Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cu-catalysed pyrazole synthesis in continuous flow†
Júlia
Comas-Barceló
a,
Daniel
Blanco-Ania
b,
Sebastiaan A. M. W.
van den Broek
c,
Pieter J.
Nieuwland
c,
Joseph P. A.
Harrity
*a and
Floris P. J. T.
Rutjes
*b
aDepartment of Chemistry, The University of Sheffield. Brook Hill, Sheffield, S3 7HF, UK. E-mail: j.harrity@sheffield.ac.uk
bInstitute for Molecules and Materials, Radboud University, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands. E-mail: f.rutjes@science.ru.nl
cFutureChemistry Holding BV, Toernooiveld 100, 6525 EC Nijmegen, The Netherlands. E-mail: p.nieuwland@futurechemistry.com
First published on 8th February 2016
Abstract
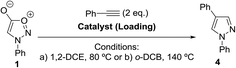
The synthesis of 1,4-disubstituted pyrazoles via the cycloaddition reaction of sydnones and terminal alkynes has been achieved employing silica-supported copper catalysts. Furthermore, this methodology has been successfully implemented in continuous flow conditions using prepacked stainless steel cartridges containing the solid-supported copper promoter. Finally, we demonstrate that this synthetic procedure can be successfully scaled up to produce practically useful amounts of pyrazole products within 2–5 hours.
Introduction
Pyrazoles are commonly found in pharmaceuticals1,2 and agrochemicals,3 and straightforward methods for their synthesis serve to underpin both the discovery of active compounds and their preparation on larger scale. Among the many synthetic approaches to these heterocycles, dipolar cycloadditions offer a convenient means to access complex pyrazoles from simple substrates, and this approach is widely used.4In this context, we have had a long standing interest in the employment of cycloaddition reactions of alkynes and sydnones for pyrazole synthesis.5–7 However, these reactions typically require high reaction temperatures (typically 160–180 °C) and long reaction times. Moreover, reaction regiocontrol can raise challenges and many unsymmetrical alkynes generate regioisomeric mixtures, which is the case for e.g. ethyl propiolate8 and 2-ethynylpyridine.9 Recently however, Taran et al. have devised a copper-catalysed cycloaddition of terminal alkynes that proceeds under milder conditions (60 °C, 16 h) and with excellent regiocontrol.10–12 In addition, we have reported the copper(II) acetate-promoted cycloaddition between sydnones and terminal alkynes that provides access to 1,4-disubstituted pyrazoles in a regioselective manner.8 Although this reaction generates 1,4-disubstituted isomers with excellent regiocontrol and using the readily available copper(II) acetate, it requires stoichiometric amounts of copper and two equivalents of the alkyne substrate. We reasoned that the excess alkyne was required to mediate the reduction of Cu(II) to Cu(I), thereby facilitating the formation of a reactive Cu-acetylide intermediate. In addition to these drawbacks, copper acetylides are known to be extremely insoluble and tend to form aggregates.13 This reduces the concentration of active reagent in solution, thus reducing the reaction rate and causes problems with mixing on larger scale as the formation of thick slurries is often observed.14,13
We envisaged that flow chemistry could provide an effective approach to address the most critical issues encountered in the copper(II) acetate-promoted cycloaddition of alkynes and sydnones. Specifically, moving from a batch process to the use of supported catalysts in a continuous flow apparatus seemed a feasible solution to addressing the formation of slurries. In this regard, Ley and co-workers have already shown that mechanical stirring employing agitating cell reactor (ACR) devices can successfully avoid clogging of the flow system, among other strategies.15
The use of supported catalysts for the promotion of dipolar cycloadditions has not been extensively reported outside of the established ‘click’ reaction of alkynes and azides to generate triazoles. In this latter case however, several different supported forms of copper have been shown to successfully promote the reaction both in batch and in flow. For example, Cu(0) powder,16 CuI supported on Amberlyst® A-21,17 and both Cu(I) and Cu(II) salts supported on amino-modified silica, have all proved to be effective.18–20 McQuade has also reported the use of Cu2O suspended in 4 Å molecular sieves for the generation of N-heterocyclic carbene complexes.21 As an alternative strategy, reactors made of copper metal have been developed that promote dipolar cycloadditions and other copper-catalysed processes such as Sonogashira cross couplings or Ullmann-type reactions. In these cases the addition of extra catalyst is no longer necessary, thus reducing the levels of complexity of the system.22 An example of this has been reported by Sach, where the synthesis of 1,2,3-triazoles under flow conditions is carried out by means of copper-made disquette reactors in the absence of extra copper sources.23
In an effort to improve the scope and versatility of Cu-promoted sydnone cycloadditions we decided to investigate the potential of supported Cu-catalysts for the synthesis of pyrazoles.
