Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The unique fluorine effects in organic reactions: recent facts and insights into fluoroalkylations
Chuanfa
Ni
and
Jinbo
Hu
*
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai 200032, China. E-mail: jinbohu@sioc.ac.cn
First published on 8th August 2016
Abstract
Fluoroalkylation reaction, featuring the transfer of a fluoroalkyl group to a substrate, is a straightforward and efficient method for the synthesis of organofluorine compounds. In fluoroalkylation reactions, fluorine substitution can dramatically influence the chemical outcome. On the one hand, the chemistry of alkylation with non-fluorinated reagents may not be applicable to fluoroalkylations, so it is necessary to tackle the fluorine effects to achieve efficient fluoroalkylation reactions. On the other hand, fluorine substitution may bring about new reactivities and transformations that cannot be realized in alkylation with non-fluorinated reagents; thus, fluorine substitution can be used to explore new synthetic methods. This tutorial review provides a brief overview of the unique fluorine effects in recently developed nucleophilic, electrophilic, radical, and transition metal-mediated fluoroalkylation reactions by comparing with either their non-fluorinated counterparts or fluorinated counterparts with different numbers of fluorine substituents.
Key learning points(1) Fluorine substitution can dramatically influence the chemical outcome of fluoroalkylation reactions.(2) The chemistry of alkylation with non-fluorinated reagents may not be applicable to fluoroalkylation chemistry. (3) Fluorine substitution may bring about new reactivities and transformations that cannot be achieved in alkylation with non-fluorinated reagents. (4) Understanding the unique fluorine effects can help to predict, design, and further develop fluoroalkylation chemistry. |
1. Introduction
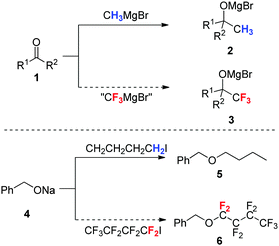
Nowadays, one of the major efforts of chemists in both academia and industry is the search for “special effect” chemicals with new structures and functions.1,2 In this context, fluorine is undoubtedly one of the elements that has attracted the highest recent research interest in several aspects of chemistry, since the judicious incorporation of the fluorine atom or the fluorinated moiety into organic compounds or polymers has become a powerful tool to discover new chemical entities possessing unique physical, chemical and/or biological properties.2–4 For instance, fluorinated materials have been widely used in liquid crystal displays of smart phones and in photovoltaic solar cells.5,618F-labeled molecules are essential radiotracers for positron emission tomography (PET), which serves as a privileged diagnostic tool for cancers and other diseases.7 Polyfluorinated (fluorous) molecules are used in 19F magnetic resonance imaging (MRI) thanks to the high sensitivity of 19F nuclei in nuclear magnetic resonance (NMR) measurements.8 The highest impact of fluorine in life sciences is associated with the development of agrochemicals and pharmaceuticals. Recently, it has been estimated that about 30% of new approved drugs (excluding biopharmaceutical products) contained fluorine atoms or fluoroalkyl groups.9–11 The fact that fluorinated organic compounds and materials often show unusual physical, chemical, and/or biological properties and behaviour (in comparison with their nonfluorinated counterparts) is often called “fluorine effects” or “fluorine magic”.4,12,13The profound effect of fluorine substitution on the chemical reactivity of organic compounds is an interesting research topic in organic chemistry.2–4 In this context, many fluoroalkylation reactions have been extensively studied over the past decade. Fluoroalkylation reactions, such as tri-, di-, and mono-fluoromethylations and other perfluoroalkylations and polyfluoroalkylations, represent one of the most straightforward and efficient methods for the incorporation of a fluoroalkyl group (such as CF3, CF2H, or CH2F group) into organic molecules.14 Traditionally, fluoroalkylation reactions can be divided into four categories: nucleophilic fluoroalkylation, electrophilic fluoroalkylation, radical fluoroalkylation, and transition metal-mediated fluoroalkylation.2–4 However, fluoroalkylation reactions are often surprisingly different from general alkylation reactions in organic chemistry, and the known knowledge of general alkylation chemistry cannot be simply used to predict the fluoroalkylation chemistry. Two typical examples are shown in Scheme 1. Firstly, Grignard reagent CH3MgBr can readily undergo nucleophilic addition to an aldehyde or ketone 1 to give product 2; however, a similar addition with CF3MgBr is very difficult, because CF3MgBr is highly unstable and readily decomposes to difluorocarbene (:CF2) and magnesium bromofluoride.2–4 Secondly, Williamson ether synthesis between sodium alkoxide 4 and n-butyl iodide gives ether product 5 through an SN2 reaction pathway; however, a similar SN2 reaction between 4 and perfluorobutyl iodide cannot proceed to give ether product 6. This is because the perfluorobutyl group in n-C4F9I is more electronegative than iodine, and the polarisation is thus reversed and nucleophile 4 attacks iodine rather than the carbon atom of the perfluorobutyl group.2–4
Therefore, the understanding of the unique fluorine effects in fluoroalkylation reactions not only facilitates the prediction, design, and further development of fluoroalkylation chemistry, but also provides deeper insights into the unique features of fluorine and its related science. In this tutorial review, we wish to highlight some fluoroalkylation reactions that have been developed recently, and discuss the unique fluorine effects involved in these “surprising” reactions. The examples selected here merely help to interpret the concept of this review; for recent developments in fluoroalkylation reactions, one can refer to some comprehensive reviews.9,14–18
2. The unique fluorine effects in nucleophilic fluoroalkylation reactions
Nucleophilic fluoroalkylation features the transfer of a fluoroalkyl group to an electrophile, in which either a free fluorocarbanion, an equivalent of a fluorocarbanion (i.e., a species that has a similar reactivity character to a fluorocarbanion, such as pentacoordinate silicon species), or a fluoroalkyl metal species (RfM, M = main group metals in most cases) is involved. Fluorine substitution can influence the generation, the thermodynamic stability, and the kinetic reactivity (including the desired nucleophilic fluoroalkylation, competitive decomposition and protonation) of a fluorinated carbanion, thus distinguishing nucleophilic fluoroalkylations from non-fluorinated transformations and their counterparts with different number of fluorine substituents.19,20 In terms of the kinetic reactivity of fluorocarbanion, the competitive decomposition, which tends to occur more easily as the number of fluorine substituents increases, always has a negative (unfavourable) influence on the desired nucleophilic fluoroalkylation reaction.19,20 Nevertheless, in some cases, the fluorine substitution can play a positive (favourable) role in nucleophilic fluoroalkylations by facilitating the generation and stabilizing the reaction intermediates. In this section, we mainly explain the influence of fluorine substitution on the reactivity of α/β-fluorocarbanions and the methods for tuning their nucleophilic fluoroalkylation reactivity by changing the countercations, the neighbouring substituents, the catalysts, the solvents, and so on.2.1 Fluoroalkylation of epoxides with fluoroalkyl sulfones: exploring new fluoroalkylations by tackling the negative fluorine effect
