Synthesis and characterization of bis(imino)pyridine complexes of divalent Mg and Zn†
Abstract
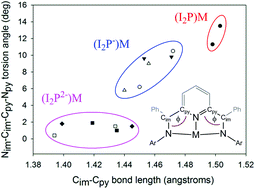
Phenyl-bis(imino)pyridine (PhI2P) complexes, (PhI2P)ZnCl2 (1), (PhI2P−)ZnCl (2) and (PhI2P−)Zn(py)Cl (3) were obtained with the I2P ligand in both the neutral and the one-electron reduced state. In all examples, the metal ion is Zn(II). Metrical parameters obtained from solid state structures of 2 and 3 indicate that the PhI2P− ligand exists as a radical which is supported at the carbon atom of the imino donor, and this electronic state is also apparent in the analogous one-electron reduced ligand Al(III) complex, (PhI2P−)AlCl2 (4), that we prepared for comparison. We were unable to obtain PhI2P Mg complexes, and so the more electron rich methyl-substituted bis(imino)pyridine ligand, MeI2P, was investigated. Reaction of two-electron reduced MeI2P with MgCl2 and Mg(OTf)2 did afford the two-electron reduced ligand complexes [(MeI2P2−)Mg(THF)]2(μ-MgCl2) (5) and (MeI2P2−)Mg(THF)2 (6), respectively (MeI2P = 2,6-bis(1-methylethyl)-N-(2-pyridinylmethylene)phenylamine). Complex 5 crystallizes as a trinuclear Mg complex consisting of two (MeI2P2−)Mg moieties bridged by MgCl2 and the (MeI2P2−) ligand is symmetric across the pyridine ring, but is not planar. In contrast, the (MeI2P2−) ligand in 6 is asymmetric across the pyridine ring and all atoms in the ligand are coplanar. Cyclic voltammetry measurements reveal that in complexes, 1, 4, 5, 6, the I2P0, I2P−, and I2P2− ligand charge states are accessible electrochemically.
- This article is part of the themed collection: Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...