Results and discussion
Preparation of supported catalysts
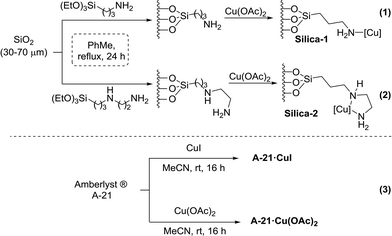
Our initial studies focused on the preparation of supported catalysts. Two main supports were chosen: Amberlyst® A-21, which is an amine-based polystyrene resin, and silica gel modified with additional amine groups to assist copper coordination. The copper sources chosen to coordinate with these supports were CuI and Cu(OAc)2 as they were readily available and represented pre-catalyst complexes at different oxidation states. The supported catalysts were prepared following procedures previously reported in the literature.18,24The two different Cu/silica supports were prepared from commercial chromatography grade silica gel (30–70 μm particle size), which was first functionalised to incorporate amino groups by reaction with the corresponding siloxanes in refluxing toluene for 24 h (Scheme 1, eqn (1) and (2)). After filtration and drying of the functionalised silica supports, they were stirred in ethanol in the presence of Cu(OAc)2 to afford the corresponding catalysts as brightly coloured blue solids.
The Amberlyst® A-21-based support was prepared from the commercially available resin, which was first treated with methanol and dichloromethane before shaking with the corresponding copper salt in acetonitrile for 16 h (Scheme 1, eqn (3)). The copper content of each supported catalyst system was established using inductively coupled plasma atomic analysis (ICP-MS), and loadings were expressed as a mass percentage (g of copper per 100 g of support). The results are summarised in Table 1.
Cycloaddition reactions in batch using supported catalysis
Preliminary cycloaddition studies highlighted that Amberlyst® A-21 beads were not suitable for this chemistry, since very low conversion for the cycloaddition reactions was observed (Table 2, entries 1–4). This polymer cannot be employed at temperatures above 100 °C due to its low thermal resistance. To enhance the performance of A-21-supported copper(I) iodide, the addition of nitrogen-containing ligands was considered. The addition of 1 equivalent of N,N,N′,N′-tetramethylethylenediamine (TMEDA), however, afforded the corresponding Glaser homocoupled 1,4-diphenyl-1,3-butadiyne product with no detectable quantities of the desired pyrazole (entry 2). On the other hand, the addition of 1 equivalent of 2,2′-bipyridine dramatically changed the outcome of the reaction since full conversion to the 1,4-disubstituted pyrazole was achieved in 6 h (entry 3). Although this copper-ligand combination led to the formation of product at 80 °C, further purification of the crude material was required to separate the bipyridine ligand.| Entry | Catalyst (loading) | Conditions | Resulta |
|---|---|---|---|
| a Conversions determined by 1H-NMR spectroscopy. b Reaction performed by adding 1 eq. of TMEDA. c Reaction performed by adding 1 eq. of 2,2′-bipyridine. d Reaction performed with 1 eq. of the alkyne. | |||
| 1 | A-21·CuI (60 mol%) | a) | No reaction |
| 2b | A-21·CuI (60 mol%) | a) | Formation of homocoupled alkyne |
| 3c | A-21·CuI (60 mol%) | a) | 100% conversion |
| 4 | A-21·Cu(OAc)2 (40 mol%) | a) | 7% conversion |
| 5d | Silica-1 (30 mol%) | b) | 100% conversion in 3 h |
| 6 | Silica-2 (30 mol%) | b) | 100% conversion in 2 h |
| 7d | Silica-2 (30 mol%) | b) | 100% conversion in 2 h, >95% yield after 4 cycles |
For this reason, silica supported alternatives at higher temperatures were then studied in order to try to find a system which would afford clean product in one step by removing the supported catalyst by filtration. Indeed, silica supported catalysts performed much better leading to full conversion in 3 h or less (entries 5–7). Satisfactorily, for both Silica-1 and Silica-2 supported catalysts, the amount of alkyne required for the cycloaddition to take place could be reduced to 1 equivalent with no effect on conversion to the pyrazole product (entries 5, 7).
