Exploring the reducing role of boron: added insights from theory†
Abstract
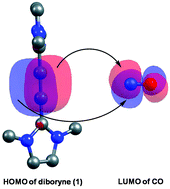
Carbon–carbon coupling in CO molecules is a challenging proposition, and very few main group complexes have been shown to effect this process. A recently reported triply bonded diboryne system (1) is notable for coupling four CO molecules to produce a (bis)boralactone species. The current full quantum chemical computational investigation with density functional theory (DFT) provides important insights into the nature of the CO coupling process by triply bonded diboryne systems. The complete reaction pathway leading to the formation of the (bis)boralactone has been determined. Factors that make this system so successful in coupling CO groups have been elucidated, and pertinent issues, such as why the coupling process stops after four CO additions, have been explored. Also, importantly, insights have been gained through the natural bond orbital (NBO) analysis into how the back-donation from diboryne activates CO.
- This article is part of the themed collection: Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...