Open Access Article
Open Access ArticleSynthesis in mesoreactors: Ru(porphyrin)CO-catalyzed aziridination of olefins under continuous flow conditions†
S.
Rossi
,
A.
Puglisi
*,
M.
Benaglia
*,
D. M.
Carminati
,
D.
Intrieri
and
E.
Gallo
Dipartimento di Chimica, Università degli Studi di Milano, Via Golgi 19, 20133, Milano, Italy. E-mail: alessandra.puglisi@unimi.it; maurizio.benaglia@unimi.it
First published on 20th April 2016
Abstract
The Ru(porphyrin)CO-catalyzed addition of aryl azides to styrenes to afford N-aryl aziridines was successfully performed for the first time in mesoreactors under continuous flow conditions. Mesofluidic technology allowed for a rapid screening of different parameters and a quick identification of the optimized reaction conditions.
Aziridines are valuable compounds largely employed as key starting materials for the synthesis of a plethora of nitrogen-containing molecules.1 Among the different available synthetic strategies,2 the metal-catalyzed addition of nitrenes to olefins represents one of the most popular and established methodologies for the preparation of aziridines.3 In order to optimize the process sustainability, the selection of a convenient nitrene source is crucial. Thus, organic azides (RN3)4 are largely used thanks to their high atom efficiency resulting in the formation of eco-friendly N2 as the sole by-product of nitrene transfer reactions. Organic azide-based aziridinations are well catalyzed by several classes of metal transition catalysts and efficient stereoselective synthetic protocols have also been developed.5
However, the high reactivity that turns aziridines into excellent building blocks for further synthetic elaboration, on the other hand, makes these valuable compounds rather hazardous reagents to handle; a limited exposure to this class of compounds is therefore highly desirable. Furthermore, the synthesis and manipulation of azides are delicate processes, due to their inherent propensity for vigorous decomposition.
We felt that continuous flow methodologies6 could provide a positive solution to some of the above mentioned issues to pave the way for a more widespread application of the metal-catalyzed synthesis of aziridines. In the last few years, flow chemistry technologies have become very popular in the synthesis of organic molecules,7 including complex natural products8 or Active Pharmaceutical Ingredients (APIs).9 The attractive features of continuous processes are short reaction times, improved reagent mixing as well as mass and heat transfer. Safer handling of hazardous reagents or very reactive intermediates may also be positively addressed.
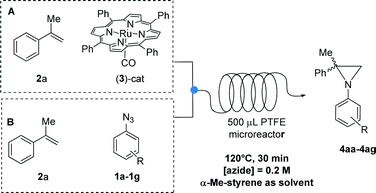
The synthesis of aziridines by flow chemistry techniques is largely underdeveloped, as evidenced by the scarce number of publications;10 notably, as far as we know, examples of catalytic aziridinations in micro- or mesoreactors have never been reported. Considering that some of us have extensively studied nitrene transfer reactions promoted by ruthenium porphyrin complexes from both the experimental11 and theoretical point of view,12 we decided to investigate the Ru(porphyrin)CO-catalyzed aziridination of styrenes by aryl azides in mesoreactors under continuous flow conditions in an attempt to develop a simple, efficient and safe synthesis of aziridines.13
In a preliminary experiment, the reaction between 3,5-bis(trifluoromethyl)phenyl azide 1a and α-methylstyrene 2a promoted by [Ru(TPP)(CO)] (TPP = dianion of tetraphenyl porphyrin) 3 was studied (Scheme 1).
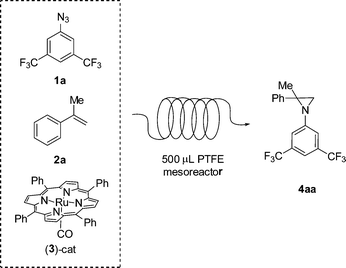 | ||
| Scheme 1 Reaction between azide 1a and α-methylstyrene 2a promoted by [Ru(TPP)(CO)] catalyst 3 in a continuous-flow system, using a 500 μL PTFE mesoreactor. | ||
The reaction was conducted in a mesoreactor made of commercially available PTFE tubing (inner diameter 0.58 mm, length 189 cm, total volume 500 μL) that was coiled in a bundle and immersed in a preheated oil bath. A New Era NE 300 syringe pump equipped with one Hamilton gastight 10 mL syringe was used in order to feed the reagents into the mesoreactor. Conversions and yields were determined by GC using biphenyl as the internal standard (see the ESI† for further details). A few selected results are reported in Table 1.