Catalyst Silica-2 was the one chosen for further studies since it provided the product in shorter reaction times (2 h), the crude products contained lower levels of impurities and the same batch of catalyst could be reused up to 4 cycles without decreasing its performance. Finally, and to our delight, we noted a dramatic reduction of the loading of copper promoter required to achieve complete conversion of the sydnone substrates. Indeed, catalyst loadings of 25–30 mol% consistently provided optimal performance.
The fact that only one equivalent of alkyne was required, suggests that the alkyne could not be involved in promoting the reduction of Cu(II) to Cu(I). Also, no homocoupled diyne, resulting from Glaser-type processes, was detected in any case under the present conditions, which is opposite to our previously reported copper-promoted cycloadditions.8
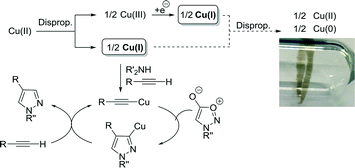
Bearing these observations in mind, an alternative explanation was required to justify the formation of a copper(I) acetylide as the active reaction intermediate. We hypothesised, based on literature precedent, that a convergent disproportionation process could be taking place when employing Cu(OAc)2 at temperatures above 120 °C. Based on studies by Mao et al. and as summarised in Scheme 2, copper(II) disproportionates to the active catalyst Cu(I) species together with Cu(III), which may also function as a source of Cu(I) after further reduction.25 The supported Cu(I) complex can then promote formation of the Cu-acetylide and, after cycloaddition, the cuprous pyrazole will exchange with alkyne to allow the catalyst to turnover. We also note the established disproportionation of Cu(I) to inactive Cu(0) and Cu(II).25–27 Indeed, we have observed the formation of copper mirrors on the glass surface after performing the reactions in batch using Schlenk tubes under inert atmosphere (Scheme 2).
The cycloaddition reactions were performed at 140 °C under inert atmosphere and during variable reaction times (1–20 h). Using these conditions, a wide scope was developed employing both aromatic and aliphatic sydnones and diverse alkynes incorporating different functionalities such as heteroaromatics, esters and alkyl residues, affording the corresponding pyrazole products in moderate to excellent yields (Scheme 3). In general, aromatic sydnones reacted faster than N-benzylsydnone, which only generated the corresponding products when employing phenylacetylene (14) or ethyl propiolate (15). No pyrazole formation was detected after 24 h with any other alkyne. Also, electron-neutral aromatic sydnones (4–8) gave better results than the electron-rich analogues (9–13). As for the alkynes, the ones bearing aromatic and heteroaromatic groups were the ones performing best (4, 8, 9, 13), whereas cyclopentylacetylene gave rather moderate results (6, 11), presumably due to the high volatility of this alkyne, which evaporated during the reaction.
