Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of riboflavin on the reduction of radionuclides by Shewanella oneidenis MR-1
Andrea
Cherkouk†
a,
Gareth T. W.
Law
b,
Athanasios
Rizoulis
a,
Katie
Law
b,
Joanna C.
Renshaw‡
a,
Katherine
Morris
a,
Francis R.
Livens
ab and
Jonathan R.
Lloyd
*a
aResearch Centre for Radwaste Disposal and Williamson Research Centre for Molecular Environmental Science, School of Earth, Atmospheric and Environmental Sciences, The University of Manchester, Manchester M13 9PL, UK. E-mail: jon.lloyd@manchester.ac.uk
bCentre for Radiochemistry Research, School of Chemistry, Manchester, M13 9PL, UK
First published on 20th November 2015
Abstract
Uranium (as UO22+), technetium (as TcO4−) and neptunium (as NpO2+) are highly mobile radionuclides that can be reduced enzymatically by a range of anaerobic and facultatively anaerobic microorganisms, including Shewanella oneidensis MR-1, to poorly soluble species. The redox chemistry of Pu is more complicated, but the dominant oxidation state in most environments is highly insoluble Pu(IV), which can be reduced to Pu(III) which has a potentially increased solubility which could enhance migration of Pu in the environment. Recently it was shown that flavins (riboflavin and flavin mononucleotide (FMN)) secreted by Shewanella oneidensis MR-1 can act as electron shuttles, promoting anoxic growth coupled to the accelerated reduction of poorly-crystalline Fe(III) oxides. Here, we studied the role of riboflavin in mediating the reduction of radionuclides in cultures of Shewanella oneidensis MR-1. Our results demonstrate that the addition of 10 μM riboflavin enhances the reduction rate of Tc(VII) to Tc(IV), Pu(IV) to Pu(III) and to a lesser extent, Np(V) to Np(IV), but has no significant influence on the reduction rate of U(VI) by Shewanella oneidensis MR-1. Thus riboflavin can act as an extracellular electron shuttle to enhance rates of Tc(VII), Np(V) and Pu(IV) reduction, and may therefore play a role in controlling the oxidation state of key redox active actinides and fission products in natural and engineered environments. These results also suggest that the addition of riboflavin could be used to accelerate the bioremediation of radionuclide-contaminated environments.
Introduction
Shewanella species are facultative anaerobic bacteria with a remarkable respiratory versatility, being able to couple the oxidation of organic matter to the reduction of terminal electron acceptors including nitrate (NO3−), Fe(III), Mn(IV), U(VI) and Tc(VII).1,2 To date, reactions involving Fe(III) and Mn(IV) have received the most attention, and here, the metals are thought to be reduced via outer membrane cytochromes which are abundant in the genome.3,4 Ferric iron is highly insoluble at neutral pH in most environments, but Shewanella species have evolved pathways to conserve energy for growth through the transfer of electrons to the insoluble Fe(III).1 At least two pathways have been proposed for electron transfer to the solid Fe(III) substrate; (1) the direct transfer of electrons from the cell surface to the Fe(III) mineral, and (2) the use of low-molecular weight soluble redox mediators or “electron shuttles” to promote extracellular electron transfer.1,2,5,6 An Fe(III) reduction deficient mutant strain of Shewanella oneidensis MR-1 was found to contain a mutation in a gene (gspD) that encodes the outer membrane porin of a type II secretion system, which is involved in the extracellular translocation of Shewanella oneidensis outer membrane cytochromes MtrC and OmcA.6,7 The lack of a properly functioning type II protein secretion system renders the organism incapable of reducing Fe(III) oxy(hydr)oxides at wild-type rates.6 However, the Fe(III) reduction activity of the type II protein secretion mutant can be rescued by the addition of exogenous electron shuttling compounds such as anthraquinone-2,6-disulfonate (AQDS).6In addition to direct reduction mechanisms via cytochromes, for more than a decade Shewanella strains have also been known to secrete soluble electron shuttles that can play a role in extracellular electron transfer.8 More recently, the flavin molecules FMN and riboflavin were identified as the electron shuttles secreted by S. oneidensis MR-1 and shown to promote growth via the reduction of poorly soluble Fe(III) oxides.2,9 These molecules have also been shown to play a role in mediating the flow of electrons to an anode surface in a microbial fuel cell containing Shewanella oneidensis MR-1 and Shewanella sp. MR-4.10
Shewanella oneidensis MR-1 can also reduce a range of redox active radionuclides. For example U(VI) is reduced directly under anoxic conditions, resulting in the precipitation of U(IV) as uraninite (UO2).11–13 Recently, it was demonstrated that c-type cytochromes of Shewanella oneidensis MR-1 are essential for the reduction of U(VI) and the subsequent formation of extracelluar UO2 nanoparticles.12,14 In particular, the outer membrane (OM) decaheme cytochrome MtrC, previously implicated in Mn(IV) and Fe(III) reduction, was shown to play a role in transferring electrons to U(VI). Additionally, deletions of mtrC and/or the gene encoding another outer membrane cytochrome omcA significantly affected the in vivo rate of U(VI) reduction relative to the wild-type MR-1 strain.12 A study on the electron transfer pathway for U(VI) reduction mediated by FMN, which is secreted by Shewanella species, demonstrated that FMN may also act as a mediator during the reduction of U(VI) to U(IV), accelerating its reduction.15