In 2006, a study of nucleophilic ring-opening fluoroalkylation of epoxides conducted by us summarized the influence of α-fluorine substitution on the chemical reactivities of various carbanions.21 Reactions with di- and monofluoro(phenylsulfonyl)methyllithium showed that α-fluorine substitution on the carbanion dramatically decreases the carbanion's reactivity towards epoxides (Scheme 2). From the product yields, the reactivity of fluorinated carbanions (Nu−) toward propylene oxide decreases in the following orders: (1) for different fluorinated anions: (PhSO2)2CF− > PhSO2CHF− > PhSO2CF2−; (2) for different halogen-substituted anions: PhSO2CCl2− > PhSO2CF2−. This unfavourable (negative) influence of fluorine substitution on the reaction of the carbanions was described as the “negative fluorine effect (NFE)”.21The NFE in many nucleophilic fluoroalkylations, including but not restricted to the above mentioned fluoroalkylation of epoxides, can be attributed to the following two aspects: (1) the low thermal stability of the fluorine-substituted carbanion caused by its high tendency to undergo α-elimination of a fluoride ion (or another leaving group such as PhSO2−); (2) its intrinsic low nucleophilicity towards a certain electrophile such as the epoxide caused by factors such as the unmatched hard/soft nature.19,20 Although the NFE could not be used to summarize all reactivity aspects of α-fluorocarbanions in nucleophilic fluoroalkylation reactions, it is at least helpful to better understand many nucleophilic fluoroalkylation reactions. The knowledge of the NFE can be used to tune and improve a nucleophilic fluoroalkylation reaction in the following manners: (1) slightly changing the neighboring groups; (2) changing the metal countercations; (3) enhancing the reactivity of the electrophiles. For a detailed discussion on the nucleophilic fluoroalkylation of various organic compounds by tackling the NFE, the readers are recommended to refer to our previous reviews.19,20
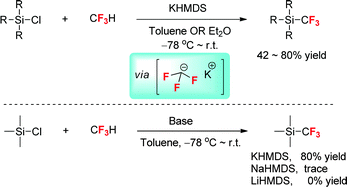
2.2 Direct trifluoromethylation with CF3H: enhancing the reactivity by changing the metal–fluorine interaction
The metal–fluorine interaction can significantly influence the outcome of nucleophilic trifluoromethylation. Previously, fluoroform (CF3H) was used for the trifluoromethylation of carbonyl compounds with a strong base in aprotic polar solvents. The in situ trapping of CF3− by a solvent such as DMF to form an adduct is deemed to be important for stabilizing the labile trifluoromethyl anion, thus facilitating the nucleophilic trifluoromethylation.22 In 2012, Prakash et al.22 disclosed that the direct nucleophilic trifluoromethylation of silicon-, boron-, sulfur-, and carbon-centers with CF3H can be best performed in THF, ether, and toluene when potassium hexamethyldisilazide (KHMDS) was used as a base (Scheme 3). In this case, CF3− generated from the deprotonation of CF3H undergoes nucleophilic trifluoromethylation rather than decomposition to the fluoride ion and singlet difluorocarbene. Indeed, CF3− with K+/18-crown-6 as a countercation has been observed in a recent study.23 Control experiments demonstrated the importance of the countercation effect.22 Taking the reaction between CF3H and Me3SiCl in toluene for an example, the use of KHMDS gives Me3SiCF3 as a major product; in sharp contrast, sodium hexamethyldisilazide (NaHMDS) gives Me3SiCF3 as a minor product, whereas lithium hexamethyldisilazide (LiHMDS) cannot give the desired product. These observations show that the countercation K+ is important in stabilizing CF3− due to a weakened metal–fluorine interaction.2.3 Trifluoro- vs. trichloromethylation of MBH-fluorides with Me3SiCF3: electron-withdrawing and electron-donating effects of fluorine on the reactivity of CF3−
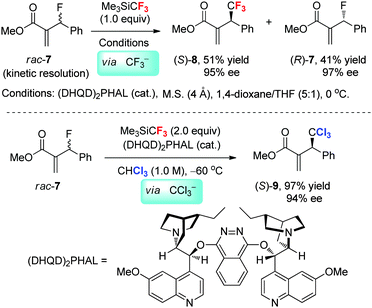
Although fluorine is more electronegative than the chlorine atom, the α-fluorine atom is less effective for stabilizing the α-fluorocarbanion due to the repulsion between the lone-pair electrons of the carbanion and the electron-rich fluorine atom.4 As a result, the methine proton of CHCl3 (pKa = 21, in H2O) is more acidic than CF3H (pKa = 30.5, in H2O). Therefore, the in situ generated CF3− from Me3SiCF3 can be used as an effective base for the deprotonation of carbon acids such as CH3CN and CHCl3. Shibata et al.24 reported that Morita–Baylis–Hillman (MBH)-type allyl fluoride 7 can undergo enantioselective nucleophilic trifluoromethylation, proceeding through the cleavage of the C–F bond of MBH-fluoride by a cooperative system that consisted of the bis(cinchona alkaloid) and Me3SiCF3 (Scheme 4). When conducting the reaction in ether solvents, the trifluoromethylation product 8 is formed via two subsequent SN2’ reactions: (1) the addition of the chiral tertiary amine catalyst at the terminal alkenyl position of one enantiomer of MBH-fluoride, which leads to the formation of a chiral allylic ammonium intermediate and the subsequent activation of Me3SiCF3; (2) the stereoselective addition of CF3− released from Me3SiCF3 to the chiral allylic ammonium gives the product. However, when CHCl3 is used instead of the ether solvents, the in situ generated CF3− readily deprotonates CHCl3 rather than undergoing nucleophilic trifluoromethylation, thus giving rise to trichloromethylation product 9.25 By taking advantage of the in situ generated CF3− as a base, the allylation of carbon acids that are more acidic than CF3H has also been achieved enantioselectively.2.4 Direct fluoromethylations with Me3SiCF2H vs. Me3SiCF3: the important inductive effect of fluorine on the reactivity of Me3SiRf
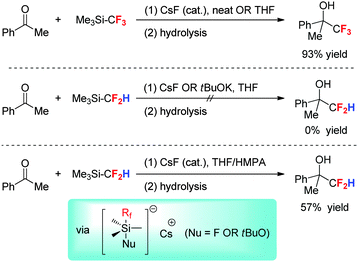
Me3SiCF2H, as an analogue of Me3SiCF3, is a potentially useful difluoromethyl anion (HCF2−) source for direct nucleophilic difluoromethylation. However, due to the weaker electron-withdrawing ability of a CF2H group than a CF3 group, the reactivity of Me3SiCF2H is distinct from the well-documented trifluoromethylation with Me3SiCF3 (Scheme 5). The trifluoromethylation with Me3SiCF3 can be readily initiated by a wide range of nucleophiles under mild conditions,9,26 whereas the initiation of a similar difluoromethylation is quite demanding. Calculations showed that the bond order of the Si–CF2H bond (0.436) is significantly higher than that of the Si–CF3 bond (0.220), which is in accordance with the observation that the cleavage of the Si–CF2H bond is more difficult than that of the Si–CF3 bond.27In 2011, we performed an exhaustive investigation on the reaction conditions and revealed that cesium fluoride (CsF)/DMF and potassium tert-butoxide (tBuOK)/THF could allow efficient difluoromethylation of various aldehydes, diaryl ketones, and imines with Me3SiCF2H at room temperature or even at −78 °C.27 However, this protocol could not efficiently difluoromethylate enolizable ketones due to either the competitive difluoromethylation of the solvent (when conducted in DMF) or the strong basicity of HCF2− (when conducted in THF). The latter can lead to enolization and subsequent condensation reaction of the ketones. Very recently, Radchenko et al.28 showed that the difluoromethylation of enolizable ketones can be achieved by performing the reaction in THF in the presence of an unreactive polar additive such as HMPA, albeit in only moderate yields. It is obvious that the additive HMPA plays an important role in inhibiting the enolization of ketones, thus improving the nucleophilic difluoromethyl anion.