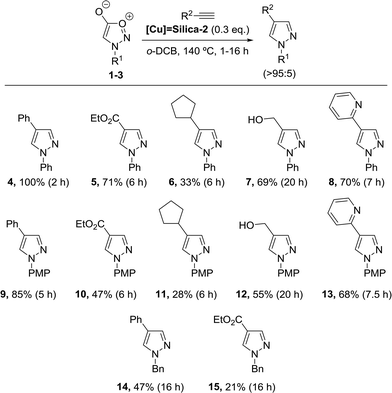 | ||
| Scheme 3 Scope for the solid-supported cycloaddition reactions in batch. PMP = p-methoxymethyl, Bn = benzyl. All yields correspond to isolated yields. | ||
Implementation of the cycloadditions in flow
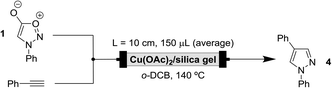
After the successful demonstration of the advantages of supported catalysts for pyrazole synthesis, the implementation of the process in flow was attempted. For this purpose, 10 cm length stainless steel tubing (1.75 mm inner diameter) was used to build the cartridges blocking the ends with cotton wool to allow confinement of the supported catalyst.The initial design of the flow reaction consisted of two syringe pumps, a T-mixer unit for the homogenisation of the reaction mixture and the positioning of a packed cartridge inside an oil bath to allow the reaction to reach 140 °C.
To optimise the residence time, N-phenylsydnone (0.20 M) and phenylacetylene (0.10 M) were each dissolved in o-dichlorobenzene and flowrate adjusted to ensure that mixing in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio was achieved inside the system. Table 3 summarises the results. For all the cycloadditions performed in flow, the volume of the cartridge corresponds to the effective volume after packing the cartridge with the supported catalyst in each case. Entry 2 highlights that a residence time of 5 minutes was required to give optimal conversion to the desired pyrazole product. In principle, longer residence times should give higher conversion but this was not observed in the current system (entries 3, 4 vs. entry 2). The previous set of experiments was performed in a 48 hour period using the same prepacked cartridge (entry 2 performed on day 1, entries 1, 3 and 4 performed on day 2). These results suggest that the catalyst loses its efficiency over time, and so for this reason, we decided to prepare fresh cartridges for the optimisation study in order to ensure that the results were comparable.
1 molar ratio was achieved inside the system. Table 3 summarises the results. For all the cycloadditions performed in flow, the volume of the cartridge corresponds to the effective volume after packing the cartridge with the supported catalyst in each case. Entry 2 highlights that a residence time of 5 minutes was required to give optimal conversion to the desired pyrazole product. In principle, longer residence times should give higher conversion but this was not observed in the current system (entries 3, 4 vs. entry 2). The previous set of experiments was performed in a 48 hour period using the same prepacked cartridge (entry 2 performed on day 1, entries 1, 3 and 4 performed on day 2). These results suggest that the catalyst loses its efficiency over time, and so for this reason, we decided to prepare fresh cartridges for the optimisation study in order to ensure that the results were comparable.
Another parameter we considered for the optimisation was the solvent. Satisfactorily, 100% conversion was achieved after 5 minutes when performing the model reaction using toluene instead of o-dichlorobenzene in the presence of a back pressure regulator (BPR) in order to raise the pressure and keep the solvent in the liquid state.
Once the optimal reaction conditions were found, we decided to study the efficiency of the catalyst over time. To do so, the model reaction was run over a period of 5 h collecting and analysing aliquots at different time points (Table 4).
| Entry | Collection time/h | Collected mass (mg) | Conversion (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)a 1)a |
|---|---|---|---|
| a Reaction conversion was determined using 400 MHz 1H NMR spectroscopy. Note that ϕSydnone = 2 × ϕAlkyne, where ϕ is flow rate. | |||
| 1 | 0:00–0:30 | 1.8 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
| 2 | 0:30–1:00 | 5.6 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 3 | 1:00–1:30 | 3.7 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 4 | 1:30–2:00 | 5.7 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 5 | 2:00–3:00 | 7.4 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 6 | 3:00–4:00 | 6.0 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 7 | 4:00–5:00 | 6.7 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
As Table 4 shows, initially an equilibration time was required to ensure complete circulation of both streams along the flow system and homogeneous mixing, which translated into an initial lower conversion (entry 1).