Tc(VII) in contrast, is generally thought to be reduced via direct interactions with hydrogenase, using hydrogen as an electron donor.16,17Shewanella oneidensis MR-1 is able to catalyse this reaction consistent with the presence of a NiFe hydrogenase in the strain.18 However, this organism can also couple lactate oxidation to a slower rate of enzymatic Tc(VII) reduction, with c-type cytochromes implicated in electron transfer to the radionuclide with this electron donor.18
The reductive capability of Shewanella putrefaciens (ATCC 8071) (now renamed S. oneidensis) has also been used for the reduction of Np(V) to lower valence (probably Np(IV)19). However, the neptunium in these experiments could not be removed from solution in this study via this direct reductive precipitation mechanism. Instead, bioreduction to Np(IV) by S. putrefaciens, together with phosphate liberation by a Citrobacter sp. with high phosphatase activity, permitted bioprecipitative removal of Np, presumably as an insoluble Np(IV) phosphate.19 A later study demonstrated that cell suspensions of S. oneidensis were able to enzymatically reduce unchelated Np-(V) to insoluble Np(IV)(s), and that the addition of citrate enhanced Np(V) bioreduction.20
Finally, the mechanism of plutonium reduction by S. oneidensis MR-1 has also been studied, and it was demonstrated that in the absence of complexants, very little Pu(III) was produced from the enzymatic reduction of Pu(IV)(OH)4. By contrast, in the presence of the complexant EDTA, most of the Pu(IV)(OH)4(am) was reduced to Pu(III).21,22 Clearly, much work remains to be done to fully understand mechanism and environmental consequence of Pu hydrous oxide reduction in this and other organisms.
The aim of the work described in this paper is to determine if flavins can be used to accelerate the reduction of a range of priority radionuclides by S. oneidensis. This information is important to help elucidate the mechanisms of radionuclide reduction in flavin-secreting metal-reducing bacteria, and also to help inform biotechnological approaches for the efficient bioremediation of radionuclide contaminated land and/or wastewaters. Wild type cells of Shewanella oneidensis MR-1 were used, and in the case of U(VI) and Tc(VII), a mutant with an in-frame deletion of gspD (ΔgspD) was used to identify the potential role of outer membrane c-type cytochromes.
Material and methods
Bacterial strains and media
Shewanella oneidensis MR-1 was obtained from University of Manchester Geomicrobiolgy group culture collection and the Shewanella oneidensis targeted in-frame deletion mutant (ΔgspD) was obtained from Thomas DiChristina (Georgia Tech, USA).6 Starter cultures of the bacteria were cultured aerobically in tryptic soy broth (Oxoid CM0876) for 10 h (30 °C) at 125 rpm and then transferred to a Shewanella minimal medium.3 In the case of the mutant, 50 μM of the antibiotic rifamycin was added to the medium. The bacteria for experimental cultures were grown to mid-exponential growth in Shewanella minimal medium with DL-sodium lactate (100 mM) as the electron donor and sodium fumarate as electron acceptor (20 mM) for 20 h at 30 °C under an atmosphere of N2.2 Cell suspensions were prepared for metal reduction assays by washing the cells twice and resuspending them in 30 mM sodium bicarbonate buffer (pH 7; degassed with N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 (80
CO2 (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20)).
20)).
U(VI) reduction assay
U(VI) reduction assays were conducted in 30 mM sodium bicarbonate buffer (pH 7) containing 500 μM U(VI) as uranyl acetate. DL-sodium lactate was used as the electron donor (10 mM) with or without the addition of 10 μM riboflavin as an extracellular electron shuttle. Controls were also prepared which contained no added electron donor or no cells. Sterile solutions of electron donor, riboflavin and S. oneidensis MR-1 cell suspension or ΔgspD mutant cells were added anaerobically to the autoclaved and degassed buffer solution. Kinetic studies were initiated by the addition of 0.2 ml of a standardised suspension of cells of S. oneidensis MR-1 or the ΔgspD mutant, resulting in a final assay density of 2 × 108 cells per ml. All experiments were conducted in triplicate. The assay tubes were incubated at 30 °C in the dark. At multiple time-points samples were collected and the U(VI) content of the supernatant (after centrifugation at 1 min, 9000g) was analysed using a colorimetric assay with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol.23Tc(VII) reduction assay
The kinetics of Tc(VII) reduction by wild-type S. oneidensis MR-1 and a ΔgspD mutant were determined using washed cell suspensions. Tc(VII) reduction assays contained a final concentration of 100 μM pertechnetate (CERCA, France) in pH 7, 30 mM sodium bicarbonate buffer purged with an N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 (80
