Structural diversity in NiII cluster chemistry: Ni5, Ni6, and {NiNa2}n complexes bearing the Schiff-base ligand N-naphthalidene-2-amino-5-chlorobenzoic acid†
Abstract
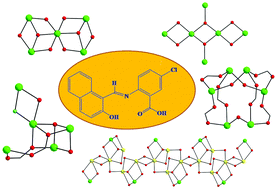
The employment of the fluorescent bridging and chelating ligand N-naphthalidene-2-amino-5-chlorobenzoic acid (nacbH2) in NiII cluster chemistry has led to a series of pentanuclear and hexanuclear compounds with different structural motifs, magnetic and optical properties, as well as an interesting 1-D coordination polymer. Synthetic parameters such as the inorganic anion present in the NiX2 starting materials (X = ClO4− or Cl−), the reaction solvent and the nature of the organic base employed for the deprotonation of nacbH2 were proved to be structure-directing components. Undoubtedly, the reported results demonstrate the rich coordination chemistry of nacbH2 in the presence of NiII metal ions and the ability of this chelate to adopt a variety of different modes, thus fostering the formation of high-nuclearity molecules with many physical properties.

 Please wait while we load your content...
Please wait while we load your content...