However, after this period, excellent conversions were maintained over a period of 4 hours, affording clean pyrazole fractions containing minor traces of unreacted sydnone (entries 2–6). Interestingly, after 4 h, conversion to product was still very high but the crude product contained higher levels of impurities (entry 7). We therefore decided to perform the cycloadditions over a period of 4–5 hours confident that this would provide high yields of pyrazole products of high purity.
Having found optimal parameters to perform these cycloaddition reactions, the expansion of the scope was next pursued employing the flow platform (Scheme 4).28
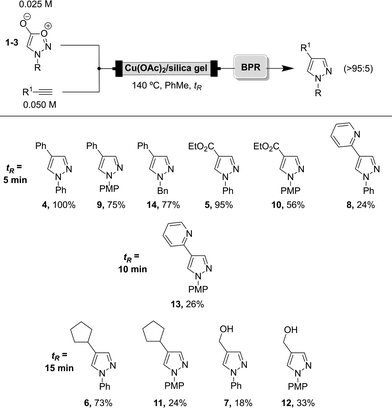 | ||
| Scheme 4 Scope for the cycloaddition reaction under flow conditions. PMP = p-methoxymethyl, Bn = benzyl. Note that ϕSydnone = 2 × ϕAlkyne, where ϕ is flow rate. | ||
Depending on the nature of the substrate, residence times from 5 minutes to 15 minutes were employed for the synthesis of the substrates already prepared by means of supported catalysis in batch.
Best results were obtained with phenylacetylene and ethyl propiolate, similarly to the batch procedure. When 2-ethynylpyridine, cyclopentylacetylene or propargyl alcohol were employed, the reaction rates were slower so that higher residence times were needed to increase conversion in most cases (tR = 10, 15 min). Interestingly, when using 2-ethynylpyridine (8, 13), moderate yields were obtained compared to the batch examples, which could be attributed to potential coordination of pyridine to the copper centres of the catalyst along the cartridge, preventing full pyrazole recovery after the reaction. Also, the low yields obtained under these conditions for the alcohol-containing pyrazoles suggest potential degradation of the products inside the microreactor. For example, as these reactions require heating at high temperatures over prolonged periods of time, it is conceivable that trans-esterification with the silica support results in some losses of the pyrazole 7, as this compound was recovered cleanly but in low amounts, and no starting material was recovered.29
Scaling-up of the flow process
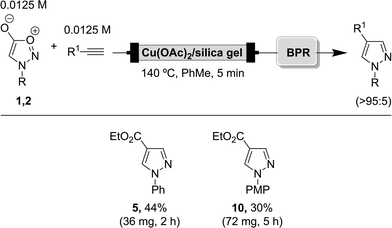
The currently developed flow system employing supported catalysis allowed the preparation of a range of 1,4-disusbtituted pyrazoles in a regioselective manner in relatively short reaction times. Although this provides proof of concept for the implementation of this technology, the absolute amount of pyrazole products generated with the setup is quite low due to the small internal volume of the cartridge. To show that these reactions can be run equally well at larger scale, we developed a system that allowed scaling up of the process to generate practically useful amounts of these products over a few hours.For this purpose, we considered that a suitable alternative to stainless steel tubing would be to employ empty stainless steel HPLC columns, since they are easy to fill with catalyst, can be reused and are thermally resistant to the required reaction conditions. In the present case, a 2 mL empty HPLC column (Knauer, A2103-1) with 7 μm frits was chosen. To complete the system, an HPLC pump and a back-pressure regulator were also employed, and the system was further simplified by using only one feed containing both the sydnone and the alkyne (no reaction between them occurs at room temperature).