CO2 (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) gas mixture. The electron donor used to support radionuclide reduction in this study was DL-sodium lactate (10 mM). In addition, 10 μM riboflavin was added as an electron shuttle. Kinetic studies were initiated by the addition of 0.2 ml of a standardised suspension of cells of S. oneidensis MR-1 or the ΔgspD mutant, resulting in a final assay density of 2 × 108 cells per ml. All experiments were conducted in triplicate and incubated at 30 °C in the dark. At multiple time-points, samples (0.2 ml) were collected and centrifuged for 4 min at 7000g to remove the cells and any precipitated radionuclide. An aliquot of the supernatant (50 μl) was analysed for total Tc concentrations by liquid scintillation counting on a Packard Tri-Carb 2100TR. Another aliquot of the supernatant (100 μl) was also complexed with tetraphenyl arsonium chloride (TPAC) and then the TPAC-TcO4− complex was extracted from the soluble phase with chloroform, prior to liquid scintillation counting (as above) to quantify the concentration of Tc(VII) in solution.18
20) gas mixture. The electron donor used to support radionuclide reduction in this study was DL-sodium lactate (10 mM). In addition, 10 μM riboflavin was added as an electron shuttle. Kinetic studies were initiated by the addition of 0.2 ml of a standardised suspension of cells of S. oneidensis MR-1 or the ΔgspD mutant, resulting in a final assay density of 2 × 108 cells per ml. All experiments were conducted in triplicate and incubated at 30 °C in the dark. At multiple time-points, samples (0.2 ml) were collected and centrifuged for 4 min at 7000g to remove the cells and any precipitated radionuclide. An aliquot of the supernatant (50 μl) was analysed for total Tc concentrations by liquid scintillation counting on a Packard Tri-Carb 2100TR. Another aliquot of the supernatant (100 μl) was also complexed with tetraphenyl arsonium chloride (TPAC) and then the TPAC-TcO4− complex was extracted from the soluble phase with chloroform, prior to liquid scintillation counting (as above) to quantify the concentration of Tc(VII) in solution.18
Np(V) reduction assay
The kinetics of aqueous Np(V) reduction by the wild-type S. oneidensis MR-1 were investigated by resting cell assays using washed cell suspensions. All experiments were carried out at pH 7, in 50 mM 3-(N-morpholino)propanesulfonic acid (MOPS) buffer, that had been purged with a N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 (80
CO2 (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) gas mixture. Three treatments were established (all with 11 μM Np added as NpO2+); (i) abiotic (without cells) containing 10 mM sodium lactate, (ii) biotic (with wild type cells) with 10 mM sodium lactate, and (iii) biotic (with wild type cells) containing 10 mM sodium lactate and 10 μM riboflavin. All medium constituents were sterilised by autoclaving or <0.22 μm filtration (in the case of riboflavin) before use. The biotic treatments were inoculated with washed S. oneidensis MR-1 cells, resulting in a final assay density of 4 × 108 cells per ml. All experiments were conducted in triplicate and incubated at 30 °C in the dark. Periodically, samples (∼400 μl) were taken aseptically using argon gas purged needles and syringes. Thereafter, samples were centrifuged for 5 min at 7000g, and then diluted into 2% HNO3 for determination of the total neptunium concentration in solution by inductively coupled plasma mass spectrometry (ICP-MS) with 232Th as an internal standard. It was assumed that all Np remaining in solution was present as Np(V).
20) gas mixture. Three treatments were established (all with 11 μM Np added as NpO2+); (i) abiotic (without cells) containing 10 mM sodium lactate, (ii) biotic (with wild type cells) with 10 mM sodium lactate, and (iii) biotic (with wild type cells) containing 10 mM sodium lactate and 10 μM riboflavin. All medium constituents were sterilised by autoclaving or <0.22 μm filtration (in the case of riboflavin) before use. The biotic treatments were inoculated with washed S. oneidensis MR-1 cells, resulting in a final assay density of 4 × 108 cells per ml. All experiments were conducted in triplicate and incubated at 30 °C in the dark. Periodically, samples (∼400 μl) were taken aseptically using argon gas purged needles and syringes. Thereafter, samples were centrifuged for 5 min at 7000g, and then diluted into 2% HNO3 for determination of the total neptunium concentration in solution by inductively coupled plasma mass spectrometry (ICP-MS) with 232Th as an internal standard. It was assumed that all Np remaining in solution was present as Np(V).
An additional experiment to ascertain the Np oxidation state at the end of the incubation in the biotic (with wild type cells) treatment containing 10 mM sodium lactate and 10 μM riboflavin was also established. Here, the Np concentration of the starting solution was increased to 50 μM whilst all other parameters remained unchanged. After 120 hours of incubation the total amount of neptunium in solution was then measured, and a cell pellet was harvested via centrifugation (5 min at 7000g) under an inert atmosphere for subsequent XAS analysis.