2.5 Mono- vs. difluoromethylation with sulfoximines: a balance between the inductive effect of fluorine and the leaving ability of the neighbouring substitution
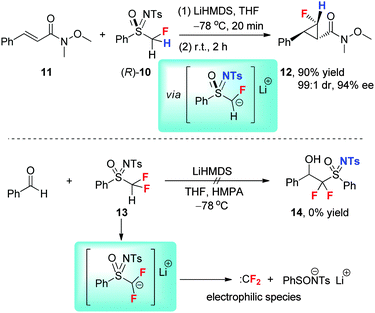
Fluorinated sulfoximines have been used as versatile fluoroalkylation reagents. In 2012, we reported the first highly diastereo- and enantioselective monofluorocyclopropanation reaction with enantiopure monofluoromethyl sulfoximine (R)-10 (Scheme 6).29 This novel reaction was carried out through the Michael-addition of a chiral α-fluorocarbanion generated from sulfoximine (R)-10 to α,β-unsaturated Weinreb amides (such as 11) followed by γ-elimination of the sulfoximidoyl group, giving optically enriched cyclopropanes (such as 12). The good leaving ability of the N-tosylated sulfoximine moiety and the moderate stability of this monofluorocarbanion are responsible for such a cyclization reaction. However, the nucleophilic difluoromethylation or gem-difluoromethylenation with the difluorinated analogue 13 was proved to be unsuccessful due to the ready decomposition of the difluorinated carbanion to form difluorocarbene (Scheme 6).29The reactivity of the difluoromethyl sulfoximine can be tuned from electrophilic to nucleophilic by changing the N-substituent from the electron-withdrawing tosyl (Ts) group to the electron-donating tert-butyldimethylsilyl (TBS) group (Scheme 7).29 A proper balance between the induction effect of the two fluorine atoms and the weak leaving ability of the N-silylated sulfoximine moiety enables the nucleophilic difluoromethylation reaction. The addition of sulfoximine (R)-15 to a variety of aryl alkyl ketones (such as 16) followed by reductive desulfoximidoylation furnishes optically enriched difluoromethyl tertiary alcohols (such as 18).
2.6 Iodo- and bromodifluoromethylation vs. (aminosulfonyl)methylenation with 2-pyridyl sulfones: fluorine substitution facilitates SO2 extrusion
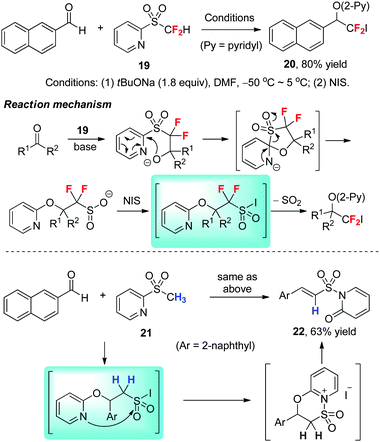
The synthesis of iodo- and bromodifluoromethylated tertiary alcohols through direct nucleophilic fluoroalkylation of ketones is particularly challenging, because of the high tendency of the decomposition of CF2Br− and CF2I− to difluorocarbene. To overcome this synthetic problem, we developed formal nucleophilic iodo- and bromodifluoromethylation by using the combination of a nucleophilic difluoroolefination reagent and an electrophilic halogenation reagent.30 In the previously disclosed Julia–Kocienski-type gem-difluoroolefination of carbonyl compounds with difluoromethyl 2-pyridyl sulfone (19),30 we found that 2-pyridyl sulfone, which is rarely used in Julia–Kocienski reaction due to its relatively weak electron-withdrawing ability, is an optimal scaffold for gem-difluoroolefination due to the fluorine effect. We noticed that the sulfinate intermediates generated in situ are relatively stable. Thus, halogenation of the sulfinate intermediates can provide halodifluoromethylation products (such as 20), which change the reaction pathway from the traditional olefination to alkylation (Scheme 8).In the case of methyl 2-pyridyl sulfone 21, a non-fluorinated sulfone, the halogenation of the sulfinate intermediate proceeds in a different manner, affording aminosulfonylated alkene (E)-22 as the main product.30 The change in the reaction pathway may result from the difference in the stabilities of the non- and difluorinated sulfonyl iodide intermediates, with the latter readily extruding SO2.
2.7 Stereoselective synthesis of monofluoroolefins via Julia–Kocienski reaction: the stereoelectronic effect of fluorine facilitates the kinetic resolution of the sulfinate intermediates
During our pursuit of carbonyl fluoroolefination with heteroaryl sulfones, we developed a spontaneous kinetic resolution of water-soluble Julia–Kocienski intermediates that facilitates liquid–liquid extractive separation of (Z)- and (E)-monofluoroolefins (Scheme 9).31 Although the carbonyl addition with the fluorinated sulfones (such as 23) is non-stereoselective, the decomposition rates of the two diastereomeric sulfinate intermediates (such as 24) from a ready Smiles rearrangement are significantly different. Intermediate 24-Z spontaneously and rapidly decomposed to monofluoroalkene (Z)-25 at room temperature, whereas its diastereomeric isomer 24-E only broke down upon addition of an acid. Thus, after separating (Z)-25via extraction of the mixture with water/ether, acidification of the aqueous phase (24-E) followed by a second water/ether extraction can afford another stereoisomer (E)-25. This kinetic resolution probably arises from the distinct energy barriers for the decomposition of the fluorinated sulfinate intermediates, which is partially influenced by the stereoelectronic effect of fluorine.31 | ||
| Scheme 9 Stereoselective synthesis of monofluoroolefins via kinetic resolution of Julia–Kocienski reaction intermediates. | ||
2.8 Difluoroalkylation with silyl difluoroenol ethers “on-water”: the remarkable accelerating effect of F–H interaction
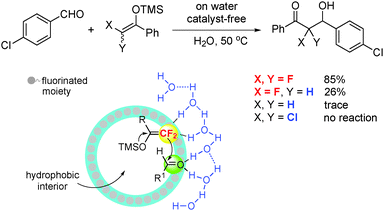
Similar to their non-fluorinated counterparts, the Mukaiyama addition reactions of silyl difluoroenol ethers normally require either a Lewis acid or a Brønsted acid to activate the carbonyl compounds or imine substrate. In 2014, Zhou et al.32 reported a highly efficient “on water”, catalyst-free nucleophilic addition reaction of silyl difluoroenol ethers (Scheme 10). The experiments are performed using water as the only solvent without adding any additives. A wide range of substrates, including aromatic aldehydes, activated ketones, and isatylidene malononitriles (Michael acceptors), worked well to give the corresponding difluoroalkylation products in high yields. Control experiments with nonfluorinated, monofluorinated, and dichlorinated enol ethers showed that the fluorine effect on the reaction is remarkable. The reaction of the nonfluorinated silyl enol ether and the aldehyde gave only trace amounts of the product, whereas the monofluorinated silyl enol ether reacted sluggishly to give the product in only 26% yield under the same reaction conditions. Moreover, the dichlorinated analogue failed to undergo the reaction. An explanation is that the C–F⋯H–O interactions between difluorinated enol ethers and the hydrogen bond network of water at the phase boundary of the reactants promote the formation of a unique microstructure, thus facilitating the “on-water” catalyst-free addition reaction.322.9 Monofluoromethylation of aldehydes with (PhSO2)2CHF: the electronic effect of fluorine facilitates the addition reaction
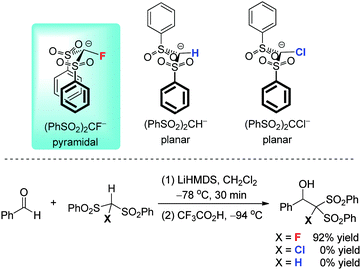
Fluorobis(phenylsulfonyl)methane [(PhSO2)2CHF] is a synthetically useful nucleophilic monofluoromethylation reagent. X-ray crystallographic studies have shown that its anion (PhSO2)2CF− has a pyramidal structure, whereas its nonfluorinated counterpart (PhSO2)2CH− and a brominated anion (C4F9SO2)2CBr− adopt planar structures. NMR and computational studies confirm that the highly electronegative fluorine substituent plays an important role in pyramidalization of the carbon center of (PhSO2)2CF− (Scheme 11).33Compared with the non-fluorinated sulfones (PhSO2)2CH2, fluorine substitution can improve the reactivity of the bis(phenylsulfonyl)methyl anion. An example is the nucleophilic addition reaction between (PhSO2)2CHF and an aldehyde reported by us (Scheme 11).34 This reaction was previously considered to be unattainable because of the steric hindrance of the (PhSO2)2CF group. We accomplished the reaction by using lithium hexamethyldisilazide (LiHMDS) as the base to promote the formation of the alcoholate. Control experiments with lithium, sodium, and potassium hexamethyldisilazides showed that LiHMDS serves as the best base for the addition reaction, indicating that the strong Li–O coordination in the alcoholate intermediate plays an important role in its stabilization. Furthermore, under the same reaction conditions, similar nucleophilic addition reactions between (PhSO2)2CH2 or (PhSO2)2CHCl and an aldehyde are unsuccessful. This suggests that the fluorine substitution, which helps to strengthen the newly formed C–C bond, is also of great importance for the nucleophilic fluoromethylation reaction. Taking together, the Li–O interaction and the fluorine substitution simultaneously promote this aldehyde addition reaction.34