When applying the previous set of conditions (140 °C, PhMe, tR = 5 min) on both aromatic sydnones and ethyl propiolate, successful results were obtained (Scheme 5). Even though this set of conditions was not optimal for this new flow system, since full conversion was not reached after 5 minutes, between 50–100 mg of pyrazole products could be produced with this larger scale setup.
Experimental
General procedure for the synthesis of 1,4-disubstituted pyrazoles in batch
To a stirred solution of sydnone (1 eq.) and supported catalyst Silica-2 (0.3 eq., 4.02% Cu) in o-dichlorobenzene (0.20–0.25 M) under N2 at room temperature, was added the alkyne (1 eq.). After stirring at room temperature for 5 minutes, the mixture was heated at 140 °C for the designated time. After cooling, the reaction mixture was filtered and the crude product was purified by flash chromatography on silica gel (eluting solvent: 5% ethyl acetate in heptane, unless otherwise stated). Further purification of the pyrazole products could be realised by recrystallisation from CH2Cl2–heptane if required.General procedure for the synthesis of 1,4-disubstituted pyrazoles in flow
Two feed solutions were employed: stream A containing the sydnone of choice in toluene (0.025 M), and stream B containing the alkyne in toluene (0.050 M), both driven by syringe pumps (ϕA = 2ϕB). These were mixed in a PEEK T-mixer connection (P-713, IDEX) before entering the microreactor, consisting of a 10 cm stainless steel cartridge packed with the supported copper catalyst, at 140 °C for 5 to 15 minutes. By removing the solvent in vacuo, the desired pyrazole products were obtained. In some cases, further purification was achieved by flash chromatography on silica gel (5–10% ethyl acetate in heptane) and/or by recrystallisation from dichloromethane/heptane.Conclusions
We have developed new methodology for the regioselective synthesis of 1,4-disubstituted pyrazoles via cycloaddition reactions between sydnones and alkynes employing a silica-supported copper catalyst. This methodology has also been implemented in flow for the first time, which led to significantly reduced reaction times, and proved the feasibility of the continuous process. Moreover, scaling-up of this flow process has successfully been achieved for the synthesis of ester-containing pyrazole derivatives in practical amounts showing the potential that this technology can offer towards the development of other solid-supported catalysed processes.Acknowledgements
This work was supported by the FP7 Marie Curie Actions of the European Commission via the ITN ECHONET Network (MCITN-2012-316379).Notes and references
- E. Arbaciauskiené, G. Vilkauskaité, G. A. Eller, W. Holzer and A. Sackus, Tetrahedron, 2009, 65, 7817–7824 CrossRef.
- S. Ishibuchi, H. Morimoto, T. Oe, T. Ikebe, H. Inoue, A. Fukunari, M. Kamezawa, I. Yamada and Y. Naka, Bioorg. Med. Chem. Lett., 2001, 11, 879–882 CrossRef CAS PubMed.
- C. Lamberth, Heterocycles, 2007, 71, 1467–1502 CrossRef CAS.
- S. Fustero, M. Sánchez-Roselló, P. Barrio and A. Simón-Fuentes, Chem. Rev., 2011, 111, 6984–7034 CrossRef CAS PubMed.
- D. L. Browne, M. D. Helm, A. Plant and J. P. A. Harrity, Angew. Chem., Int. Ed., 2007, 46, 8656–8658 CrossRef CAS PubMed.
- D. L. Browne, J. F. Vivat, A. Plant, E. Gomez-Bengoa and J. P. A. Harrity, J. Am. Chem. Soc., 2009, 131, 7762–7769 CrossRef CAS PubMed.