Neptunium LIII-edge X-ray absorption spectroscopy
Under an inert atmosphere the Np labelled cell-pellet was mixed with boron nitride to attain a solid sample with ∼400 ppm Np. The sample was then carefully packed into an airtight XAS sample tube and stored under an inert atmosphere at −80 °C prior to analysis. Neptunium XAS analysis was conducted at the INE Beamline for actinide research at the ANKA synchrotron light source, Karlsruhe, Germany. Neptunium LIII edge XANES spectra were collected in fluorescence mode by a 5 element solid-state geranium detector with the sample maintained at cryo-temperatures throughout analysis. Parallel measurements of a Zr foil were also taken in transmission for energy calibration. Spectra summation and analysis was conducted using the ATHENA software.Pu(IV) reduction assay
Pu(IV) reduction assays contained a final concentration of 1 mM 242Pu in 30 mM sodium bicarbonate buffer purged with an N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 (80
CO2 (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) gas mixture. DL-sodium lactate (10 mM) was used as an electron donor, with or without added riboflavin (10 μM). Control solutions contained no cells. All experiments were conducted in triplicate and incubated at 30 °C in the dark. 242Pu concentrations were measured by liquid scintillation counting.24 Solution concentrations in supernatant samples were measured after centrifugation (14
20) gas mixture. DL-sodium lactate (10 mM) was used as an electron donor, with or without added riboflavin (10 μM). Control solutions contained no cells. All experiments were conducted in triplicate and incubated at 30 °C in the dark. 242Pu concentrations were measured by liquid scintillation counting.24 Solution concentrations in supernatant samples were measured after centrifugation (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, for 5 min). For 242Pu experiments, precipitates and cells were collected by centrifugation (14
000g, for 5 min). For 242Pu experiments, precipitates and cells were collected by centrifugation (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, for 5 min), dissolved in 1 M HCl and UV-visible-near infrared spectra were recorded from the resulting supernatants using the method described by Boukhalfa and coworkers.21
000g, for 5 min), dissolved in 1 M HCl and UV-visible-near infrared spectra were recorded from the resulting supernatants using the method described by Boukhalfa and coworkers.21
Results
The influence of riboflavin on U(VI) reduction
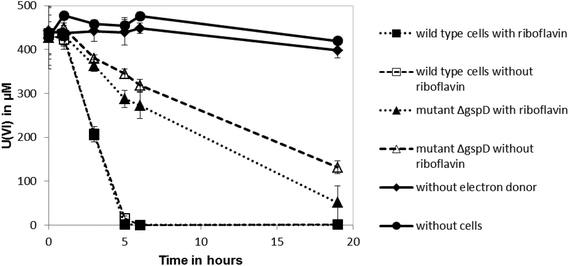
Shewanella oneidensis MR-1 can reduce U(VI) under anoxic conditions, which results in the precipitation of uraninite (UO2).11–13 Outer membrane-associated c-type cytochromes are thought to be involved in U(VI) reduction,12 although the involvement of secreted flavin redox shuttles has not been investigated. To test if riboflavin could enhance the rate of U(VI) reduction by S. oneidensis MR-1, 10 μM of the flavin was added to cell suspensions and the impact on the rate of U(VI) reduction was quantified. The added riboflavin had no significant influence on the reduction rate of U(VI) by the anaerobically-grown wild type cells (Fig. 1), suggesting that addition of the endogenous electron shuttle does not play a significant role in enhancing reduction of this radionuclide. The cell pellets collected in these cultures after centrifugation were also stained black, indicating the likely formation of reduced, insoluble uraninite.11 However, the ΔgspD mutant that was unable to secrete outer membrane cytochromes required for the reduction of extracellular electron acceptors, reduced U(VI) at a slower rate than the wild type (Fig. 1). This ΔgspD mutant reduced U(VI) at a slightly faster rate with 10 μM riboflavin added, but the rate of uranium reduction did not reach the reduction rate noted in the cultures of wild type cells.The influence of riboflavin on Tc(VII) reduction
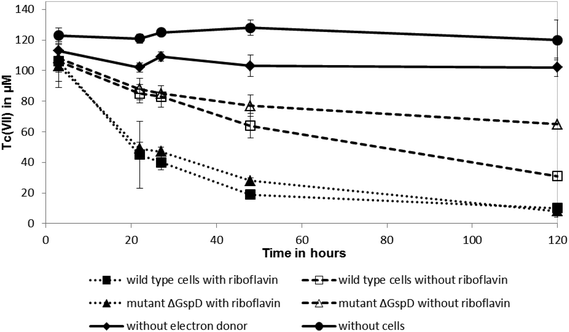