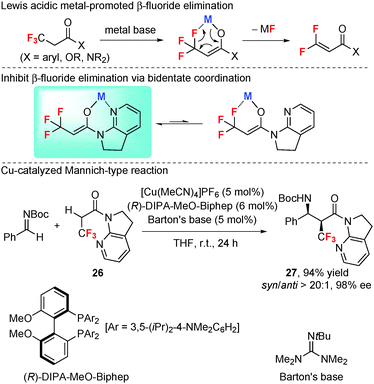
2.10 Asymmetric Mannich-type reaction of α-CF3 amide through bidentate coordination: stabilization effect of CF3vs. β-fluoride elimination
Although the strong electron-withdrawing α-trifluoromethyl group is more effective than α-fluorine atom for the stabilization of a carbanion electronically, α-CF3 carbanions with “hard” metal countercations usually readily eliminate a β-fluoride to form gem-difluoroolefins due to the strong metal–fluorine interaction (Scheme 12).35 This undesired β-fluoride elimination reaction is a main obstacle for effective synthetic application of α-CF3 carbanions.α-Trifluoromethyl enolates are active nucleophiles for synthesizing CF3-bearing functionalized organic molecules. However, methods for tuning their nucleophilic reactivity are very limited. Very recently, Kumagai, Shibasaki et al.35 developed a catalytic method for the generation of α-CF3 enolate through bidentate coordination of a soft Lewis acidic metal countercation with the enolate oxygen and an amide auxiliary group (Scheme 12). The inhibition of the metal–fluorine interaction enables the catalytic generation of α-trifluoromethyl enolate without competitive fluoride elimination. Thus, the catalytic asymmetric Mannich-type reaction using an α-CF3 amide 26via soft Lewis acid/hard Brønsted base cooperative catalysis affords the Mannich products (such as 27) in excellent yields with high diastereo- and enantioselectivity.
3. The unique fluorine effects in electrophilic fluoroalkylation reactions
Despite the strong electron-withdrawing ability of fluorine atoms, the α-fluorine atom can stabilize carbocations through the interaction between the lone-pair electron of fluorine and the unoccupied p-orbital of the cationic carbon center. Studies have indicated that the stability of fluorinated carbocations decreases as follows: HCF2+ > CH2F+ > CF3+ > CH3+. Although the fluorine effect is complex for CF3+, this trend at least shows that α-fluorine substitution is effective for stabilizing carbocations.4However, in real electrophilic fluoroalkylation reactions, the fluoroalkylating agents are usually equivalents of fluorinated carbocations, rather than free fluorocarbocations. Thus, the electrophilic fluoroalkylations commence with the reaction of a nucleophilic centre (usually negatively charged) with an electrophilic fluorinated carbon centre, and conclude with the formation of a fluorinated sp3-carbon centre. In this section, we will give a brief summary on the influence of fluorine substitution on the chemoselectivity and reaction mechanism of electrophilic fluoroalkylations.
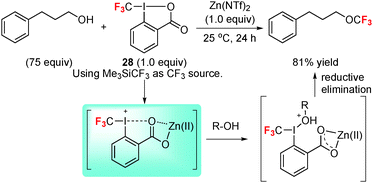
3.1 O-Trifluoromethylation with a hypervalent iodine reagent: flipping the reactivity of trifluoromethyl from nucleophilic to electrophilic
As mentioned in the Introduction part, the direct perfluoroalkylation of a hard nucleophile with Rf–I is difficult to achieve due to the reversed polarisation of Rf by the fluorine substitution. Since 2006, Togni et al.18,36 have introduced (per)fluoroalkylated hypervalent iodine compounds for electrophilic introduction of the trifluoromethyl group and longer (per)fluorinated alkyl chains. Enhancing the valence of the iodine atom is an elegant method to change the reaction pathway to the desired electrophilic perfluoroalkylation.Trifluoromethyl ethers are useful in materials, agricultural, and pharmaceutical sciences; however, their traditional synthesis relies on multi-step transformations. In 2009, Togni et al.18 reported a practical O-trifluoromethylation of aliphatic alcohols under the activation of CF3–I(III) reagent 28 by Lewis acid Zn(NTf2)2 (Scheme 13). The reaction probably proceeds via Zn(II)-promoted ligand exchange with alcohol followed by reductive elimination of the product.
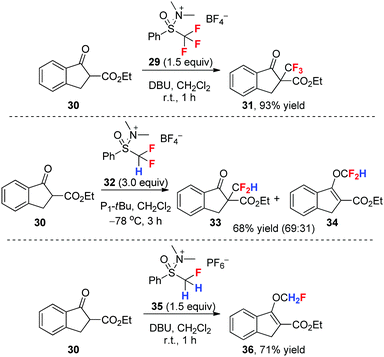
3.2 C- vs. O-fluoroalkylation of ketoesters with a fluorinated sulfoximinium salt: the influence of fluorine on the chemoselectivity
Sulfoximinium salt is also a useful scaffold for electrophilic fluoroalkylations (Scheme 14).37S-Trifluoromethyl sulfoximinium salt 29 is an electrophilic trifluoromethylation reagent. Its reaction with β-ketoester 30 affords C-trifluoromethylation product 31. The control experiment showed that adding nitrobenzene does not decrease the yield, thus suggesting a normal electrophilic trifluoromethylation pathway. However, the reaction of 32 and β-ketoester 30 gives a mixture of C- and O-difluoromethylation products 33 and 34. More interestingly, the monofluorinated analogue 35 is an efficient electrophilic monofluoromethylation reagent with inherent O-selectivity. Its reaction with β-ketoester 30 gives O-monofluoromethyl compound 36 in high yield.Computational studies provided some insights into the different chemoselectivity of the tri-, di and monofluoromethylation of β-ketoester.37 The C-/O-selectivity should be attributed to the electronic character of tri-, di- and monofluoromethyl cations or radicals. Trifluoromethylation with C-selectivity possibly involves the formation of a more cationic species like CF3+. For difluoromethylation, it may involve both radical and cationic species, thus providing a mixture of C- and O-difluoromethylation products. Monofluoromethylation probably proceeds involving a more radical-like species.37
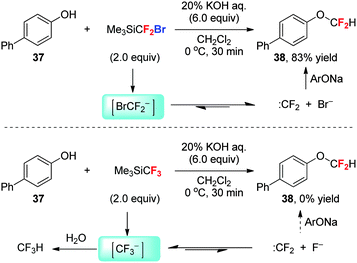
3.3 O-Difluoromethylation of phenols with Me3SiCF3vs. Me3SiCF2Br: the inhibition effect of a third fluorine substitution on the generation of difluorocarbene in an aqueous system
Difluorocarbene (:CF2, with a singlet ground state) is the most stable dihalocarbene owing to the interaction of the lone pairs of its fluorine substituents with the carbene center. The combined destabilization effect by the negative inductive effect of fluorine and stabilization effect by π-donation from the fluorine to the vacant p-orbital of carbon render :CF2 a moderately reactive electrophilic species.38 In the past few years, we and Prakash et al. developed Me3SiCF3, Me3SiCF2Cl and Me3SiCF2Br as novel difluorocarbene reagents. These three fluoromethylsilanes can generate :CF2 effectively and have been used for the gem-difluorocyclization of alkynes and alkenes via [2+1] cycloaddition in organic solvents.38However, the heteroatom-difluoromethylation usually needs an alkaline base (such as KOH) to activate the pronucleophiles (such as phenols) by deprotonation. Hence, an aqueous solution of base is preferred for the activation of both the substrates and the difluorocarbene reagents. Me3SiCF2Br and Me3SiCF2Cl are suitable reagents for difluoromethylation of phenol 37 in a mixed solvent system consisting of CH2Cl2 and water, but Me3SiCF3 fails to react with 37 under the same conditions, being hydrolysed to CF3H completely (Scheme 15).39 The electronic nature of α-halo substituents determines the feasibility of the difluorocarbene pathway. In the reaction using Me3SiCF3 and aqueous KOH, the strong electron-withdrawing ability of fluorine stabilizes the CF3 anion, rending α-fluoro elimination much difficult; therefore, the protonation of CF3 anion prevails.