| Entry | [Azide]a (M) | Solvent | Residence time (min) | Temp (°C) | Conversion of 1ab,c (%) | Yield of 4aab,d (%) |
|---|---|---|---|---|---|---|
| a Molarity values correspond to the effective concentration inside the microreactor. b Monitored by GC using biphenyl as the internal standard. c Calculated as mmol of consumed 1a per mmol of starting 1a. d Calculated as mmol of 4aa per mmol of 1a. | ||||||
| 1 | 0.02 | Benzene | 10 | 90 | 49.4 | 39.7 |
| 2 | 0.02 | Benzene | 30 | 90 | 62.8 | 46.6 |
| 3 | 0.02 | Benzene | 60 | 90 | 89.3 | 84.7 |
| 4 | 0.2 | Benzene | 30 | 90 | 59.9 | 51.7 |
| 5 | 0.02 | α-Me-styrene | 5 | 90 | 44.1 | 41.9 |
| 6 | 0.02 | α-Me-styrene | 30 | 90 | 55.3 | 40.9 |
| 7 | 0.02 | α-Me-styrene | 5 | 120 | 54.8 | 41.6 |
| 8 | 0.2 | α-Me-styrene | 5 | 120 | 39.9 | 32.1 |
As initial conditions, a catalyst/azide/olefin ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 and a 0.2 M solution of azide 1a in dry benzene were chosen in order to reproduce the batch conditions.11 From previous work, it is known that refluxing benzene (typically 30 mL for a standard reaction procedure) was necessary in order to achieve high azide conversions, so the reactions were run at 90 °C. A small amount of 3,5-bis(trifluoromethyl)aniline (usually less than 3%) was detected as a by-product, in agreement with literature reports.11 No product 4aa was formed when working at room temperature for short reaction times.
250 and a 0.2 M solution of azide 1a in dry benzene were chosen in order to reproduce the batch conditions.11 From previous work, it is known that refluxing benzene (typically 30 mL for a standard reaction procedure) was necessary in order to achieve high azide conversions, so the reactions were run at 90 °C. A small amount of 3,5-bis(trifluoromethyl)aniline (usually less than 3%) was detected as a by-product, in agreement with literature reports.11 No product 4aa was formed when working at room temperature for short reaction times.
Results of this initial screening are reported in Table 1. For short residence times, a low conversion was recorded for the catalytic reaction in benzene (entries 1–2, Table 1); a good yield of product 4aa (85%) was obtained only by running the reaction for 60 min. It is interesting to note that changing the concentration of azide 1a and olefin 2a without modifying their stoichiometric ratios (0.2 M azide, olefin/azide/catalyst = 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) does not affect the yield of aziridine 4aa appreciably (entry 2 vs. 4, Table 1). During all experiments, no clogging problems were encountered, but after 24 h, 11.4% of aziridine 4aa was detected in the feeding syringe.
1) does not affect the yield of aziridine 4aa appreciably (entry 2 vs. 4, Table 1). During all experiments, no clogging problems were encountered, but after 24 h, 11.4% of aziridine 4aa was detected in the feeding syringe.
α-Methylstyrene 2a was then used as the reaction solvent, but no improvements were obtained (entries 5 and 6, Table 1) at 90 °C, while a higher conversion was detected at 120 °C (entry 7, Table 1). Using a 0.2 M solution of azide 1a yielded product 4aa with no substantial improvement; however, we noted a high tendency of catalyst 3 to crystallize inside the feeding syringe.
On the basis of these preliminary results, we decided to change the feeding system by splitting the addition of the reactants into two different syringes (Scheme 2). A New Era NE 300 syringe pump equipped with two Hamilton gastight 2.5 mL syringes was used to feed the reagents into the microreactor through a T-junction (syringe A: [Ru(TPP)(CO)] 3 dissolved in α-methylstyrene 2a;‡ syringe B: azide 1a and biphenyl as the internal NMR standard dissolved in α-methylstyrene 2a). For convenience, 0.2 M solution of azide 1a was used; selected results are reported in Table 2.