Shewanella oneidensis MR-1 couples the rapid reduction of Tc(VII) to the oxidation of hydrogen, in common with other well studied Tc(VII)-reducing cultures, but can also use lactate as an electron donor for this reaction, albeit at a slower rate.18,25,26 To test if riboflavin accelerateds the reduction rate of Tc(VII) by S. oneidensis MR-1 with lactate as an electron donor (presumably coupling to electron transfer from c-type cytochromes that are abundant in the cell), 10 μM of riboflavin was added to resting cell suspensions challenged with Tc(VII). The addition of 10 μM of riboflavin enhanced significantly the rate of Tc(VII) reduction catalysed by the wild type S. oneidensis MR-1 cells and also the ΔgspD mutant (Fig. 2). In the presence of riboflavin, the MR-1 strain and the ΔgspD mutant were both able to reduce ∼55% of the Tc(VII) within 22 h. In contrast, without riboflavin both cultures reduced only 20% of the Tc(VII) within this time period. Within 48 h ∼80% of the Tc(VII) was reduced by both the MR-1 strain and the ΔgspD mutant, and all the detectable Tc was reduced after 120 hours in the presence of the added flavin. Without added riboflavin, there was a clear difference in the amount of Tc(VII) reduced after 120 hours, with the wild type strain reducing 71% ± 6% compared to only 40% ± 7% of the initial Tc(VII) in cultures of the ΔgspD mutant (Fig. 2). A black cell-associated precipitate was noted in all cultures where Tc(VII) reduction occurred consistent with the formation of insoluble Tc(IV)O2.27The influence of riboflavin on Np(V) reduction
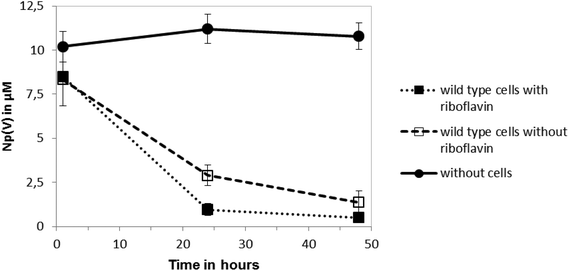
The reduction of Np(V) by Shewanella species has been noted previously,19,20 although interestingly other Gram-negative Fe(III)-reducing bacteria such as Geobacter sulfurreducens cannot reduce this radionuclide.28 As the mechanism of Np(V) reduction in Shewanella species remains poorly characterised, in this study we investigated the impact of the addition of 10 μM riboflavin on the reduction of Np(V) by Shewanella oneidensis MR-1. As shown in Fig. 3, the addition of 10 μM of riboflavin enhanced slightly the rate of reduction of Np(V) by S. oneidensis MR-1, which was mainly noticeable at the 24 hour time-point.The wild type cells of S. oneidensis removed >90% of the available Np from solution after incubation for 48 h. As shown in Fig. 4, comparison of XANES spectra collected from experimental cultures, with those from Np(V) and (IV) standards29,30 showed spectra from the 50 μM Np(V) experiment end point were consistent with the presence of Np(IV). Specifically, the XANES lacked the multiple scattering feature on the high energy side of the absorption edge which is attributed to scattering along the neptunyl axial oxygens. Indeed, a simple two end-member linear combination model of the sample between the oxic Np(V) and reduced Np(IV) standards, suggested 80% of the Np was speciated as Np(IV). This highlighted the dominance of Np(IV) in these systems with only a modest, if any, contribution from Np(V) sorption, and confirmed the reductive precipitation of Np(V) to Np(IV).
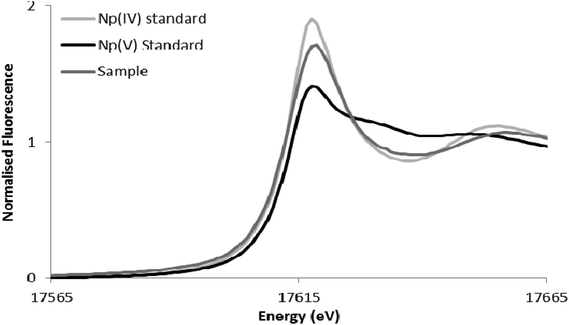 | ||
| Fig. 4 Neptunium LIII-edge XANES spectra collected from the 50 μM Np experiment alongside pure oxidation state Np(V) and Np(IV) standards from the AcReDaS database.29,30 | ||
The influence of riboflavin on Pu(IV) reduction
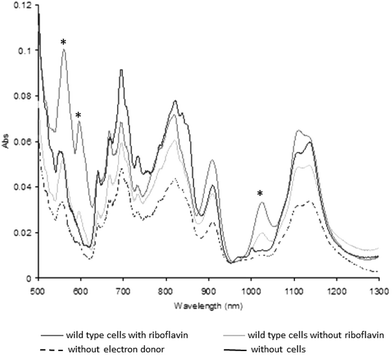
Previous studies have shown that washed cell suspensions of Shewanella oneidensis MR-1 produce very little Pu(III) enzymatically from Pu(IV)(OH)4,21,22 whereas the presence of the complexant EDTA enhances the reduction of the Pu(IV)(OH)4(am) to Pu(III),21 presumably by increasing the solubility and bioavailability of Pu(IV). To determine if the addition of riboflavin enhances the reduction of Pu(IV), 10 μM of riboflavin was added to washed cell suspensions with 1 mM Pu(IV), and the ability of the cells to reduce the radionuclide investigated.Plutonium was removed rapidly from solution (within 10 min).22 After 24 h incubation, UV-visible-near infrared spectra of the dissolved cell pellets (Fig. 5) were altered, with increases in the amplitude of diagnostic peaks for Pu(III) at 561, 601 and 1025 nm associated with the cell suspensions supplemented with lactate or lactate and riboflavin.22 The diagnostic peaks for Pu(III) at 601 nm and 1025 were missing in the control samples without electron donor and without cells, while the third peak at 561 nm showed a higher intensity in the samples with and without riboflavin compared to the control samples. Additionally, the intensity of the diagnostic peaks of Pu(III) in the sample containing riboflavin were much higher than without riboflavin, confirming that riboflavin accelerates Pu(IV) reduction by S. oneidensis MR-1.