3.4 C-Difluoromethylation of ester with CF3H: an electrophilic lithiodifluoromethylation pathway facilitated by intermolecular lithium–fluorine interaction
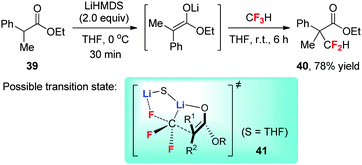
In contrast to the negative role of F–Li interaction in nucleophilic trifluoromethylation with CF3Li, this interaction can promote electrophilic difluoromethylation with CF3Li. Mikami et al. reported the direct α-difluoromethylation of carbonyl compounds such as ester 39 with CF3H under the promotion of lithium hexamethyldisilazide (LiHMDS) (Scheme 16).40 Among various alkali metals (Li, Na, K, Cs) used for both deprotonations, only LiHMDS, which contains the most Lewis acidic metal countercation, works for this difluoromethylation. Moreover, the amount of the base used is found to be crucial for the reaction; two equivalents (relative to the substrate) give the highest yields. Computational studies40 showed that the reaction proceeds through transition state 41 involving a bimetallic difluoromethylene carbenoid species [Li–F–CF2–Li]+. Nucleophilic attack of the enolate carbon on the electrophilic difluoromethylene carbenoid through an SN2-like mechanism followed by protonation furnishes this difluoromethylation.4. The unique fluorine effects in radical fluoroalkylation reactions
Fluoroalkyl radicals, as very important reactive intermediates in organofluorine chemistry, not only participate in fluoroalkyl addition to unsaturated systems, but also play roles in fluoroalkylation of nucleophiles (via single electron-transfer mechanism).α-Fluorination has a special effect on the structure and stabilization of methyl radicals. The trifluoromethyl radical is essentially tetrahedral, and difluoromethyl and monofluoromethyl radicals are pyramidal, while the methyl radical is planar.4 The kinetic analysis on the C–C bond homolytic cleavage of various fluorinated tert-butoxy radicals by Jiang et al. showed that the relative rate for the formation of methyl and fluoromethyl radicals decreases as follows: HCF2˙ (10.2) > CH2F˙ (9.0) > CH3˙ (1.0) > CF3˙ (0.08).2,4 C–H bond dissociation energy calculations for fluorinated methanes also suggest that the thermodynamic stability of fluoromethyl radicals decreases in a similar order: CH2F˙ > HCF2˙ > CH3˙ > CF3˙.2,4 These data indicate that both mono- and difluorination stabilize the methyl radical, whereas trifluorination destabilizes the radical.4 Calculations suggest that fluorine substitution destabilized the α-radicals inductively, but slightly stabilizes α-radicals through resonance; overall, the tetrahedral trifluoromethyl radical becomes the most unstable one among fluoromethyl radicals due to the diminished resonance stabilization.4
The reactivity of a fluoroalkyl radical can be divided into nucleophilicity and electrophilicity. On the one hand, due to the electron-donating resonance effect of lone-pair electrons on the α-fluoro substituent, all fluoromethyl radicals have lower ionization potential than CH3˙, with HCF2˙ being more nucleophilic than CH3˙; on the other hand, due to the strong inductive effect of the α-fluorine, CF3˙ is more electrophilic than CH3˙.4,41
In radical fluoroalkylation with reagents of various forms, not only the stability and reactivity of the fluoroalkyl radicals, but also other factors such as the reduction potential and the stability of the fluoroalkyl radical precursors can influence the whole chemical outcome.
4.1 Fluoroalkylation of alkenes with sulfonyl chlorides: the influence of fluorine substitution on the generation and reactivity of fluoroalkyl radicals
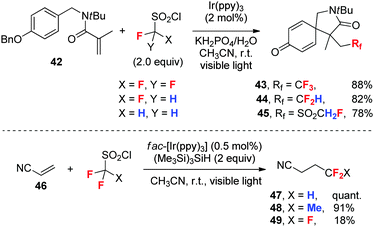
Fluoroalkanesulfonyl chlorides are excellent fluoroalkyl radical precursors, which can be reduced more readily through single electron transfer (SET) than fluoroalkyl iodides owing to the stronger electron-withdrawing ability of the fluoroalkanesulfonyl group than a simple fluoroalkyl group. Fluoroalkanesulfonyl chlorides have been used for radical fluoroalkylation of arenes and alkenes.14 In spite of their relatively high reactivity, the influence of fluorine substitution on the generation and reactivity of fluoroalkyl radicals is still significant.In a photoredox-catalyzed dearomative radical cyclization of N-benzylacrylamides such as 42 (Scheme 17), Dolbier et al.42 showed that the reaction with CF3SO2Cl or HCF2SO2Cl at room temperature provides tri- or difluoromethylation products 43 and 44, respectively, with the loss of SO2. However, the reaction with CH2FSO2Cl under the same conditions gives monofluoromethanesulfonylation product 45 with the retaining of SO2. Obviously, the weak electron-withdrawing ability of the CH2F group makes the loss of SO2 at a low temperature difficult.