- R. S. Foster, J. Huang, J. F. Vivat, D. L. Browne and J. P. A. Harrity, Org. Biomol. Chem., 2009, 7, 4052–4056 CAS.
- J. Comas-Barceló, R. S. Foster, B. Fiser, E. Gomez-Bengoa and J. P. A. Harrity, Chem. – Eur. J., 2015, 21, 3257–3263 CrossRef PubMed.
- R. S. Foster, H. Jakobi and J. P. A. Harrity, Tetrahedron Lett., 2011, 52, 1506–1508 CrossRef CAS.
- S. Kolodych, E. Rasolofonjatovo, M. Chaumontet, M.-C. Nevers, C. Créminon and F. Taran, Angew. Chem., Int. Ed., 2013, 52, 12056–12060 CrossRef CAS PubMed.
- S. Specklin, E. Decuypere, L. Plougastel, S. Aliani and F. Taran, J. Org. Chem., 2014, 79, 7772–7777 CrossRef CAS PubMed.
- E. Decuypere, S. Specklin, S. Gabillet, D. Audisio, H. Liu, L. Plougastel, S. Kolodych and F. Taran, Org. Lett., 2015, 17, 362–365 CrossRef CAS PubMed.
- C. Theunissen, M. Lecomte, K. Jouvin, A. Laouiti, C. Guissart, J. Heimburger, E. Loire and G. Evano, Synthesis, 2014, 1157–1166 Search PubMed.
- J. F. Normant, Synthesis, 1972, 63–80 CrossRef CAS.
- D. L. Browne, B. J. Deadman, R. Ashe, I. R. Baxendale and S. V. Ley, Org. Process Res. Dev., 2011, 15, 693–697 CrossRef CAS.
- M. Fuchs, W. Goessler, C. Pilger and C. O. Kappe, Adv. Synth. Catal., 2010, 352, 323–328 CrossRef CAS.
- I. R. Baxendale, S. V. Ley, A. C. Mansfield and C. D. Smith, Angew. Chem., Int. Ed., 2009, 48, 4017–4021 CrossRef CAS PubMed.
- T. Miao and L. Wang, Tetrahedron Lett., 2007, 48, 95–99 CrossRef CAS.
- Q. Wu and L. Wang, Synthesis, 2008, 2007–2012 CAS.
- T. Miao and L. Wang, Synthesis, 2008, 363–368 CAS.
- S. M. Opalka, J. K. Park, A. R. Longstreet and D. T. McQuade, Org. Lett., 2013, 15, 996–999 CrossRef CAS PubMed.
- J. Bao and G. K. Tranmer, Chem. Commun., 2015, 51, 3037–3044 RSC.
- A. R. Bogdan and N. W. Sach, Adv. Synth. Catal., 2009, 351, 849–854 CrossRef CAS.
- C. Girard, E. Önen, M. Aufort, S. Beauvière, E. Samson and J. Herscovici, Org. Lett., 2006, 8, 1689–1692 CrossRef CAS PubMed.
- Z. Mao, Z. Wang, Z. Xu, F. Huang, Z. Yu and R. Wang, Org. Lett., 2012, 14, 3854–3857 CrossRef CAS PubMed.
- A. E. King, T. C. Brunold and S. S. Stahl, J. Am. Chem. Soc., 2009, 131, 5044–5045 CrossRef CAS PubMed.
- A. E. King, L. M. Huffman, A. Casitas, M. Costas, X. Ribas and S. S. Stahl, J. Am. Chem. Soc., 2010, 132, 12068–12073 CrossRef CAS PubMed.
- ICP-MS analysis of pyrazoles 5 and 9 (×2) highlighted only small quantities of leaching (<400 ppm) under conditions highlighted in Scheme 4 over a 2 hour period.
- We are grateful to a reviewer for this suggestion.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra. See DOI: 10.1039/c5cy02247a |
| This journal is © The Royal Society of Chemistry 2016 |