Discussion
The secretion of endogenous flavin electron shuttles by Shewanella species has been shown to promote the dissimilatory reduction of extracellular electron acceptors including poorly soluble Fe(III) oxides2,9 and anodes in microbial fuel cells.10,31 However, the impact of secreted flavin molecules on the redox state of radionuclides has not been studied intensively to date, even though in many cases they may be present at the surface of microbial cells; microbial uptake of actinides into the microbial cell remains a controversial subject, and bioavailable U(VI) can be sorbed to minerals in contaminated land.32 The information provided here is important to help understand the mechanisms of electron transfer to contaminant metals and radionuclides, and to aid in the design and optimisation of bioremediation approaches. Shewanella species have the widest radionuclide substrate range of metal-reducing prokaryotes studied to date, are able to mediate Tc(VII) reduction via novel non hydrogenase-mediated mechanisms and also have the capacity to enzymatically reduce Np(V), unlike the few Geobacter species that have been studied.19,25,28 The aim of our study was to identify novel mechanisms of radionuclide reduction in Shewanella oneidensis MR-1, and in particular to investigate the influence of riboflavin on the reduction of the actinides U(VI), Np(V) and Pu(IV) as well as the fission product Tc(VII).The addition of 10 μM riboflavin increased significantly the reduction rate of Tc(VII) by wild type cultures of S. oneidensis MR-1, while having a more modest, but significant, impact on the reduction rate of Np(V). In addition, more Pu(III) was also detected in the presence of riboflavin than without (17% to 3%). Interestingly, there was no significant change in the reduction rate of U(VI) by S. oneidensis MR-1 when riboflavin was added, suggesting that under the conditions of study, secreted flavins do not play a significant role in mediating U(VI) reduction by this organism. This is in contrast to a study on a potential flavin mononucleotide (FMN)-mediated electron pathway for microbial U(VI) reduction by Shewanella putrefaciens (ATCC 8072), which suggested that the addition of FMN promoted U(VI) reduction.15 It is noteworthy that there were several key differences between this study and our experiments: another Shewanella strain was used, with FMN (instead of riboflavin), which was added at higher concentrations (50 μM and 500 μM), and in combination with U(VI) citrate (instead of uranyl acetate in our experiments). Thus direct comparisons are difficult, although these data do suggest that very high concentrations of flavin molecules could enhance U(VI) reduction in some experimental systems.15
Our results also demonstrated that a Shewanella oneidensis ΔgspD mutant reduces U(VI) and Tc(VII) at slower rates than the wild-type. The S. oneidensis ΔgspD mutant, that lacks a functioning type II protein secretion systems, is also incapable of reducing iron(oxy)(hydr)oxides and Mn(IV) oxides at wild-type rates.6,33 These data are to be expected as the outer membrane c-type cytochromes of S. oneidensis MR-1 (e.g. MtrC, also known as OmcB and OmcA) are thought to be essential for lactate-dependent reduction of Fe(III), and also U(VI) and Tc(VII), presumably mediated at the cell surface.12,18 Indeed, these electron transfer proteins are translocated across the outer membrane to this site by the type II protein-secretion pathway.7 The disruption of the type II secretion system in the S. oneidensis ΔgspD mutant seemingly prevents the delivery of the c-type cytochromes to this key exposed and reactive compartment of the Gram-negative bacterial cell, thus lowering the rate of reduction of U(VI) and Tc(VII).
It was demonstrated previously that the addition of the humic analogue and extracellular electron shuttle anthraquinone-2,6-disulfonate (AQDS) reinstates the ability of the ΔgspD mutant to reduce extracellular insoluble Fe(III) oxides.6 Our results showed that the addition of riboflavin to cultures of the ΔgspD mutant also results in an increase in the rate of Tc(VII) reduction, restoring it to the levels noted for the wild-type cells. Thus, the reduction of Tc(VII) may be mediated directly by (reduced) riboflavin, which would have access to c-type cytochromes that were not secreted, for example CymA which is localised to the periplasm of the Gram-negative cell.34 Similar predictions can be made for the actinides Np(V) and Pu(III), on the basis of enhanced reduction in the wild type cultures, when riboflavin was added. Clearly further work is required on these more radiologically challenging elements, to help define the precise mechanism of bioreduction in Shewanella cultures, and other environmental systems. However, the situation with uranium is distinct, and here the rate of reduction of U(VI) by the ΔgspD mutant did not reach the same rate noted in the wild-type cultures even in the presence of riboflavin. This suggests that the outer membrane c-type cytochromes of S. oneidensis MR-1 most likely play a defining role in the direct reduction of U(VI) at the cell surface which cannot be compensated for by the addition of the electron shuttle. This is consistent with other studies, which have shown that the correct processing of surface exposed outer membrane c-type cytochromes is a key determining factor in extracellular electron transfer in S. oneidensis MR-1.35 This would not seem to be true of Tc(VII) and potentially Np(V) and Pu(IV), and it seems for these radionuclides riboflavin can likely mediate directly between the enzymatic electron transfer machinery of the cell and the radionuclide. This is in contrast to U(VI), which seems unlikely to couple directly to the reduced flavin molecule under the conditions used in our experiments. It is conceivable that the reduced riboflavin-Tc(VII) interactions could be in free solution, or mediated by flavin groups bound to the outer membrane cytochromes as noted recently.36
Shewanella species and other microorganisms37 that actively secrete flavins and use them as electron shuttles could have an adaptive advantage in environments that contain significant concentrations of metals and radionuclides that are not easily to access (e.g. intergrain areas38 or low porosity rocks and mineral assemblages). The concentration of flavins secreted by anaerobically grown cultures of S. oneidensis was measured at the relatively low levels of 0.1 to 0.6 μM, but it is expected that the local concentrations of secreted flavins in microenvironments such as biofilms or micropores in minerals are potentially much higher, approaching the levels used in our experiments.2 However, the addition of riboflavin (available in large quantities as a relatively inexpensive food additive) could also be used to accelerate the bioremediation of radionuclide contaminated environments, for example in sediments contaminated with Tc(VII) and Np(V). Pure culture experiments such as those described here can help identify the fate of key radionuclides in environmental systems, and the underpinning mechanisms catalysing biocycling reactions. Although it is clear that there is a need for follow up experiments on more complex environmental systems, including those using mixed microbial consortia, realistic groundwater chemistries and appropriate sediment/mineral phases, it should be noted the reductive endpoints for U, Np, and Tc,39–41 and potentially Pu22,42 noted in these experiments are broadly in line with those recorded in sediment microcosms that have been driven anaerobic by complex, extant microbial communities. With advances in analytical tools for flavin analyses in complex environmental systems, and the use of sensitive meta-omic tools targeting flavin secretion pathways, and other electron transfer systems, it should be possible to assess the precise role of these pathways in controlling radionuclide species in anoxic systems.