A study on hydrodifluoroalkylations of electron-deficient alkenes such as acrylonitrile (46) under photoredox catalysis showed that difluoroalkylations (including difluoromethylation) readily take place, while trifluoromethylation is sluggish (Scheme 17).41 Computational studies using acrylonitrile and propene as model substrates showed that the nucleophilic/electrophilic nature of various fluoroalkyl groups is consistent with their electron-donating/-withdrawing ability.41 The reactions of RCF2˙ with acrylonitrile, an electron-deficient alkene, prefer the charge-transfer (CT) transition state RCF2+/[CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCN]−, which significantly lowers the activation barrier. In the reactions of CF3˙ with propene, an electron-rich alkene, the preferential formation of the CT state CF3−/[CH2
CHCN]−, which significantly lowers the activation barrier. In the reactions of CF3˙ with propene, an electron-rich alkene, the preferential formation of the CT state CF3−/[CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3]+ is expected, thus lowering its barrier to a value smaller than that for acrylonitrile. For the reaction of CF3˙ with acrylonitrile, although the activation barrier is similar to that for RCF2˙ due to its large enthalpy, the observed low yield probably arises from the competitive hydrogen abstraction reaction of CF3˙.41
CHCH3]+ is expected, thus lowering its barrier to a value smaller than that for acrylonitrile. For the reaction of CF3˙ with acrylonitrile, although the activation barrier is similar to that for RCF2˙ due to its large enthalpy, the observed low yield probably arises from the competitive hydrogen abstraction reaction of CF3˙.41
4.2 Tri- vs. difluoromethylation of arenes with sulfinate salts: the influence of fluorine substitution on site-selectivity
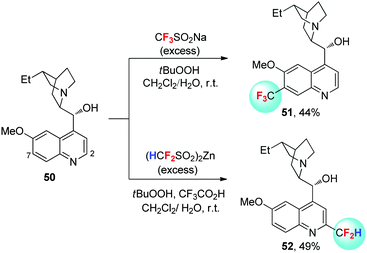
The site-selectivity in the fluoroalkylation of hetero(aromatic) compounds is another case for interpreting the influence of fluorosubstitution on the nucleophilicity/electrophilicity of alkyl radicals. Baran et al. reported the oxidative tri- and difluoromethylation of hetero(arenes) with sulfinate salts CF3SO2Na and (HCF2SO2)2Zn as the radical sources (Scheme 18).43 Dihydroquinine (50) reacts with CF3SO2Na in the presence of TBHP, to give product 51, with site-selectivity toward the o-position of the methoxy group (C-7 site), demonstrating the electrophilic nature of CF3˙. In contrast, difluoromethylation of dihydroquinine with (HCF2SO2)2Zn under similar conditions takes place at the electron-deficient C-2 site to give 52, coinciding with the nucleophilic nature of HCF2˙.4.3 Fluoroalkylation of isocyanides with sulfones: fluorine effects on the generation of fluoroalkyl radicals
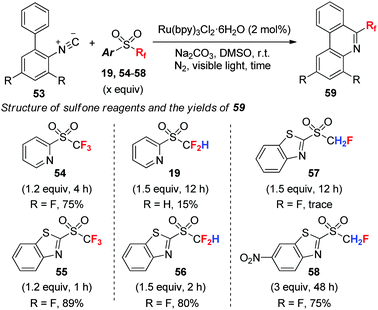
Fluorinated sulfones have been used as versatile radical precursors. We showed that under visible-light photoredox catalysis, a wide range of readily available mono-, di-, and trifluoromethyl heteroaryl sulfones readily fluoroalkylate isocyanides (such as 53) (Scheme 19).44 The electronic nature the heteroaryl group is pivotal for the generation of fluoroalkyl radicals. Trifluoromethyl sulfones with both 2-pyridyl (54) and 2-benzo[d]thiazolyl (BT) (55) groups readily undergo trifluoromethylation due to the strong electron-withdrawing ability of the CF3 group. For difluoromethylation, only the more electron-deficient 2-BT group (56) works well. Nevertheless, in the case of monofluoromethylation, increasing the reduction potential of monofluoromethyl sulfone by introducing a nitro group (58) on the 2-BT moiety is required to promote the monofluoromethylation reaction.444.4 Monofluoromethylation of phenols with fluorinated sulfoximine: the influence of fluorine substitution on the reactivity
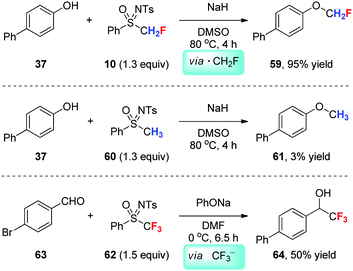
We reported the monofluoromethylation of phenols with N-tosyl-S-monofluoromethyl sulfoximine 10 (Scheme 20),29 which is also a nucleophilic fluoroalkylation reagent as mentioned previously (see Scheme 6). Compared with its non-fluorinated counterpart 60, reagent 10 reacts with phenol 37 at a much faster reaction rate. This phenomenon is inconsistent with a typical SN2 reaction pathway, in which α-fluorine substitution should decrease the reactivity of an electrophile. Therefore, a single-electron transfer (SET) mechanism involving CH2F˙ is proposed, which is supported by radical inhibition experiments.29 In contrast, the reaction between PhONa and trifluoromethyl sulfoximine 62, gives CF3H instead of an O-trifluoromethylation product. The addition of aldehyde 63 to the reaction system affords the nucleophilic trifluoromethylation product 64, supporting the generation of CF3− during the reaction (Scheme 14).29It is obvious that the degree of fluorine substitution significantly affects the reactivity of mono-, di- and trifluoromethyl sulfoximines. In comparison with radical monofluoromethylation with PhSO(NTs)CH2F (10) via CH2F˙ and electrophilic difluoromethylation with PhSO(NTs)CF2H (13) via :CF2 (see Scheme 6), PhSO(NTs)CF3 (62) is a nucleophilic trifluoromethylation reagent via CF3−.
5. Unique fluorine effects in transition metal-mediated fluoroalkylation reactions
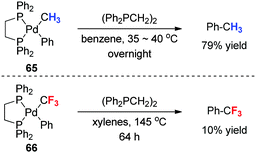
Transition metals, which may be considered as the countercations of fluoroalkyl anions, can significantly influence the fluoroalkylation reactions due to their varying hard/soft nature, oxidation sate and ligand effect. The stability and reactivity problems associated with fluoroalkyl metal species are major hurdles in developing transition metal mediated fluoroalkylation reactions. For example, unlike perfluoroalkyl copper reagents such as CuCF3, the CuCF2H reagent prepared by a metathesis reaction between Cd(CF2H)2 and CuBr undergoes a rapid decomposition to give 1,1,2,2-tetrafluoroethane and (Z)-1,2-difluoroethene at temperatures above −30 °C.45 As another example (Scheme 21), Ph–Pd(II)–CF3 complex 65 with a bidentate phosphine ligand (Ph2PCH2)2 is reluctant to undergo reductive elimination to form Ph–CF3 even at an elevated temperature (145 °C), while its nonfluorinated analogue 66 readily releases Ph-CH3 at relatively low temperatures (15–40 °C).17 In recent years, great progress has been achieved in transition-metal mediated fluoroalkylations by changing the metal species and the ligands. In this section, we only introduce some typical examples that are related to the fluorine effect, including trifluoromethylation, difluoroalkylation, monofluoroalkylation, and β-fluoroalkylation reactions.5.1 Palladium-catalyzed trifluoromethylation of aryl chlorides: tackling the fluorine effect by changing the ligand
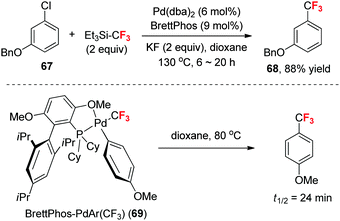
As mentioned above, it is usually difficult for the Ph–Pd(II)–CF3 complex to undergo reductive elimination due to the electronic effect of the CF3 group.17 The strong electron-withdrawing CF3 group renders the palladium more Lewis acidic, which significantly improves the activation barrier for Ph-CF3 elimination and impedes the development of a Pd-catalyzed nucleophilic trifluoromethylation of aryl halides with normal phosphine ligands.In 2010, Buchwald et al.46 developed the first Pd-catalyzed trifluoromethylation of aryl chlorides (such as 67) by using the steric hindered electron-rich phosphine ligand BrettPhos (Scheme 22). Stoichiometric reaction showed that the Ar–Pd–CF3 complex 69, which is ligated with only one BrettPhos ligand, can easily undergo reductive elimination at 80 °C. Computational studies showed that in comparison to the ground states, the Pd–CF3 distance in the transition states is substantially elongated, whereas the distance between the Pd atom and the aryl ring remains essentially unchanged, suggesting that the main contribution of BrettPhos to the activation energy is the breaking of the strong Pd–CF3 bond.46
5.2 Nucleophilic mono- vs. trifluoromethylation of arynes: tackling the fluorine effect by changing the metal countercation
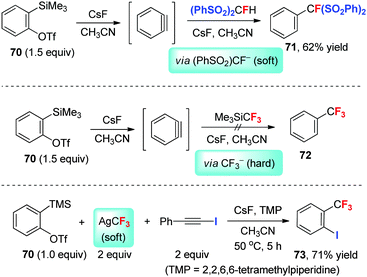
Arynes, with their low-lying lowest unoccupied molecular orbital (LUMO), can be considered as soft electrophiles. During our investigation of the hard/soft nature of α-fluoro carbanions, we developed the nucleophilic monofluoromethylation of arynes (in situ generated from precursors such as 70) by using a soft monofluoromethaide anion equivalent, (PhSO2)2CF− (Scheme 23).47However, arynes failed to react with the trifluoromethyl anion generated from TMSCF3 and a fluoride salt such as CsF, presumably because of the low stability of CF3− and the mismatch between the soft aryne and the hard CF3− species (due to the fluorine substitution) under the provided conditions. This synthetic problem was finally solved by using AgCF3, a trifluoromethylmetallic species possessing increased stability and softness in comparison with the “naked” CF3−.47 Thus, in situ-generated aryne species reacts with AgCF3 to form an o-(trifluoromethyl)arylsilver complex, which further reacts with the alkynyl iodide (an iodination reagent) to give vicinally iodotrifluoromethylated aromatics such as 73.
