 9755 RSC; (d) A. A. Dar, S. K. Sharma and R. Murugavel, Inorg. Chem., 2015, 54, 4882 CrossRef CAS PubMed; (e) A. A. Dar, S. Sen, S. K. Gupta, G. N. Patwari and R. Murugavel, Inorg. Chem., 2015, 54, 9458 CrossRef CAS PubMed.
- For selected examples, see: (a) M. G. Walawalkar, R. Murugavel, A. Voigt, H. W. Roesky and H.-G. Schmidt, J. Am. Chem. Soc., 1997, 119, 4656 CrossRef CAS; (b) M. G. Walawalkar, R. Murugavel, H. W. Roesky and H.-G. Schmidt, Inorg. Chem., 1997, 36, 4202 CrossRef CAS; (c) M. Ganapati Walawalkar, R. Murugavel, H. W. Roesky and H.-G. Schmidt, Organometallics, 1997, 16, 516 CrossRef; (d) Y. Yang, H.-G. Schmidt, M. Noltemeyer, J. Pinkas and H. W. Roesky, J. Chem. Soc., Dalton Trans., 1996, 3609 RSC; (e) G. Anantharaman, M. G. Walawalkar, R. Murugavel, B. Gábor, R. Herbst-Irmer, M. Baldus, B. Angerstein and H. W. Roesky, Angew. Chem., Int. Ed., 2003, 42, 4482 CrossRef CAS PubMed.
- (a) Y. Yang, J. Pinkas, M. Noltemeyer, H.-G. Schmidt and H. W. Roesky, Angew. Chem., Int. Ed., 1999, 38, 664 CrossRef CAS; (b) G. Anantharaman, V. Chandrasekhar, M. G. Walawalkar, H. W. Roesky, D. Vidovic, J. Magull and M. Noltemeyer, Dalton Trans., 2004, 1271 RSC.
- M.-C. Lacasse, C. Poulard and A. B. Charette, J. Am. Chem. Soc., 2005, 127, 12440 CrossRef CAS PubMed.
- For selected examples, see: (a) W. Bury, I. Justyniak, D. Prochowicz, Z. Wróbel and J. Lewiński, Chem. Commun., 2012, 48, 7362 RSC; (b) K. Sokołowski, W. Bury, I. Justyniak, D. Fairén-Jiménez, K. Sołtys, D. Prochowicz, S. Yang, M. Schröder and J. Lewiński, Angew. Chem., Int. Ed., 2013, 52, 13414 CrossRef PubMed; (c) W. Bury, I. Justyniak, D. Prochowicz, A. Rola-Noworyta and J. Lewiński, Inorg. Chem., 2012, 51, 7410 CrossRef CAS PubMed; (d) D. Prochowicz, K. Sokołowski, I. Justyniak, A. Kornowicz, D. Fairen-Jimenez, T. Friščić and J. Lewiński, Chem. Commun., 2015, 51, 4032 RSC; (e) A. Grala, M. Wolska-Pietkiewicz, A. Wojewódzka, M. Dabergut, I. Justyniak and J. Lewiński, Organometallics, 2015, 34, 4959 CrossRef CAS; (f) K. Sokołowski, W. Bury, A. Tulewicz, A. M. Cieślak, I. Justyniak, D. Kubicki, E. Krajewska, A. Milet, R. Moszyński and J. Lewiński, Chem. – Eur. J., 2015, 21, 5496 CrossRef PubMed; (g) Z. Wróbel, I. Justyniak, I. Dranka and J. Lewiński, Dalton Trans., 2016, 45, 7240 RSC.
- (a) A. Grala, M. Wolska-Pietkiewicz, W. Danowski, Z. Wróbel, J. Grzonka and J. Lewiński, Chem. Commun., 2016, 52, 7340 RSC; (b) P. Krupiński, A. Kornowicz, K. Sokołowski, A. M. Cieślak and J. Lewiński, Chem. – Eur. J., 2016, 22, 7817 CrossRef PubMed; (c) J. Paczesny, M. Wolska-Pietkiewicz, I. Binkiewicz, M. Wadowska, Z. Wróbel, K. Matuła, W. Nogala, J. Lewiński and R. Hołyst, ACS Appl. Mater. Interfaces, 2016, 8, 13532 CrossRef CAS PubMed; (d) J. Paczesny, M. Wolska-Pietkiewicz, I. Binkiewicz, Z. Wróbel, M. Wadowska, K. Matuła, I. Dzięcielewski, D. Pociecha, J. Smalc-Koziorowska, J. Lewiński and R. Hołyst, Chem. – Eur. J., 2015, 21, 16941 CrossRef CAS PubMed; (e) K. Sokołowski, I. Justyniak, W. Bury, J. Grzonka, Z. Kaszkur, Ł. Mąkolski, M. Dutkiewicz, A. Lewalska, D. Kubicki, K. Wójcik, K. Kurzydłowski and J. Lewiński, Chem. – Eur. J., 2015, 21, 5488 CrossRef PubMed; (f) W. Bury, E. Krajewska, M. Dutkiewicz, K. Sokołowski, I. Justyniak, Z. Kaszkur, K. J. Kurzydłowski, T. Płociński and J. Lewiński, Chem. Commun., 2011, 47, 5467 RSC.
- C. G. Lugmair, T. D. Tilley and A. L. Rheingold, Chem. Mater., 1997, 9, 339 CrossRef CAS.
- D. E. C. Corbridge, The Structural Chemistry of Phosphorus, Elsevier, Amsterdam, 1974 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: All experimental details and characterization data. CCDC 1499962–1499965. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt03324e |
| ‡ These authors contributed equally to the manuscript. |
| This journal is © The Royal Society of Chemistry 2016 |