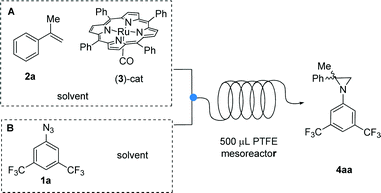 | ||
| Scheme 2 Reaction between azide 1a and α-methylstyrene 2a promoted by [Ru(TPP)(CO)] catalyst 3 in a two-syringe continuous-flow system, using a 500 μL PTFE mesoreactor. | ||
| Entry | Solvent | Residence time (min) | Temp (°C) | Conversion of 1aa (%) | Selectivity of 4aab (%) | Yield of 4aa (%) |
|---|---|---|---|---|---|---|
| a Monitored by GC using biphenyl as the internal standard. b Calculated as (mmol of aziridine)/(mmol of azide − mmol of azide residue) × 100. | ||||||
| 1 | Benzene | 5 | 90 | 12.4 | 77.4 | 9.6 |
| 2 | Benzene | 10 | 90 | 19.1 | 91.1 | 17.4 |
| 3 | Benzene | 30 | 90 | 31.7 | 85.5 | 27.1 |
| 4 | Benzene | 60 | 90 | 57.8 | 51.5 | 29.8 |
| 5 | α-Me-styrene | 5 | 90 | 68.8 | 86.8 | 59.7 |
| 6 | α-Me-styrene | 10 | 90 | 66.8 | 85.5 | 57.1 |
| 7 | α-Me-styrene | 30 | 90 | 89.0 | 85.3 | 75.9 |
| 8 | α-Me-styrene | 5 | 120 | 90.6 | 96.7 | 87.6 |
| 9 | α-Me-styrene | 10 | 120 | 97.7 | 97.4 | 95.1 |
| 10 | α-Me-styrene | 30 | 120 | 98.9 | 99.1 | 98.0 |
Running the reaction in α-methylstyrene 2a as the solvent had a beneficial effect on the reaction course in flow, resulting in higher azide conversions and in turn higher aziridine yields (entries 1–4 vs. entries 5–7, Table 2). In benzene, aziridine 4aa was obtained in 27% yield after 30 min residence time, while the reaction run for the same time in α-methylstyrene 2a achieved 75.9% yield of the desired product 4aa; the reaction selectivity was comparable in both cases (compare entries 3 and 7 of Table 2). Note that the replacement of benzene with α-methylstyrene 2a has a beneficial effect in terms of reaction eco-compatibility. In fact, solvent-free reactions are cleaner especially when the substrate, used as the reaction solvent, can be recycled in a continuous flow process.
Raising the temperature from 90 to 120 °C earned higher aziridine yields in short reaction times (entries 8–10, Table 2); 4aa was produced in 95% yield after 10 min only and in almost a quantitative yield after 30 min residence time. Again, comparable selectivities were obtained in the examined cases.
Having identified the best reaction parameters and feeding mode, the scope of the ruthenium-catalyzed aziridination was studied; the reactivity of different aryl azides 1a–1g was investigated in the reaction with α-methylstyrene 2a (Table 3).
| Entry | R | Azide residuea (%) | Product yielda,b (%) | Selectivityc | |
|---|---|---|---|---|---|
| a Determined by 1H NMR. b Calculated as (mmol of aziridine)/(mmol of azide) × 100. c Calculated as (mmol of aziridine)/(mmol of azide − mmol of azide residue) × 100. | |||||
| 1 | 3,5-(CF3)2 | 1.1 | 4aa | 98 | 99 |
| 2 | 4-Cl | 34 | 4ab | 53 | 80 |
| 3 | 4-NO2 | 22 | 4ac | 64 | 82 |
| 4 | 4-CF3 | 16 | 4ad | 73 | 87 |
| 5 | 4-Br | 20 | 4ae | 63 | 80 |
| 6 | 3,5-Cl2 | 5 | 4af | 93 | 98 |
| 7 | 4-CN | 31 | 4ag | 55 | 80 |
Aziridines 4aa–4ag were all obtained in a selectivity higher than 80% in 30 min residence time. For very reactive azides 1a and 1f, bearing strongly electron-withdrawing groups, the selectivity was almost quantitative (entries 1 and 6, Table 3). The results were comparable to those reported in the literature.11
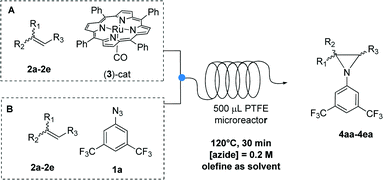
We then focused our attention on the study of the aziridination reaction of different olefins 2a–2e by 3,5-bis(trifluoromethyl)phenyl azide 1a (Table 4). As expected, styrene gave the desired product 4ba in quantitative yield (entry 2, Table 4). 4-Br-styrene proved to be less reactive, affording aziridine 4ca in 64% yield (entry 3, Table 4). β-Methylstyrene gave the corresponding product 4da as a mixture of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cis
1 cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : trans isomers in a quantitative conversion but only 55% yield (entry 4, Table 4). 1,1-Diphenylethylene afforded aziridine 4ea in 98% yield (entry 5, Table 4). All the results were in agreement with literature data and with the reaction mechanism that favors terminal olefins with respect to internal olefins.11
: trans isomers in a quantitative conversion but only 55% yield (entry 4, Table 4). 1,1-Diphenylethylene afforded aziridine 4ea in 98% yield (entry 5, Table 4). All the results were in agreement with literature data and with the reaction mechanism that favors terminal olefins with respect to internal olefins.11
| Entry | R1 | R2 | R3 | Azide residue (%) | Product yield (%) | Selectivityc | |
|---|---|---|---|---|---|---|---|
a Monitored by GC using biphenyl as the internal standard.