5.3 Palladium-catalyzed regiodivergent allylic fluoroalkylation: the electronic effect of fluorine on the yield and regioselectivity
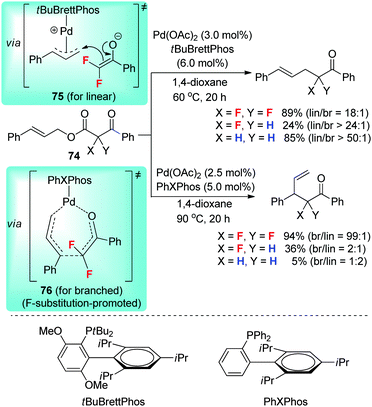
In 2015, Altman et al.48 reported a palladium-catalyzed regiodivergent allylic fluoroalkylation with difluorinated β-ketoesters 74 as the substrates (Scheme 24). By changing the ligand structure, either linear or branched α-allyl-α,α-difluoroketone is obtained in good yield and regioselectivity. Interestingly, the ligand-controlled regioselective reaction only works for the difluorinated ketoesters, and the analogous mono- and nonfluorinated substrates cannot provide branched products with a similar chemical outcome. It is obvious that the fluorine substitution on the substrate facilitates the generation of the branched product.An analysis of the relationship between the electronic properties of the difluorinated substrates and the regioselectivities of the catalytic reactions implies two distinct pathways accounting for the linear and branched products.48 On the one hand, under the catalytic conditions with tBuBrettPhos as the ligand, electron-rich cinnamyl esters provides linear products with lower selectivity than electron-deficient cinnamyl esters, which is in accordance with a normal outer-sphere mechanism involving transition state 75. On the other hand, under the catalytic conditions with PhXPhos as the ligand, the electronic properties of cinnamyl esters do not influence the regioselectivity significantly. Moreover, this reaction is consistent with the accelerating effect of fluorine in non-metal-catalyzed 3,3-sigmatropic rearrangements of allyl α,α-difluoroenol ethers. Therefore, the combined unique electronic effect of the fluorine atom and the ligand effect of PhXPhos may facilitate an analogues rearrangement, in which an η1-allyl palladium(enolate) intermediate undergoes the sigmatropic rearrangement via palladacyclic transition state 76 to afford the branched product.48
5.4 AgF-mediated fluorinative cross-coupling of two olefins: the electronic effect of fluorine on the reactivity of olefins
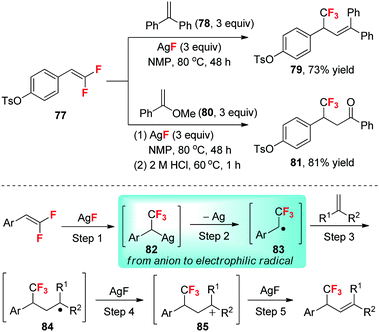
As mentioned previously (see Section 2.10), the β-fluoride elimination of the α-CF3 carbanion is a competitive reaction that influences the synthetic application of such an anion. Because the electron-withdrawing inductive effect of the fluorine atoms together with electron repulsion between the double bond and the fluorine atoms render the difluorinated carbon atom of gem-difluoroolefin very electrophilic, we conceived that the reverse fluoride addition to difluoroolefin may be a possible solution to the β-fluoride elimination problem. However, one challenge associated with this method is the spontaneous protonation of the α-CF3 carbanion when an alkali metal salt is used as the fluoride source.We found that AgF can be used to react with gem-difluorostyrene derivatives (such as 77) and to stabilize the intermediates (Scheme 25).49 The ready homocoupling reaction indicates that the intermediates are α-CF3-benzylsilver species 82 with radical reactivity.49 Thus, the use of electron-rich terminal olefins (such as 78 and 80) as the α-CF3-benzyl radical acceptor leads to a AgF-mediated fluorinative cross-coupling reaction between gem-difluoroolefins and non-fluorinated olefins, providing a facile access to both α-CF3 alkenes (such as 79) and β-CF3 ketones (such as 81) that otherwise remain challenging to be directly prepared.49
5.5 Nickel-mediated enantioselective Suzuki cross-coupling of unactivated 1-halo-1-fluoroalkanes: the steric effect of fluorine substituents on the enantioselectivity
Although the van der Waal radii of fluorine (1.47 Å) is only a little larger than that of hydrogen (1.20 Å) and is usually recognized as a mimic of hydrogen, the steric effect of fluorine is remarkable in many transformations.4Very recently, Gandelman et al.50 reported a Ni-mediated Suzuki-cross coupling of unactivated 1-halo-1-fluoroalkanes with alkylboranes for the synthesis of secondary alkyl fluorides (Scheme 26). Racemic 1-halo-1-fluoroalkanes (such as 87) bearing directing groups such as phenyl, keto, and sulfonamide substituents at various distant positions efficiently participate in stereoconvergent enantioselective Suzuki reactions with good enantioselectivity. The enantioselectivity of the product and the absence of kinetic resolution for 1-halo-1-fluoroalkane 8750 indicate that the coupling reaction may proceed through a single electron-transfer process involving α-fluoroalkyl radicals.
 | ||
| Scheme 26 Nickel-mediated enantioselective Suzuki cross-coupling of unactivated 1-halo-1-fluoroalkanes. | ||
6. Conclusions
Incorporating fluorine atom(s) via fluoroalkylation has become a useful strategy in drug design and new functional material development. In fluoroalkylation reactions, the fluorine substitutions can dramatically influence the chemical outcome. As a result, the chemistry of alkylation with their non-fluorinated counterparts may not be applicable to fluoroalkylations. Therefore, tackling the unique fluorine effects is necessary to achieve desired fluoroalkylation reactions. On the other hand, the fluorine substitution can bring about new reactivities and transformations that cannot be achieved in alkylation with non-fluorinated reagents, thus fluorine substitution is also a tool to explore new synthetic methods.Fluorine effects are ubiquitous in fluoroalkylations, which are either negative or positive for the reactions. Understanding the unique fluorine effects in fluoroalkylation reactions not only facilitates the prediction, design, and further development of fluoroalkylation chemistry, but also provides deeper insights into unique features of fluorine and its related science. It is worthwhile to note that Cahard and Bizet12 also published a tutorial review on fluorine effects, concentrating on asymmetric synthesis using fluorinated substrates and fluorinated catalysts. Our Tutorial Review mainly discusses fluoroalkylation reactions, that is, the organic synthesis using fluorinated reagents. These two reviews complement each other, providing readers an opportunity to deeply understand the unique fluorine effects in organic chemistry.
Acknowledgements
We thank the National Basic Research Program of China (2015CB931900 and 2012CB821600), the National Natural Science Foundation of China (21421002, 21372246, and 21472221), the Chinese Academy of Sciences, Shanghai Academic Research Leader Program (15XD1504400), Shanghai Rising-Star Program (16QA1404600), and the Youth Innovation Promotion Association CAS (2014231) for financial support.Notes and references
-
P. Vollhardt and N. Schore, Organic Chemistry: Structure and Function, Freeman, New York, 7th edn, 2014 Search PubMed
.
-
R. D. Chambers, Fluorine in Organic Chemistry, Blackwell, Oxford, 2004, and reference therein Search PubMed
.
-
P. Kirsch, Modern Fluoroorganic Chemistry. Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, 2nd edn, 2013 Search PubMed
.