Acknowledgements
This work was supported by the BIGRAD consortium under the UK Natural Environmental Research Council, (NE/H007768/1). JRL acknowledges support from the Royal Society. The authors acknowledge the use of the AcReDaS an Actinide reference database for XAS, EELS, IR, Raman and NMR Spectroscopy (https://www.hzdr.de/db/acxas.www.welcome) and INE Beamline for actinide research at the ANKA synchrotron light source, Karlsruhe, Germany.References
- T. J. DiChristina, D. J. Bates, J. L. Burns, J. R. Dale, Jr. and A. N. Payne, in Past and Present Water Column Anoxia, ed. L. N. Neretin, 2006, vol. 64, pp. 443–469 Search PubMed.
- H. von Canstein, J. Ogawa, S. Shimizu and J. R. Lloyd, Appl. Environ. Microbiol., 2008, 74, 615–623 CrossRef CAS PubMed.
- C. R. Myers and K. H. Nealson, Science, 1988, 240, 1319–1321 CAS.
- C. R. Myers and J. M. Myers, Appl. Environ. Microbiol., 2004, 70, 5415–5425 CrossRef CAS PubMed.
- D. R. Lovley, D. E. Holmes and K. P. Nevin, in Advances in Microbial Physiology, ed. R. K. Poole, 2004, vol. 49, pp. 219–286 Search PubMed.
- M. Zhang, J. R. Dale, T. J. DiChristina and A. G. Stack, Geomicrobiol. J., 2009, 26, 83–92 CrossRef CAS.
- L. Shi, S. Deng, M. J. Marshall, Z. Wang, D. W. Kennedy, A. C. Dohnalkova, H. M. Mottaz, E. A. Hill, Y. A. Gorby, A. S. Beliaev, D. J. Richardson, J. M. Zachara and J. K. Fredrickson, J. Bacteriol., 2008, 190, 5512–5516 CrossRef CAS PubMed.
- D. K. Newman and R. Kolter, Nature, 2000, 405, 94–97 CrossRef CAS PubMed.
- D. E. Ross, S. L. Brantley and M. Tien, Appl. Environ. Microbiol., 2009, 75, 5218–5226 CrossRef CAS PubMed.
- E. Marsili, D. B. Baron, I. D. Shikhare, D. Coursolle, J. A. Gralnick and D. R. Bond, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3968–3973 CrossRef CAS PubMed.
- D. R. Lovley, E. J. P. Phillips, Y. A. Gorby and E. R. Landa, Nature, 1991, 350, 413–416 CrossRef CAS.
- M. J. Marshall, A. S. Beliaev, A. C. Dohnalkova, D. W. Kennedy, L. Shi, Z. M. Wang, M. I. Boyanov, B. Lai, K. M. Kemner, J. S. McLean, S. B. Reed, D. E. Culley, V. L. Bailey, C. J. Simonson, D. A. Saffarini, M. F. Romine, J. M. Zachara and J. K. Fredrickson, PLoS Biol., 2006, 4 CAS , e268.
- J. D. Wall and L. R. Krumholz, Annu. Rev. Microbiol., 2006, 60, 149–166 Search PubMed.
- R. Bencheikh-Latmani, S. Middleton Williams, L. Haucke, C. S. Criddle, L. Wu, J. Zhou and B. M. Tebo, Appl. Environ. Microbiol., 2005, 71, 7453–7460 CrossRef CAS PubMed.
- Y. Suzuki, Y. Kitatsuji, T. Ohnuki and S. Tsujimura, Phys. Chem. Chem. Phys., 2010, 12, 10081–10087 RSC.
- J. R. Lloyd, J. A. Cole and L. E. Macaskie, J. Bacteriol., 1997, 179, 2014–2021 CAS.
- G. De Luca, P. De Philip, Z. Dermoun, M. Rousset and A. Vermeglio, Appl. Environ. Microbiol., 2001, 67, 4583–4587 CrossRef CAS PubMed.
- M. J. Marshall, A. E. Plymale, D. W. Kennedy, L. Shi, Z. M. Wang, S. B. Reed, A. C. Dohnalkova, C. J. Simonson, C. X. Liu, D. A. Saffarini, M. F. Romine, J. M. Zachara, A. S. Beliaev and J. K. Fredrickson, Environ. Microbiol., 2008, 10, 125–136 CAS.
- J. R. Lloyd, P. Yong and L. E. Macaskie, Environ. Sci. Technol., 2000, 34, 1297–1301 CrossRef CAS.
- G. A. Icopini, H. Boukhalfa and M. P. Neu, Environ. Sci. Technol., 2007, 41, 2764–2769 CrossRef CAS PubMed.