b Calculated by NMR using 3,5-dinitrotoluene as the internal standard.
c Calculated as (mmol of aziridine)/(mmol of azide − mmol of azide residue) × 100.
d Product was obtained as a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of cis 1 mixture of cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : trans isomers. : trans isomers.
|
|||||||
| 1 | CH3 | Ph | H | 1.1a | 4aa | 98a | 99 |
| 2 | H | Ph | H | 3.4a | 4ba | 90.1a | 93 |
| 3 | H | 4-Br-C6H5 | H | 2.9a | 4ca | 62a | 64 |
| 4 | H | Ph | CH3 | 1.5b | 4da | 55b,d | 44 |
| 5 | Ph | Ph | H | 2.0b | 4ea | 89b | 91 |
It is noteworthy that the productivity§ of the flask process for the synthesis of aziridine 4aa calculated according to literature conditions (90 °C, 30 min, 97% yield)11 was 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 617; a comparable productivity, 87
617; a comparable productivity, 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 h−1, was obtained for the flow process, working at 120 °C for 5 min residence time only (88% yield). We also report in Table 5 the space-time yields for the two processes, measured as [mass (product)/(vol(reactor) × reaction time) expressed as kg m−3 s−1; this value takes into account the volume of the reactor (or the flask) used to perform the reaction.
600 h−1, was obtained for the flow process, working at 120 °C for 5 min residence time only (88% yield). We also report in Table 5 the space-time yields for the two processes, measured as [mass (product)/(vol(reactor) × reaction time) expressed as kg m−3 s−1; this value takes into account the volume of the reactor (or the flask) used to perform the reaction.
| Entry | Reactor | Time (min) | Temp (°C) | Yield (%) | Reactor volume (mL) | Space-time yielda |
|---|---|---|---|---|---|---|
| a Measured as [mass (product)/(vol(reactor) × reaction time) expressed as kg m−3 s−1. b See ref. 11. c Run in α-methylstyrene as the reaction solvent. d This work. | ||||||
| 1b | Flask | 30 | 90 | 97 | 30 | 3.78 × 10−3 |
| 2c | Flask | 5 | 120 | 99 | 30 | 23.17 × 10−3 |
| 3d | PTFE | 5 | 120 | 87.6 | 0.5 | 20.16 × 10−3 |
| 4d | PTFE | 10 | 120 | 95.1 | 0.5 | 10.95 × 10−3 |
| 5d | PTFE | 30 | 120 | 98.0 | 0.5 | 3.75 × 10−3 |
Data show that the two systems give comparable space-time yields (entries 2 and 3, Table 5). However, the flow process has the advantage of performing reactions in compact devices and small reaction volumes rather than in batch systems with larger reaction volumes.
Conclusions
In conclusion, the addition of aryl azides to styrenes for the synthesis of N-aryl aziridines was successfully accomplished in a PTFE mesoreactor under continuous-flow conditions, in the presence of a ruthenium porphyrin-based catalyst. Yields and selectivities of the flow process favorably compare with those of the batch reaction, with the undeniable advantage of operating with smaller reaction volumes. The procedure without solvent becomes even more appealing when functionalized, more expensive styrene derivatives are used, with the possibility of recycling the substrate in a continuous flow process. The methodology opens new opportunities for a very convenient synthesis of functionalized aziridines with a safe, fast and experimentally simple procedure.Acknowledgements
S. R. thanks the University of Milan for a postdoctoral fellowship.Notes and references
- For reviews, see: (a) L. Degennaro, P. Trinchera and R. Luisi, Chem. Rev., 2014, 114, 7881–7929 CrossRef CAS PubMed; (b) G. Callebaut, T. Meiresonne, N. De Kimpe and S. Mangelinckx, Chem. Rev., 2014, 114, 7954–8015 CrossRef CAS PubMed; (c) A. L. Cardoso and T. M. V. D. Pinho e Melo, Eur. J. Org. Chem., 2012, 6479–6501 CAS; (d) S. Stanković, M. D'hooghe, S. Catak, H. Eum, M. I. Waroquier, V. Van Speybroeck and N. De Kimpe, Chem. Soc. Rev., 2012, 41, 643–665 RSC; (e) H. Ohno, Chem. Rev., 2014, 114, 7784–7814 CrossRef CAS PubMed.
- See ref. 1 and (a) H. Pellissier, Adv. Synth. Catal., 2014, 356, 1899–1935 CrossRef CAS; (b) R. Chawla, A. K. Singh and L. D. S. Yadav, RSC Adv., 2013, 3, 11385–11403 RSC; (c) J. B. Sweeney and A. Yudin, Aziridines and Epoxides in Organic Synthesis, Wiley-VCH Verlag GmbH & Co, KGaA, 2006 Search PubMed.
- (a) A. Fingerhut, O. V. Serdyuka and S. B. Tsogoeva, Green Chem., 2015, 17, 2042–2058 RSC; (b) S. Fantauzzi, A. Caselli and E. Gallo, Dalton Trans., 2009, 5434–5443 RSC.
- Recent reviews: (a) K. Shin, H. Kim and S. Chang, Acc. Chem. Res., 2015, 48, 1040–1052 CrossRef CAS PubMed; (b) D. Intrieri, P. Zardi, A. Caselli and E. Gallo, Chem. Commun., 2014, 50, 11440–11453 RSC.
- (a) L.-M. Jin, X. Xu, H. Lu, X. Cui, L. Wojtas and X. P. Zhang, Angew. Chem., Int. Ed., 2013, 52, 5309–5313 CrossRef CAS PubMed; (b) Y. Fukunaga, T. Uchida, Y. Ito, K. Matsumoto and T. Katsuki, Org. Lett., 2012, 14, 4658–4661 CrossRef CAS PubMed; (c) C. Kim, T. Uchida and T. Katsuki, Chem. Commun., 2012, 7188–7190 RSC; (d) T. Uchida and T. Katsuki, Chem. Rec., 2014, 14, 117–129 CrossRef CAS PubMed; (e) N. Jung and S. Bräse, Angew. Chem., Int. Ed., 2012, 51, 5538–5540 CrossRef CAS PubMed; (f) S. A. Cramer and D. M. Jenkins, J. Am. Chem. Soc., 2011, 133, 19342–19345 CrossRef CAS PubMed.
- Selected recent reviews: (a) F. Darvas, V. Hessel and G. Dorman, Flow Chemistry, De Gruyter, Berlin, 2014 Search PubMed; (b) K. F. Jensen, B. J. Reizmana and S. G. Newman, Lab Chip, 2014, 14, 3206 RSC; (c) T. Wirth, Microreactors in Organic Synthesis and Catalysis, 2nd edn, Wiley-VCH, Weinheim, 2013 Search PubMed; (d) C. Wiles and P. Watts, Green Chem., 2012, 14, 38–54 RSC; (e) J. Wegner, S. Ceylan and A. Kirschning, Chem. Commun., 2011, 47, 4583–4592 RSC.
- For recent reviews on stereoselective catalytic reactions in flow see: (a) I. Atodiresei, C. Vila and M. Rueping, ACS Catal., 2015, 5, 1972–1985 CrossRef CAS; (b) A. Puglisi, M. Benaglia, R. Porta and F. Coccia, Curr. Organocatal., 2015, 2, 79–101 CrossRef CAS; (c) R. Munirathinam, J. Huskens and W. Verboom, Adv. Synth. Catal., 2015, 357, 1093–1123 CrossRef CAS; (d) C. Rodríguez-Escrich and M. A. Pericàs, Eur. J. Org. Chem., 2015, 1173–1188 CrossRef; (e) A. Puglisi, M. Benaglia and V. Chiroli, Green Chem., 2013, 15, 1790–1813 RSC; (f) T. Tsubogo, T. Ishiwata and S. Kobayashi, Angew. Chem., Int. Ed., 2013, 52, 6590–6604 CrossRef CAS PubMed; (g) D. Zhao and K. Ding, ACS Catal., 2013, 3, 928–944 CrossRef CAS; For some very recent examples about continuous flow synthetic methodologies see: (h) J.-S. Poh, D. N. Tran, C. Battilocchio, J. M. Hawkins and S. V. Ley, Angew. Chem., Int. Ed., 2015, 54, 7920–7923 CrossRef CAS PubMed; (i) D. C. Fabry, M. A. Ronge and M. Rueping, Chem. – Eur. J., 2015, 21, 5350–5354 CrossRef CAS PubMed; (j) D. N. Tran, C. Battilocchio, S.-B. Lou, J. M. Hawkins and S. V. Ley, Chem. Sci., 2015, 6, 1120–1125 RSC.
- For a comprehensive review on the synthesis of natural products in flow see: J. C. Pastre, D. L. Browne and S. V. Ley, Chem. Soc. Rev., 2013, 42, 8849–8869 RSC For a recent example see: S. Newton, C. F. Carter, C. M. Pearson, L. de C. Alves, H. Lange, P. Thansandote and S. V. Ley, Angew. Chem., Int. Ed., 2014, 53, 4915–4920 CrossRef CAS PubMed.
- (a) B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed., 2015, 54, 6688–6728 CrossRef CAS PubMed; (b) R. Porta, M. Benaglia and A. Puglisi, Org. Process Res. Dev., 2016, 20, 2–25 CrossRef CAS.
- (a) K. G. Maskill, J. P. Knowles, L. D. Elliott, R. W. Alder and K. I. Booker-Milburn, Angew. Chem., Int. Ed., 2013, 52, 1499–1502 CrossRef CAS PubMed; (b) A. Giovine, B. Musio, L. Degennaro, A. Falcicchio, A. Nagaki, J.-I. Yoshida and R. Luisi, Chem. – Eur. J., 2013, 19, 1872–1876 CrossRef CAS PubMed; (c) P. Salice, E. Rossi, A. Pace, P. Maity, T. Carofiglio, T. E. Menna and M. Maggini, J. Flow Chem., 2014, 4, 79–85 CrossRef CAS; (d) M. Baumann and I. R. Baxendale, Synlett, 2016, 27, 159–163 CrossRef CAS.
- (a) G. Tseberlidis, P. Zardi, A. Caselli, D. Cancogni, M. Fusari, L. Lay and E. Gallo, Organometallics, 2015, 34, 3774–3781 CrossRef CAS; (b) P. Zardi, A. Savoldelli, D. M. Carminati, A. Caselli, F. Ragaini and E. Gallo, ACS Catal., 2014, 4, 3820–3823 CrossRef CAS; (c) P. Zardi, A. Caselli, P. Macchi, F. Ferretti and E. Gallo, Organometallics, 2014, 33, 2210–2218 CrossRef CAS; (d) D. Intrieri, M. Mariani, A. Caselli, F. Ragaini and E. Gallo, Chem. – Eur. J., 2012, 18, 10487–10490 CrossRef CAS PubMed.
- (a) G. Manca, E. Gallo, D. Intrieri and C. Mealli, ACS Catal., 2014, 823–832 CrossRef CAS; G. Manca, C. Mealli, D. M. Carminati, D. Intrieri and E. Gallo, Eur. J. Inorg. Chem., 2015, 4885–4893 Search PubMed.
- (a) S. Fantauzzi, E. Gallo, A. Caselli, C. Piangiolino, F. Ragaini and S. Cenini, Eur. J. Org. Chem., 2007, 6053–6059 CrossRef CAS; (b) P. Zardi, A. Pozzoli, F. Ferretti, G. Manca, C. Mealli and E. Gallo, Dalton Trans., 2015, 44, 10479–10489 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cy00207b |
| ‡ Catalyst 3 was heated until completely dissolved (see the ESI†). |
| § For the definition of productivity and numbers, see the ESI.† |
| This journal is © The Royal Society of Chemistry 2016 |