Synthesis of biaryls using aryne intermediates
Abstract
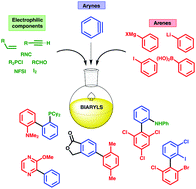
The synthesis of biaryls from benzyne intermediates offers an alternative strategy to conventional metal-catalyzed cross-coupling approaches. The concept is as old as benzyne itself, being the basis of Wittig's seminal observations on biphenyl synthesis from phenyl lithium and fluorobenzene in 1940. In the intervening 75 years, the transformation has grown to encompass a remarkable scope of reaction classes, and continues to develop as new benzyne precursors enable inventive biaryl syntheses under mild conditions. This review will cover all aryne methods relevant to biaryl synthesis, drawing together key ideas from the older literature involving halobenzene precursors, with a more comprehensive coverage of modern methods using 2-(trimethylsilyl)phenyl triflates and tri-ynes as the source of benzyne. Collectively, we hope to highlight the power of aryne chemistry to access a huge range of biaryl structures from a versatile and highly customizable set of substrates.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...