- H. Boukhalfa, G. A. Icopini, S. D. Reilly and M. P. Neu, Appl. Environ. Microbiol., 2007, 73, 5897–5903 CrossRef CAS PubMed.
- J. C. Renshaw, N. Law, A. Geissler, F. R. Livens and J. R. Lloyd, Biogeochemistry, 2009, 94, 191–196 CrossRef CAS.
- D. A. Johnson and T. M. Florence, Anal. Chim. Acta, 1971, 53, 73–79 CrossRef CAS.
- O. J. Marsden, L. Abrahamsen, N. D. Bryan, J. P. Day, L. K. Fifield, C. Gent, P. S. Goodall, K. Morris and F. R. Livens, Sedimentology, 2006, 53, 237–248 CrossRef CAS.
- J. R. Lloyd and L. E. Macaskie, Appl. Environ. Microbiol., 1996, 62, 578–582 CAS.
- R. E. Wildung, Y. A. Gorby, K. M. Krupka, N. J. Hess, S. W. Li, A. E. Plymale, J. P. McKinley and J. K. Fredrickson, Appl. Environ. Microbiol., 2000, 66, 2451–2460 CrossRef CAS PubMed.
- K. Morris, F. R. Livens, J. M. Charnock, I. T. Burke, J. M. McBeth, J. D. C. Begg, C. Boothman and J. R. Lloyd, Appl. Geochem., 2008, 23, 603–617 CrossRef CAS.
- J. C. Renshaw, L. J. C. Butchins, F. R. Livens, I. May, J. M. Charnock and J. R. Lloyd, Environ. Sci. Technol., 2005, 39, 5657–5660 CrossRef CAS PubMed.
- A. Rossberg, A. C. Scheinost, N. Schmeisser, J. Rothe, P. Kaden, D. Schild, T. Wiss and R. Daehn, 2014, https://www.hzdr.de/acredas.
- C. Hennig, Phys. Rev. B: Condens. Matter, 2007, 035120 CrossRef.
- S. B. Velasquez-Orta, I. M. Head, T. P. Curtis, K. Scott, J. R. Lloyd and H. von Canstein, Appl. Microbiol. Biotechnol., 2010, 85, 1373–1381 CrossRef CAS PubMed.
- G. T. W. Law, A. Geissler, I. T. Burke, F. R. Livens, J. R. Lloyd, J. M. McBeth and K. Morris, Geomicrobiol. J., 2011, 28, 497–506 CrossRef CAS.
- O. Bretschger, A. Obraztsova, C. A. Sturm, I. S. Chang, Y. A. Gorby, S. B. Reed, D. E. Culley, C. L. Reardon, S. Barua, M. F. Romine, J. Zhou, A. S. Beliaev, R. Bouhenni, D. Saffarini, F. Mansfeld, B. H. Kim, J. K. Fredrickson and K. H. Nealson, Appl. Environ. Microbiol., 2007, 73, 7003–7012 CrossRef CAS PubMed.
- C. Schwalb, S. K. Chapman and G. A. Reid, Biochemistry, 2003, 42, 9491–9497 CrossRef CAS PubMed.
- R. A. Bouhenni, G. J. Vora, J. C. Biffinger, S. Shirodkar, K. Brockman, R. Ray, P. Wu, B. J. Johnson, E. M. Biddle, M. J. Marshall, L. A. Fitzgerald, B. J. Little, J. K. Fredrickson, A. S. Beliaev, B. R. Ringeisen and D. A. Saffarini, Electroanalysis, 2010, 22, 856–864 CrossRef CAS.
- A. Okamoto, K. Hashimoto, K. H. Nealson and R. Nakamura, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 7856–7861 CrossRef CAS PubMed.
- R. Balasubramanian, B. T. Levinson and A. C. Rosenzweig, Appl. Environ. Microbiol., 2010, 76, 7356–7358 CrossRef CAS PubMed.
- C. Liu, J. M. Zachara, L. Zhong, S. M. Heald, Z. Wang, B. H. Jeon and J. K. Fredrickson, Environ. Sci. Technol., 2009, 43, 4928–4933 CrossRef CAS PubMed.
- L. Newsome, K. Morris and J. R. Lloyd, Chem. Geol., 2014, 363, 164–184 CrossRef CAS.
- G. T. W. Law, A. Geissler, J. R. Lloyd, F. R. Livens, C. Boothman, J. D. C. Begg, M. A. Denecke, J. Rothe, K. Dardenne, I. T. Burke, J. M. Charnock and K. Morris, Environ. Sci. Technol., 2010, 44, 8924–8929 CrossRef CAS PubMed.
- J. M. McBeth, G. Lear, J. R. Lloyd, F. R. Livens, K. Morris and I. T. Burke, Geomicrobiol. J., 2007, 24, 189–197 CrossRef CAS.
- R. L. Kimber, C. Boothman, P. Purdie, F. R. Livens and J. R. Lloyd, Mineral. Mag., 2012, 76, 567–578 CrossRef CAS.
Footnotes |
| † Present address: Formerly Geissler Helmholtz-Zentrum Dresden-Rossendorf, Institute of Resource Ecology, Bautzner Landstrasse 400, 01328 Dresden, Germany. |
| ‡ Present address: Department of Civil and Environmental Engineering, University of Strathclyde, Glasgow G1 1XQ. Scotland, UK. |
| This journal is © The Royal Society of Chemistry 2016 |