-
K. Uneyama, Organofluorine Chemistry, Blackwell, Oxford, 2006, and references therein Search PubMed
.
- P. Kirsch, J. Fluorine Chem., 2015, 177, 29–36 CrossRef CAS
.
- A. C. Stuart, J. R. Tumbleston, H. Zhou, W. Li, S. Liu, H. Ade and W. You, J. Am. Chem. Soc., 2013, 135, 1806–1815 CrossRef CAS PubMed
.
- S. Preshlock, M. Tredwell and V. Gouverneur, Chem. Rev., 2016, 116, 719–766 CrossRef CAS PubMed
.
- I. Tirotta, V. Dichiarante, C. Pigliacelli, G. Cavallo, G. Terraneo, F. B. Bombelli, P. Metrangolo and G. Resnati, Chem. Rev., 2015, 115, 1106–1129 CrossRef CAS PubMed
.
- Y. Zhou, J. Wang, G. Zhanni, S. Wang, W. Zhu, J. L. Acena, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422–518 CrossRef CAS PubMed
.
- E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS PubMed
.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC
.
- D. Cahard and V. Bizet, Chem. Soc. Rev., 2014, 43, 135–147 RSC
.
- D. O'Hagan, Chem. Soc. Rev., 2008, 37, 308–309 RSC
.
- C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765–825 CrossRef CAS PubMed
, and references therein.
- M.-C. Belhomme, T. Besset, T. Poisson and X. Pannecoucke, Chem. – Eur. J., 2015, 21, 12836–12865 CrossRef CAS PubMed
.
- C. Alonso, E. M. de Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847–1935 CrossRef CAS PubMed
.
- O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475–4521 CrossRef CAS PubMed
, and references therein.
- J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650–682 CrossRef CAS PubMed
, and references therein.
- C. Ni and J. Hu, Synlett, 2011, 770–782 CAS
, and references therein.
- C. Ni, W. Zhang and J. Hu, Top. Curr. Chem., 2012, 308, 25–44 Search PubMed
, and references therein.
- C. Ni, Y. Li and J. Hu, J. Org. Chem., 2006, 71, 6829–6833 CrossRef CAS PubMed
.
- G. K. S. Prakash, P. V. Jog, P. T. D. Batamack and G. A. Olah, Science, 2012, 338, 1324–1327 CrossRef CAS PubMed
, and references therein.
- G. K. S. Prakash, F. Wang, Z. Zhang, R. Haiges, M. Rahm, K. O. Christe, T. Mathew and G. A. Olah, Angew. Chem., Int. Ed., 2014, 53, 11575–11578 CrossRef CAS PubMed
.
- T. Nishimine, K. Fukushi, N. Shibata, H. Taira, E. Tokunaga, A. Yamano, M. Shiro and N. Shibata, Angew. Chem., Int. Ed., 2014, 53, 517–520 CrossRef CAS PubMed
.
- T. Nishimine, H. Taira, E. Tokunaga, M. Shiro and N. Shibata, Angew. Chem., Int. Ed., 2016, 55, 359–363 CrossRef CAS PubMed
.
- R. P. Singh, G. Cao, R. L. Kirchmeier and J. M. Shreeve, J. Org. Chem., 1999, 64, 2873–2876 CrossRef CAS PubMed
.
- Y. Zhao, W. Huang, J. Zheng and J. Hu, Org. Lett., 2011, 13, 5342–5345 CrossRef CAS PubMed
, and references therein.
- O. M. Michurin, D. S. Radchenko and I. V. Komarov, Tetrahedron, 2016, 72, 1351–1356 CrossRef CAS
, and references therein.
- X. Shen and J. Hu, Eur. J. Org. Chem., 2014, 4437–4451 CrossRef CAS
, and references therein.
- Y. Zhao, B. Gao and J. Hu, J. Am. Chem. Soc., 2012, 134, 5790–5793 CrossRef CAS PubMed
, and references therein.
- Y. Zhao, F. Jiang and J. Hu, J. Am. Chem. Soc., 2015, 137, 5199–5203 CrossRef CAS PubMed
. In this paper, the authors found that the ground state structure of 24-E adopts a conformation in which the C(1)–H bond is anti-periplanar to the C(2)–F bond.
- J.-S. Yu, Y.-L. Liu, J. Tang, X. Wang and J. Zhou, Angew. Chem., Int. Ed., 2014, 53, 9512–9516 CrossRef CAS PubMed
, and references therein.
- G. K. S. Prakash, F. Wang, N. Shao, T. Mathew, G. Rasul, R. Haiges, T. Stewart and G. A. Olah, Angew. Chem., Int. Ed., 2009, 48, 5358–5362 CrossRef CAS PubMed
.
- X. Shen, L. Zhang, Y. Zhao, L. Zhu, G. Li and J. Hu, Angew. Chem., Int. Ed., 2011, 50, 2588–2592 CrossRef CAS PubMed
.
- L. Yin, L. Brewitz, N. Kumagai and M. Shibasaki, J. Am. Chem. Soc., 2014, 136, 17958–17961 CrossRef CAS PubMed
, and references therein.
- V. Matoušek, J. Václavík, P. Hájek, J. Charpentier, Z. E. Blastik, E. Pietrasiak, A. Budinská, A. Togni and P. Beier, Chem. – Eur. J., 2016, 22, 417–424 CrossRef PubMed
.
- Y.-D. Yang, X. Lu, G. Liu, E. Tokunaga, S. Tsuzuki and N. Shibata, ChemistryOpen, 2012, 1, 221–226 CrossRef CAS PubMed
, and references therein.
- C. Ni and J. Hu, Synthesis, 2014, 842–863 Search PubMed
, and references therein.
- L. Li, F. Wang, C. Ni and J. Hu, Angew. Chem., Int. Ed., 2013, 52, 12390–12394 CrossRef CAS PubMed
.
- K. Honda, T. V. Harris, M. Hatanaka, K. Morokuma and K. Mikami, Chem. – Eur. J., 2016, 22, 8796–8800 CrossRef CAS PubMed
, and references therein.
- X.-J. Tang, Z. Zhang and W. R. Dolbier, Jr., Chem. – Eur. J., 2015, 21, 18961–18965 CrossRef CAS PubMed
, and references therein.
- Z. Zhang, X.-J. Tang and W. R. Dolbier, Jr., Org. Lett., 2016, 18, 1048–1051 CrossRef CAS PubMed
.
- Y. Fujiwara, J. A. Dixon, R. A. Rodriguez, R. D. Baxter, D. D. Dixon, M. R. Collins, D. G. Blackmond and P. S. Baran, J. Am. Chem. Soc., 2012, 134, 1494–1497 CrossRef CAS PubMed
, and references therein.
- J. Rong, L. Deng, P. Tan, C. Ni, Y. Gu and J. Hu, Angew. Chem., Int. Ed., 2016, 55, 2743–2747 CrossRef CAS PubMed
.
- J. Hu, W. Zhang and F. Wang, Chem. Commun., 2009, 7465–7478 RSC
, and references therein.
- E. J. Cho, T. D. Senecal, T. Kinzel, Y. Zhang, D. A. Watson and S. L. Buchwald, Science, 2010, 328, 1679–1681 CrossRef CAS PubMed
.
- Y. Zeng, L. Zhang, Y. Zhao, C. Ni, J. Zhao and J. Hu, J. Am. Chem. Soc., 2013, 135, 2955–2958 CrossRef CAS PubMed
, and references therein.
- M.-H. Yang, D. L. Orsi and R. A. Altman, Angew. Chem., Int. Ed., 2015, 54, 2361–2365 CrossRef CAS PubMed
.
- B. Gao, Y. Zhao and J. Hu, Angew. Chem., Int. Ed., 2015, 54, 638–642 CAS
, and references therein.
- X. Jiang and M. Gandelman, J. Am. Chem. Soc., 2015, 137, 2542–2547 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |