Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
New insights into the pretargeting approach to image and treat tumours
Malay
Patra
a,
Kristof
Zarschler
*b,
Hans-Jürgen
Pietzsch
b,
Holger
Stephan
b and
Gilles
Gasser
 *a
*a
aDepartment of Chemistry, University of Zurich, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland. E-mail: gilles.gasser@chem.uzh.ch; Web: www.gassergroup.com Tel: +41 44 635 46 30
bHelmholtz-Zentrum Dresden-Rossendorf, Institute of Radiopharmaceutical Cancer Research, Bautzner Landstraße 400, D-01328 Dresden, Germany. E-mail: k.zarschler@hzdr.de; Tel: +49 351 260 3678
First published on 27th September 2016
Abstract
Tumour pretargeting is a promising strategy for cancer diagnosis and therapy allowing for the rational use of long circulating, highly specific monoclonal antibodies (mAbs) for both non-invasive cancer radioimmunodetection (RID) and radioimmunotherapy (RIT). In contrast to conventional RID/RIT where the radionuclides and oncotropic vector molecules are delivered as presynthesised radioimmunoconjugates, the pretargeting approach is a multistep procedure that temporarily separates targeting of certain tumour-associated antigens from delivery of diagnostic or therapeutic radionuclides. In principle, unlabelled, highly tumour antigen specific mAb conjugates are, in a first step, administered into a patient. After injection, sufficient time is allowed for blood circulation, accumulation at the tumour site and subsequent elimination of excess mAb conjugates from the body. The small fast-clearing radiolabelled effector molecules with a complementary functionality directed to the prelocalised mAb conjugates are then administered in a second step. Due to its fast pharmacokinetics, the small effector molecules reach the malignant tissue quickly and bind the local mAb conjugates. Thereby, corresponding radioimmunoconjugates are formed in vivo and, consequently, radiation doses are deposited mainly locally. This procedure results in a much higher tumour/non-tumour (T/NT) ratio and is favourable for cancer diagnosis and therapy as it substantially minimises the radiation damage to non-tumour cells of healthy tissues. The pretargeting approach utilises specific non-covalent interactions (e.g. strept(avidin)/biotin) or covalent bond formations (e.g. inverse electron demand Diels–Alder reaction) between the tumour bound antibody and radiolabelled small molecules. This tutorial review descriptively presents this complex strategy, addresses the historical as well as recent preclinical and clinical advances and discusses the advantages and disadvantages of different available variations.
Key learning points(1) Monoclonal antibodies (mAbs) are excellent tools to target tumour associated antigens, but the straightforward approach of utilising directly radiolabelled mAbs for radioimmunodetection (RID) and radioimmunotherapy (RIT) of tumours is affected by their unfavourable biodistribution.(2) The pretargeting approach is a multistep procedure that temporarily separates targeting of tumour associated antigens by mAb conjugates from delivery of diagnostic or therapeutic radionuclides. (3) The pretargeting approach can boost the tumour/non-tumour ratio and thus limits the radiation dose for healthy tissues. (4) The pretargeting approach utilises specific non-covalent interactions (e.g. strept(avidin)/biotin or hybridisation of oligonucleotides) as well as covalent bond formations (e.g. inverse electron demand Diels–Alder reaction) between the tumour bound antibody conjugates and radiolabelled small molecules. |
1. Introduction
Early diagnoses, quantitative imaging, efficient treatment and precise monitoring of anticancer therapy require extremely sensitive detection methodologies such as the nuclear medicinal imaging techniques single-photon emission computing tomography (SPECT) and positron emission tomography (PET). In addition to these, the use of a highly tumour-specific vehicle labelled with a radionuclide as reporter for radioimmunodetection (RID) or warhead for radioimmunotherapy (RIT) is essential to achieve high sensitivity and specificity.1 These affine oncotropic vector molecules with appropriate pharmacokinetic and pharmacodynamic properties become increasingly important as an alternative to, or in combination with, conventional treatments such as surgery, chemotherapy and external radiotherapy.2Monoclonal antibodies (mAbs) bind cognate antigens with excellent specificity and thus represent indispensable molecules for therapeutic and diagnostic applications. Since their introduction more than 60 years ago, radiolabelled mAbs have been extensively evaluated in preclinical and clinical settings for delivering radioactivity to tumour sites to visualise, characterise, and treat tumour malignancies including primary and metastatic cancers.3 The potential of this so called conventional RID/RIT is evidenced by the approval of two radioimmunotherapeutic mAbs, namely ibritumomab tiuxetan (Zevalin®) and tositumomab (Bexxar®), by the U.S. Food and Drug Administration (FDA) for treatment of non-Hodgkin's lymphoma (NHL). While both Zevalin® and Bexxar® are composed of mAbs directed against the CD20 antigen which is overexpressed in B-cell lymphomas, they bear different radionuclides. In the clinic, the 111In-labelled form of Zevalin® is used for diagnostic imaging or RID, whereas 90Y-Zevalin® is used for RIT. Bexxar® was approved in combination with the radioisotope 131I for treatment of NHL.4 The principles, issues and challenges associated with RIT, radionuclides used in RIT as well as the latest progress in this field have been the subject of numerous comprehensive reviews,2,3,5,6 and the interested reader is directed to these to obtain further information on that particular topic.
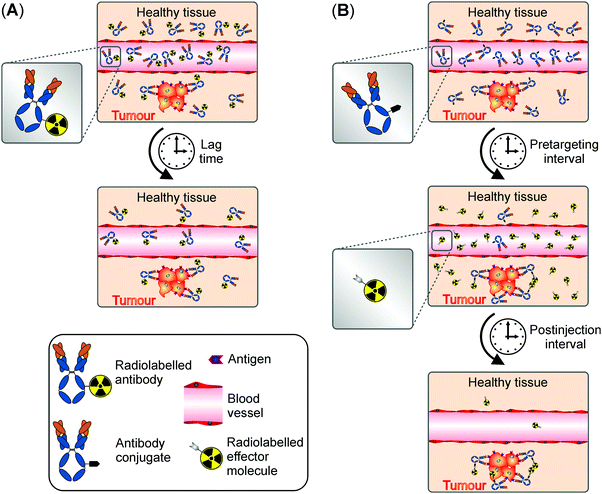
Despite their success in the clinic, the concept of utilising radioimmunotherapeutic mAbs is afflicted with several important shortcomings.4,7 mAbs are known for their high stability and prolonged circulation in the blood, slow biodistribution kinetics and slow clearance from the body. Due to the poor tissue penetration abilities of radiolabelled mAbs, a delayed clearance from the blood is required to achieve higher accumulation of radioactivity in tumours. But, at the same time, this prolong circulation appears as one of the most important drawbacks of conventional RID/RIT.3 It causes undesired radiation burden in healthy tissues, especially in the bone marrow, which is extremely sensitive to radiation. This limits the opportunity of enhancing the efficacy of therapy by increasing the dose of radiolabelled mAbs. Additionally, slow clearance of radiolabelled mAbs from blood and healthy tissues often results in a lower tumour/non-tumour (T/NT) ratio and – for RID – it requires extensive waiting periods before image acquisition. Rapid clearance of radiolabelled mAbs from the blood and non-targeted tissue is therefore beneficial in order to obtain a higher T/NT ratio. As a consequence, the pharmacokinetic properties of mAbs appear as the main dilemma in conventional RID/RIT approach.7 Fortunately, however, several of these shortcomings can be eliminated by implementing the pretargeting strategy allowing for the rational use of long circulating, high-affinity mAbs for both non-invasive cancer RID and RIT.8
2. Detailed explanation of the pretargeting strategy
In contrast to conventional RID/RIT where the radionuclides and oncotropic vector molecules are delivered as presynthesised radioimmunoconjugates (Fig. 1A), the pretargeting strategy is a multistep procedure that decouples targeting of tumour associated antigens from radionuclide delivery (Fig. 1B). All pretargeting systems share in principle two basic components: (i) a target specific component, often a non-radioactive mAb conjugate capable of binding a certain tumour antigen as well as (ii) a small radiolabelled effector component. As schematically represented in Fig. 1, unlabelled, highly tumour specific mAb conjugates are in a first step administrated into a patient. After injection, sufficient time, also referred to as pretargeting interval, is allowed for blood circulation, accumulation at the tumour site and subsequent elimination of excess mAb conjugates from the body. Importantly, as the mAb conjugates themselves are not radiolabelled, the pretargeting interval can be extended to several days for optimal T/NT ratios without the risk of radiation exposure to healthy tissues. At the time when the concentration of mAb conjugates at the tumour site is at its peak and much higher than in healthy tissues, small fast-clearing radiolabelled effector molecules with a complementary functionality directed to the prelocalised mAb conjugates are administered in a second step. Due to its fast pharmacokinetics, the small effector molecules reach the malignant tissue quickly and bind the local mAb conjugates, thereby forming radioimmunoconjugates in vivo. The excess effector molecules are rapidly excreted from the blood or healthy tissues, resulting in a higher T/NT ratio which is favourable for diagnostics and therapy. A hallmark of all pretargeting procedures therefore represents the minimised radiation exposure of non-targeted tissue as well as limited haematological toxicity.3 Following its initial demonstration by Reardan and his team in 1985,9 the pretargeting strategy has become an area of extensive research and extremely promising results have been reported over the last decades.10,11Although the pretargeting approach improves the T/NT ratio significantly over conventional RID/RIT, it should be, however, remembered that the pretargeting strategy comes with several complexities which are absent in conventional RID/RIT. Conventional targeting has two variables, namely the doses of radiolabelled mAbs and time of detection. In general, provided the mAb binding sites are unsaturated, the accumulation of radioactivity in tumour is only slightly influenced by the doses of the radiolabelled mAb but greatly varies with the detection time. The optimisation of conventional RID/RIT in clinical setting is therefore relatively easy. On the contrary, even the simplest two-step pretargeting strategy involves four variables, namely the doses of pretargeting mAb conjugates, the pretargeting interval, the doses of radiolabelled effector molecules and the time of detection. The accumulation of radioactivity, which is now not in the mAb but in the secondary effector agent, is highly influenced by the changes in any of these four variables. Additionally, there are three system-associated variables in the pretargeting approach (e.g. the mAb conjugates, the radiolabelled secondary effector agent and the type of tumour) compared to only two in conventional RID/RIT (e.g. the radiolabelled vector molecule and the type of tumour). Complexity further increases with increasing the number of steps, making the optimisation very difficult in clinical settings. The optimisation of a pretargeting system is carried out experimentally in practice by varying different variables using trial and error methods. The optimised parameters are also not broadly applicable to any system, but strictly specific to a particular pretargeting system. Therefore, optimisation of variables is required for each pretargeting system undergoing clinical trial and such optimisation is not very practical. Liu and Hnatowich proposed a semiempirical model for pretargeting RID/RIT, which is capable of estimating how the optimal tumour accumulation and T/NT ratio is influenced by the different combination of variables. The model is based on several quantitative equations relating the dependence of different pretargeting variables on the outcome. For the details of this model, from the design to the applicability, readers are directed to the excellent review published by the pioneer of this model.12 This semiempirical model has thus far been applied only to a MORFs/cMORFs (MORF = phosphodiamidate morpholino oligomers) pretargeting system. More and more experimental data on other pretargeting systems is required to predict the true applicability of this semiempirical model on the optimisation of pretargeting variables without an exhaustive trial and error method.
All the pretargeting systems introduced to date can be classified into two broad categories depending on the interactions between the pretargeted mAb conjugates and the radiolabelled small effector molecules: (i) non-covalent high affinity interaction and (ii) covalent bond formation using bioorthogonal chemistry. These two categories are presented in detail below.
3. Non-covalent high affinity interactions
3.1 Recognition systems based on bispecific antibodies
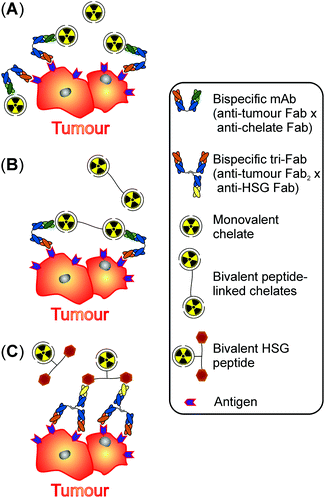
The initial pretargeting concept described more than 30 years ago pursued the strategy to apply bispecific (bs) monoclonal antibodies (mAbs) capable of binding both tumour antigens as well as radiolabelled chelates (Fig. 2).11The first generation of bs mAbs-based pretargeting systems originally involved bs mAbs consisting of a single anti-tumour Fab fragment that was chemically conjugated to a single anti-chelate Fab fragment resulting in both monovalent binding to the tumour antigen as well as to the radiometal–chelate complex (Fig. 2A).7 In the initial preclinical and clinical studies using this pretargeting system, Fab fragments against the carcinoembryonic antigen (CEA) were combined with Fab fragments binding to DTPA. Since antibodies bound to CEA do not readily internalise and thus remain accessible at the surface of tumour cells, this particular tumour antigen is especially suitable for pretargeting. Taking full advantage of this behaviour, an extended time period for clearance of the bs mAbs of up to eight days can be allowed before injection of the fast-clearing radiometal–chelate complexes. Compared to directly radiolabelled but long circulating anti-CEA IgG antibodies, improved tumour-to-blood and tumour-to-liver ratios were obtained using the two-step pretargeting method due to the fast clearance of the radiolabelled chelates.13 Although this pretargeting system was successfully tested in patients with primary colorectal cancer,14 the radiolabelled chelates were rapidly released from the tumour site due to their weak monovalent interactions with the pretargeted bs mAbs.
Higher tumour uptake and retention were achieved by gradually improving the different components of the monovalent binding system. The second generation of bs mAbs based pretargeting systems deployed short peptides linking two radiometal–chelate complexes instead of monomeric radiolabelled chelates (Fig. 2B).7 These bivalent probes interact with the anti-chelate Fab fragment of the prelocalised bs mAbs with enhanced avidity resulting in enhanced tumoural uptake and retention.11 The group of Kraeber-Bodéré intensively investigated this second generation pretargeting procedure regarding the treatment of CEA-producing medullary thyroid carcinoma (MTC) in several preclinical as well as clinical studies10 and critically compared the toxicity and efficacy of pretargeted RIT with those of conventional RIT.15
The first as well as second generation of bs mAbs-based pretargeting systems utilised anti-chelate antibodies with an exceptional specificity for a certain radiometal–chelate complex, e.g.111In-loaded DTPA. The major drawback of these approaches was that the binding affinity dropped substantially, when other radiometals were complexed by the same chelator as observed for 90Y– or 177Lu–DTPA. This limited flexibility necessitated, in the worst case, the generation of new antibodies with high affinity for the desired radiometal–chelate pairs, e.g. directed against 90Y– or 177Lu–DOTA, in order to expand these pretargeting systems to a wide variety of radionuclides.13
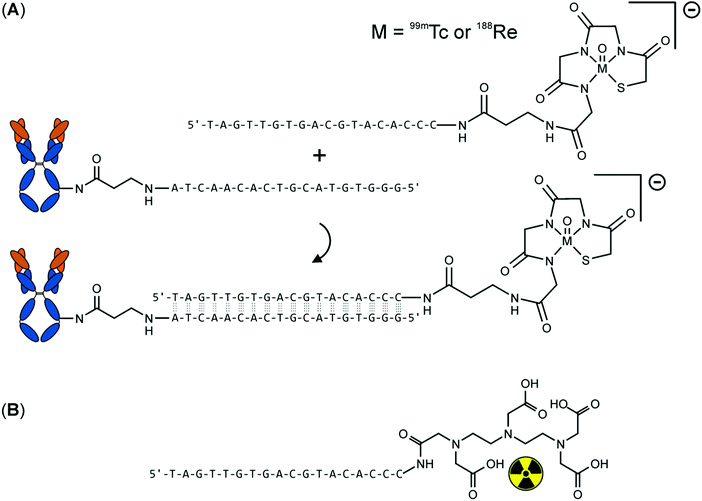
To circumvent this limitation, the anti-chelate Fab fragment of the bs mAbs was replaced by a Fab fragment binding to an inert compound composed of histamine-succinyl-glycine (HSG) with nanomolar affinity. An additional small peptide core of three to four D-amino acids serves on one hand as a metabolically stable scaffold to link two HSG moieties resulting in a bivalent HSG-peptide. On the other, the peptide can be modified in different ways to attach diverse ligands capable of binding the radionuclide of choice. This included the (3-thiosemicarbazonyl)glyoxylcysteinyl (Tscg-Cys) moiety for complexing of 99mTc and 188Re as well as DOTA for chelating 111In, 68Ga, 177Lu and 90Y for diagnostic or therapeutic applications, respectively. Furthermore, incorporation of a tyrosine residue into the peptide sequence also allowed for radioiodination.11
For the third generation of bs mAbs-based pretargeting systems, bs mAbs with two binding sites for the tumour antigen and one for the HSG-peptide were prepared by a modular approach termed Dock-and-Lock procedure (Fig. 2C).7 This innovative and sophisticated system stably joins one Fab fragment of an anti-HSG antibody and two Fab fragments of an anti-tumour antibody (tri-Fab bs mAbs) in a manner to allow for bivalent binding to the tumour antigen and monovalent binding of the radiolabelled HSG-peptide. Due to its bivalency, a single HSG-peptide can crosslink two nearby tri-Fab bs mAbs at the surface of tumour cells leading to the formation of a stable complex. For detailed information on the Dock-and-Lock method the interested reader is directed to an excellent review of Rossi et al.16
Several clinical trials for identifying optimal treatment parameters and assessing safety as well as tolerance of the third generation of bs mAbs-based pretargeting system in humans were started in patients with metastatic colorectal and lung cancer as well as medullary thyroid carcinoma, respectively. The corresponding results of these optimisation studies using anti-CEA tri-Fab bs mAbs in combination with 111In and 68Ga (for imaging) or 177Lu (for therapy) labelled bivalent HSG-peptides were reported recently.17–20 Points of particular note are in this context the fast and selective tumour visualisation within hours after injection of the radiolabelled di-HSG-peptides subsequently to pretargeting with the anti-CEA tri-Fab bs mAbs as well as fast clearance from non-targeted tissues and organs resulting in high tumour-to-normal tissue ratios. Moreover, injected radiation doses of 177Lu ranging from 2.5–7.4 GBq resulted only in limited haematological toxicity, and no signs or symptoms of renal toxicity were observed.
Of possible importance for repeated administration of bs mAbs is the appearance of immune responses in a number of patients such as infusion reactions and the formation of human antibodies against the tri-Fab bs mAbs although humanised recombinant antibody constructs were injected. The presence of such antibodies during a therapy cycle may affect the pharmacokinetics of the bs mAbs and influence the quality of tumour targeting, whereas the former adverse event is manageable by appropriate anti-allergic premedication with antihistamines and corticosteroids injected.17,19
In summary, the bs mAbs-based pretargeting systems have been intensively investigated in preclinical as well as several clinical studies for RID and RIT of cancer and documented in detail. These systems appear on one hand suitable for RID by immuno-PET, when the effector molecules are labelled with short half-life positron-emitters such as 68Ga or 64Cu. On the other hand, ongoing clinical trials will analyse their performance for RIT as promising preclinical studies have demonstrated the very fast delivery of high radiation doses to tumour tissues with limited exposure of non-targeted tissues.
3.2 Avidin/streptavidin–biotin recognition system
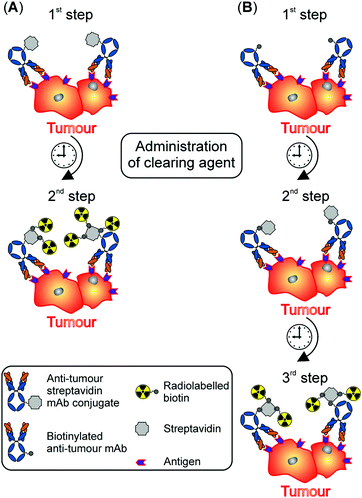
The avidin/streptavidin–biotin system relies on the very high affinity of (strept)avidin for biotin and was initially introduced by Donald J. Hnatowich and members of his group.21 Up to four biotin molecules (also known as vitamin B7, vitamin H or coenzyme R) can bind to the ∼66 kDa glycoprotein avidin with an dissociation constant Kd of 10−15 M. This is one of the strongest known non-covalent interactions, whereas their association is rapid, and, once formed, this complex is stable to extremes of pH and in the presence of denaturing agents or organic solvents. Of note, the non-glycosylated bacterial analogue of avidin, streptavidin (∼56 kDa), is interchangeable with avidin in in vitro studies. However, for in vivo applications, the difference in glycosylation between the two proteins makes them suitable for different purposes as naturally glycosylated avidin, in contrast to non-glycosylated streptavidin, is rapidly sequestered by the liver.22 Worthy of note, streptavidin was reported to show less non-specific binding to tissues compared to avidin.21 The avidin/streptavidin–biotin system requires either the conjugation of (strept)avidin to mAbs or the generation of biotinylated mAbs. Both configurations have been reported frequently and differ in their application regime.22The two-step method starts with the intravenous injection of anti-tumour streptavidin mAb conjugates (Fig. 3A). Their high molecular weight (∼210 kDa) results in a slow blood clearance and necessitates the removal of excess streptavidin mAb conjugates from the blood pool by administration of a clearing agent prior to the injection of the radiolabelled biotin molecules. Once the concentration of the streptavidin mAb conjugates peaks at the tumour site, the clearing agent (e.g. biotinylated galactose-conjugated human serum albumin) is given to transport circulating streptavidin mAb conjugates to the liver, where they are metabolised and withdrawn from circulation.22
In contrast, the three-step method of the avidin/streptavidin–biotin system uses biotinylated antitumour mAbs and glycosylated avidin as clearing agent, which is given once the biotinylated mAbs have achieved maximum tumour uptake (Fig. 3B). After the circulating complex composed of avidin and biotinylated mAbs is transported to the liver, unlabelled streptavidin is administered. Upon reaching the malignant tissue, these multivalent proteins strongly bind to the prelocalised biotinylated mAbs. Finally, radiolabelled biotin molecules are injected and interact with tumour-bound complexes of streptavidin and biotinylated mAbs.22
In their seminal paper, Hnatowich and co-workers investigated the in vivo application of mAbs linked to (strept)avidin or biotin followed by the administration of 111In-labelled (strept)avidin or biotin. They could demonstrate that the intravenous administration at first of avidin mAb conjugates followed by radiolabelled biotin was more beneficial than the reverse procedure (i.e. injection of biotin mAb conjugates followed by radiolabelled streptavidin). Indeed, the authors observed an unfavourable slow blood clearance of 111In-labelled streptavidin and its accumulation in normal tissue. Promisingly, using protein-A conjugated beads to simulate tumours, Hnatowich and his team showed that T/NT ratios were increased by more than two orders of magnitude over the conventional use of a single intravenous administration with radiolabelled mAb.21
Following this pioneering work, several research groups have employed this in vivo recognition system in view of imaging and treatment of tumours using different radionuclides (e.g.111In, 153Sm, 90Y, 177Lu and 211At).23,24
Due to space constrains, it is impossible to report individually each study on this recognition system. Thus, we have decided, in the next paragraph, to only highlight the results of clinical studies that have utilised the avidin/streptavidin–biotin system in pretargeting. For detailed information on the application of this recognition system in cancer diagnosis and therapy, the interested reader is directed to two excellent reviews by Lesch et al. and Stoldt et al., respectively, as well as references therein.23,24
To the best of our knowledge, Hnatowich and his team were the first researchers to report a clinical study. Specifically, streptavidin was conjugated to mouse mAbs directed against a component of human milk fat globule membranes and reacting strongly with a range of neoplasms. These streptavidin mAb conjugates were intravenously administered to ten patients with documented squamous cell carcinoma of the lung. Two to three days later, 111In-labelled biotin was injected and patients were imaged 2 h after the administration for detection of lung cancer lesions. Importantly, no toxicity for the patients at the doses tested was evidenced in this study. Background radioactivity levels were found to be low in liver and kidneys as well as in other normal tissues and blood. In fact, the radioactivity levels in liver and kidney were improved by at least one order of magnitude and by a factor of five, respectively, compared to conventional approach using directly radiolabelled mAbs.23
Shortly afterwards, the Milanese group of Giovanni Paganelli and co-workers described two diagnostic clinical trials on the two-step as well as three-step method of the avidin/streptavidin–biotin system. The former study was conducted on ovarian cancer patients and the latter focussed on carcinoembryonic antigen-positive patients. Both trials utilised biotinylated mAbs and provided a deep insight into pharmacokinetics, biodistribution and tumour localisation. In particular, their elaborated three-step pretargeting protocol avoids the issues of slow blood clearance of radiolabelled (strept)avidin and its accumulation in normal tissues. According to the authors, the advantages of this three-step method over other pretargeting approaches or direct radiolabelling of mAbs are manifold. Firstly, the small size of the radiolabelled biotin molecules ensures their rapid renal elimination and allows for diagnostic imaging already shortly after injection. As the retention time in the body is thereby reduced, the radiation exposure of liver and bone marrow is minimised. Secondly, circulating biotinylated antitumour mAbs interact with the clearing agent avidin and both molecules are metabolised by the mononuclear phagocyte system of the liver as non-radioactive complex leading to background reduction. Thirdly, the mild reaction conditions applied for biotinylation of the mAbs preserve their immunoreactivity. As the mAbs are not directly labelled, also their radiolysis is prevented. Fourthly, signal amplification can potentially be achieved through the multivalency of streptavidin. In theory, multiple streptavidin molecules can bind to prelocalised biotinylated mAbs and multiple radiolabelled biotin molecules can finally interact with tumour-bound complexes of streptavidin and biotinylated mAbs. And fifthly, the three-step pretargeting protocol resulted in substantial improvement of T/NT ratios within a few hours after injection of the radiolabelled effector molecules expanding the range of suitable radionuclides.24
Finally, the authors mentioned that their technique presents a few drawbacks, namely repeated injections at defined time intervals and the immunogenicity of avidin. The latter, very important point will be discussed later in this section of the review.
The same research group conducted in the following years several diagnostic and therapeutic studies on patients with different cancer entities including head and neck cancer as well as glioblastoma.13,25 Of note, the delivery of therapeutic radionuclides, in particular 90Y, by the three-step method of the avidin/streptavidin–biotin system in patients with high-grade glioma resulted in tumour mass reduction and survival benefit. In comparison to conventional RIT using directly labelled mAbs, this pretargeting approach indeed allows for the administration of high 90Y activities enabling delivery of a high dose to the tumour and sparing red marrow and other normal organs.13
At this stage, although promising clinical trials were indeed undertaken, as already discussed above, it is important to highlight that the avidin/streptavidin–biotin recognition system still presents several drawbacks. As mentioned by Goldenberg et al., who debated with Giovanni Paganelli and Marco Chinol on the viability of this recognition system in pretargeting, immunogenicity is problematic.26,27 The immune system of patients recognises the foreign biotin and produces antibodies against the biotin after the first injection. Upon a second application, the biotin can initiate a severe immune reaction. Also, the production of the antibody–protein conjugate is relatively complicated and its diffusion/adsorption is relatively slow due to its relatively large size.
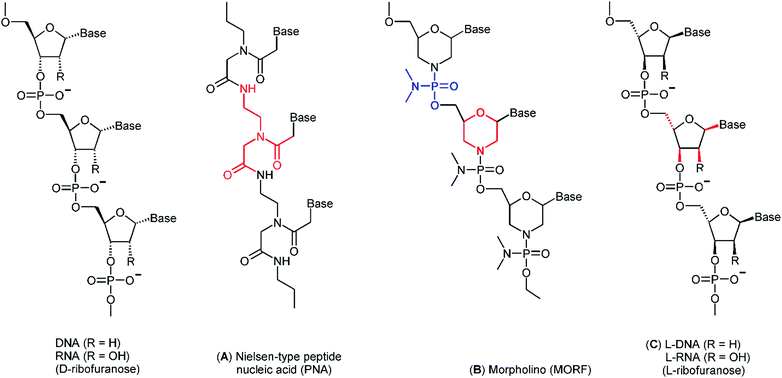
3.3 Recognition systems based on in vivo hybridisation of synthetic complementary oligonucleotides
DNA as well as RNA oligonucleotides of a certain sequence rapidly bind their complementary strands with a very high specificity and affinity. However, natural occurring nucleic acids are highly susceptible to nuclease-mediated hydrolysis and possess therefore an insufficient in vivo stability. For that reason, several synthetic non-immunogenic DNA analogues with excellent chemical and metabolic stability have been developed as recognition systems for tumour pretargeting including peptide nucleic acids, phosphorodiamidate morpholino oligomer and mirror-imaged oligonucleotides (Fig. 4).The anti-CEA antibody MN14, conjugated with amine-modified MORF, hybridises with 99mTc-labelled MAG3–cMORF-conjugate to achieve pretargeting in tumour-bearing mice. MAG3 (mercaptoacetyl triglycine) is a tripeptide chelator especially designed for stable binding of 99mTc in its oxidation state +5. Biodistribution studies demonstrated good radioactivity uptake in tumour. However, the 99mTc-labelled cMORF effector molecule also accumulated in the kidneys and intestines. This accumulation may be problematic for tumour imaging (or tumour therapy) within the abdomen. To overcome this obstacle, changing the radionuclide, and consequently, the chelator can be considered. It is well-documented that radiolabelling with different radionuclides via different chelators may alter the pharmacokinetics of even large molecules such as peptides or antibodies. Consequently, Hnatowich et al. compared pharmacokinetics of 99mTc–MAG3–cMORF and 111In–DTPA–cMORF in normal mice in a further study.29 Therein tumour-bearing mice were pretargeted with the appropriate MORF-antibody. 48 h later 99mTc–MAG3–cMORF and 111In–DTPA–cMORF, respectively, were then injected and the mice were reimaged. The authors clearly demonstrated that the biodistribution of the cMORF effector was influenced by the appropriate chelate unit both in normal and pretargeted tumour-bearing mice. As illustrated in Fig. 6, accumulation of 111In–DTPA–cMORF in the intestines was minimal compared with the intestinal accumulation of 99mTc–MAG3–cMORF.
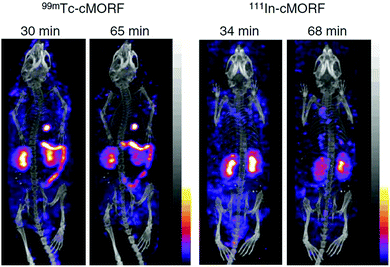 | ||
| Fig. 6 Anterior projections of the SPECT/CT fused acquisitions obtained at two time points in normal mice receiving either 99mTc–cMORF (left panel) or 111In–cMORF (right panel). Bladder 99mTc and 111In radioactivity were digitally removed in all projections, as was the 111In contamination on the animal's coat in the 68 min 111In projection. Adapted with permission from ref. 29, copyright Springer. | ||
After demonstrating the rapid tumour accumulation and normal tissue clearance of the 99mTc-labelled MAG3–cMORF/MN14–MORF pretargeting system, Hnatowich et al. reported the first preclinical mouse-model of MORF pretargeting for RIT using the β−-emitting radionuclide 188Re. 188Re was selected as the therapeutic radionuclide because of its attractive properties for radiotherapy. It is a generator-based nuclide with a half-life of 17 h and an average β-energy of about 2 MeV. A γ-radiation component of 0.155 MeV allows for SPECT imaging. Furthermore, diagnostic 99mTc and therapeutic 188Re form a so-called “matched pair” of radionuclides showing similar coordination properties. This similarity often results in radiolabelled agents with similar, if not identical, properties.
The proof-of-principle study was performed using 188Re–MAG3–cMORF/MN14–MORF with 14.8 GBq 188Re per mouse and three control groups (untreated mouse, injection of MN14–MORF alone, injection of 188Re–MAG3–cMORF alone) and resulted in fast accumulation of 188Re in tumour accompanied by rapid clearance from normal tissues. The tumour accumulation was about four times higher than in the kidney and blood, which were the healthy tissues with highest radioactivity uptake. Tumour growth in the study group ceased one day after radioactivity injection, whereas tumours continued to grow at the same rate among the three control groups. The therapeutic success was confirmed by measuring the tumour size. On the fifth day after treatment, the average net tumour weight was significantly lower for the study group compared with the three control groups.30
A second preclinical study treating LS174T-tumour bearing mice using 188Re–MAG3–cMORF and an MORF-modified antiTAG-72 mAb CC49 was published by Hnatowich et al. four years later. As shown by SPECT/CT, the radioactivity uptake was highly tumour specific for the two time points given: 3.5 and 10 h after injection (Fig. 7). Except for the intense urine radioactivity at 3.5 h, the radioactivity is essentially restricted to tumour at both time points. Tumour growth was inhibited for the treated mice and at the highest 188Re dose, a complete but temporary tumour remission was evident in three out of five animals. Histological examination of tissues from these animals showed no evidence of cytotoxicity to normal tissues but obvious radiation damage to the tumour.31
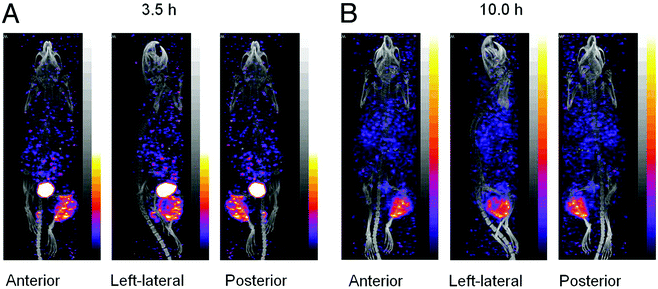 | ||
| Fig. 7 Anterior, left lateral and posterior projections for each SPECT/CT acquisition of one pretargeted mouse imaged at 3.5 h (A) and 10 h (B) after injection of 188Re–cMORF. Adapted with permission from ref. 31, copyright Taylor & Francis Ltd. | ||
From a radiopharmaceutical point of view, the trivalent metals In3+ and Y3+ constitute another “matched pair” of radionuclides. 90Y is considered as an alternative to 188Re with comparable β−-energy but of longer half-life (2.2 MeV, 64 h) that can potentially improve radiotherapy effectiveness. 90Y will provide higher tumour-to-organ absorbed radiation dose because a larger portion of the circulating 90Y will be cleared without depositing its energy in the normal tissues.
Based on the pretargeting study described above using 188Re–MAG3–cMORF, Hnatowich and co-workers performed a therapeutic pretargeting experiment using 90Y-labelled cMORF sequences.32 Due to the different coordination chemistry of yttrium compared to rhenium, the chelator MAG3 was substituted by the macrocyclic ligand DOTA forming a 90Y–DOTA–cMORF/MORF–CC49 pretargeting system. The biodistribution was measured in both normal and LS174T-tumour bearing mice, and the pharmacokinetics of the 90Y–DOTA–cMORF in both the normal the pretargeted mice was comparable to that observed previously with 188Re–MAG3–cMORFs. While the 90Y–DOTA–cMORF cleared rapidly from normal tissues, tumour clearance was very slow and tumour radioactivity accumulation was constant for at least seven days allowing for the T/B ratio to increase linearly from 6 to 25 over this period. Therefore, by extrapolation, normal tissue toxicities following administration of therapeutic doses of 90Y may be comparable to that observed for 188Re in which the T/B ratio increased from 5 to 20 over a 7 day period. Furthermore, due to the longer physical half-life, the tumour-to-non-tumour absorbed radiation dose ratios were improved in most organs and especially in blood.
Of note, the authors clearly showed that both the pharmacokinetics and pretargeting behaviour of the cMORF effector is generally not influenced by the nature of the chelator and the radionuclide using 188Re–MAG3–cMORF and 90Y–DOTA–cMORF.
In summary, the preclinical studies performed by the group of Hnatowich have clearly demonstrated the suitability and effectiveness of the morpholino-based pretargeting system, with high T/B ratios but no clinical trials have been initiated yet.
The potential of PNAs as an efficient pretargeting system has been explored by the group of Donald J. Hnatowich for the first time in 1997.36 A 15-mer PNA oligomer equipped with MAG3 as chelator was radiolabelled with 99mTc to show rapid renal clearance in mice. In vivo hybridisation was confirmed using polymeric beads transplanted into mouse thighs that contain the complementary PNA strand. The highest amount of radioactivity was found after 23 h in the transplanted beads. At this time, radioactivity could only be detected in beads, kidneys and bladder.36
We recently described active tumour pretargeting with PNA bioconjugates as complementary system. More specifically, we modified on one hand epidermal growth factor receptor (EGFR) specific therapeutic mAbs (cetuximab) with PNA oligomers consisting of 17 bases. On the other, we attached dipicolyl (DPA: N,N-bis(2-picolyl)amine) chelators to complementary PEGylated PNA oligomers with optimised pharmacological properties.37 It is worth highlighting that PEGylation of complementary effector PNAs led to an increased blood residence time without substantially influencing their hybridisation properties. Furthermore, these PNA conjugates rapidly cleared from the body via the kidneys and already 1 h postinjection, radioactivity was mostly detected in the kidneys and bladder (Fig. 8A). Very importantly, rapid and efficient hybridisation of the complementary 99mTc-labelled effector PNAs with pretargeted mAb–PNA conjugates was observed in in vivo experiments using EGFR-positive tumour xenografts (Fig. 8B and C). This active tumour pretargeting approach permits a clear visualisation of the tumour just 1 h postinjection of the radiolabelled effector, achieving tumour-to-muscle ratios >8.37
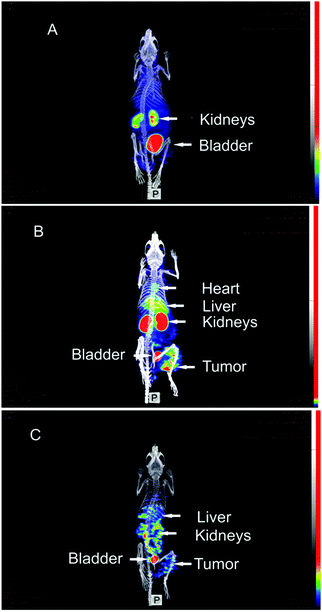 | ||
| Fig. 8 SPECT/CT maximum intensity projection images of [99mTc](Tc-Dpa)–(Cys-PEG10kDa)–PNA in murine A431 tumour xenograft (NMRI nu/nu mice; tumour located at right thigh). (A) 1 h postinjection of [99mTc](Tc-Dpa)–(Cys-PEG10kDa)–PNA without preinjection of (NOTA)3-C225-Cys-c-PNA. (B) 1 h post injection of radiotracer; (NOTA)3-C225-Cys-c-PNA was administered 24 h before injection of radiotracer. (C) 20 h postinjection of radiotracer; (NOTA)3-C225-Cys-c-PNA was administered 24 h before injection of radiotracer. Adapted with permission from ref. 37, copyright Royal Society of Chemistry. | ||
Altogether, PNAs are promising candidates for further preclinical studies. In this perspective, it is advantageous that PNA derivatives can be produced with a PNA synthesiser, allowing for rapid access to a broad range of PNA constructs in an appropriate amount.
Schlesinger et al. described the synthesis and radiopharmacological characterisation of 68Ga- and 86Y-labelled DOTA–12mer-L-RNA conjugates as molecular probes for PET.39 Biodistribution studies of the 86Y-labelled 12mer-L-RNA oligonucleotide in healthy male Wistar rats showed very fast renal elimination and low retention of the compound in tissues and organs. The same group investigated the influence of the 86Y–DOTA chelate moiety on the biodistribution of the 86Y–DOTA–12mer-L-RNA conjugate in healthy rats.40
The very fast blood clearance combined with high kidney accumulation of radiolabelled L-oligonucleotides requires optimisation to design an efficient pretargeting system. The objective was to minimise the kidney uptake and non-specific accumulation, and to increase the blood availability of radiolabelled L-oligonucleotides. Introduction of high-molecular-weight polyethylene glycol (PEG) moieties into a pharmacologically active substance is a well-established tool to influence its pharmacokinetics. Therefore, Foerster et al. investigated the impact of increasing (2, 5, 10 and 20 kDa) PEG sizes of 68Ga- and 64Cu-labelled L-oligonucleotide–PEG conjugates on their distribution and PET kinetics in Wistar rats.41 It could be demonstrated that tuning of pharmacokinetics is possible by successive PEGylation resulting in a series of suitable L-oligonucleotides with reduced kidney uptake, favourable half-life of circulation and very low unspecific tissue accumulations. The introduction of NOTA or DOTA as chelating moieties had no influence on the pharmacokinetics and allows radiolabelling with various metallic radionuclides for theranostic applications.
To conclude, the use of mirror-imaged oligonucleotides as recognition units in pretargeting systems showed great promise but is only in its infancy. Further studies are definitely required concerning dosimetry and proof-of-feasibility in vivo.
4. Covalent bond formation using bioorthogonal chemistry
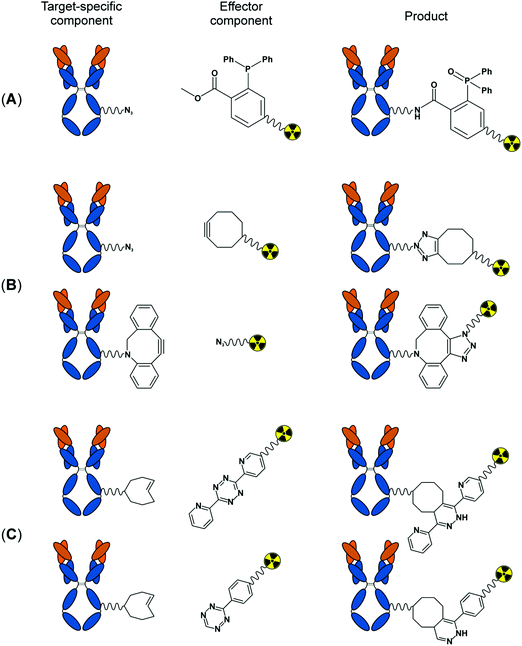
All pretargeting systems evaluated in the past are promising but each of them has specific advantages as well as limitations. For example, the streptavidin and biotin couple offers the highest binding affinity and was found to be one of the promising pretargeting systems thus far in clinical settings. However, as discussed in more detail above, the system is not free from limitations, such as the induction of immunogenicity caused by the administration of streptavidin. Moreover, complexity in engineering, the high cost of production of streptavidin or of the biotin-attached mAb, and the perturbation of antibody affinity while attached to streptavidin are additional drawbacks of this system.42In the recent past, bioorthogonal reactions have been tested as an alternative to non-covalent interactions in the pretargeting strategy. These chemical procedures are relatively straightforward, less likely to induce immunogenicity and rely on covalent bond formation between the tumour bound pretargeted mAbs and the radiolabelled effectors. In this context, it is important to note that a chemical reaction needs to meet certain criteria to become truly bioorthogonal and to be useful for this purpose. Those criteria are, for example, a high stability of the reaction components in aqueous/biological media, a fast ligation kinetics even at very low reagents concentration, an occurrence without external reagent/catalyst, a selective reaction between the components and no cross reaction with biological reactants in cellular environment, a good yield and no generation of toxic by-products.43 A few click chemistry reactions, namely, the Staudinger ligation, the strain promoted alkyne–azide cycloaddition (SPAAC) and the inverse electron demand Diels–Alder reaction (IEDAA), satisfy most of the above criteria and have been evaluated for their potential in pretargeting in cultured cells as well as in mice (Fig. 9). The progress made especially with tumour pretargeting using bioorthogonal chemistry was summarised in a few excellent reviews published recently.42,44,45 We therefore decided to limit our discussion to brief overviews of the historical developments and a few specific landmark studies on the pretargeting strategy using bioorthogonal reactions. Additionally, we cover the very recent developments on bioorthogonal tumour pretargeting that were not included in the previous review articles.
4.1 Staudinger ligation
In 1919, Staudinger and Meyer reported for the first time a reaction between an azide and a triphenylphosphine resulting in an iminophosphorane intermediate which undergoes hydrolysis spontaneously in the presence of water to give a primary amine and corresponding phosphine oxide. More than 80 years later, Bertozzi and co-workers were the first to realise the potential of this reaction for biological applications.43 The classical Staudinger reaction satisfy many of the above mentioned criteria: it occurs rapidly in water at room temperature and both reactants are abiotic and react selectively without cross-reaction with biological media or cell surface proteins. Therefore, this reaction holds the promise for being applicable in biological environments. However, the main limitation of this reaction was the aqueous instability of the iminophosphorane covalent adduct, that hydrolyses in the presence of water. Using an engineered triarylphosphine consisting of an ester group as electrophilic trap within the phosphine, Bertozzi and colleagues were able to trap the iminophosphorane intermediate intramolecularly, so that it results in a stable covalent adduct after the rearrangement. Incorporation of this specific modification transformed the Staudinger reaction into the Staudinger ligation which is sometimes also termed as the Staudinger–Bertozzi ligation (Fig. 9A).43 Over the years, this click reaction became a very popular tool and found a widespread use including metabolic labelling of cell surfaces in vitro and in vivo.43In 2011, van Dongen and co-workers were the first to make an attempt to evaluate the possibility of the application of the Staudinger ligation for tumour pretargeting. The authors therefore modified anti-CD44v6 mAbs with several triazide functionalities and developed a series of rapidly clearing radiolabelled phosphines as effector molecules. In order to evaluate the ligation efficiency in vivo, radiolabelled phosphines were injected in non-tumour bearing mice 2 h after the administration of triazide–mAbs. Unfortunately, no evidence of in vivo Staudinger ligation was detected. These negative results presumably originate from low reaction rates of the Staudinger ligation at low reagent concentrations. Moreover, rapid oxidation of phosphine to unreactive phosphine oxide in the blood was also observed representing another concern of this ligation strategy.45
4.2 Strain promoted alkyne–azide cycloaddition (SPAAC)
A click reaction between cyclooctyne and azide to form a 1,2,3-triazole unit was first reported by Krebs and Wittig in 1961 (Fig. 9B). The reaction is analogues to the original copper mediated Huisgen [3+2] alkyne–azide cycloaddition. However, it proceeds without the need of Cu, which is toxic to living tissues, but via the release of the strain of the cyclooctyne moiety. The reaction is termed as strain promoted alkyne–azide cycloaddition (SPAAC). Furthermore, the reagents are more compatible to in vivo conditions and the rate of the reaction is superior compared to the Staudinger ligation.43 At the moment, to the best of our knowledge, there are only two reports where SPAAC was evaluated for tumour pretargeting and the findings were described in recent reviews.42,45In 2013, the group of Rossin and Robillard prepared triazide-conjugated anti-CD20 mAbs (Rituximab) as well as a number of 177Lu-labelled DOTA-conjugated cyclooctyne derivatives to assess the reactivity profile of the triazide–mAbs and the radiolabelled effector probes in the circulation in non-tumour-bearing mice. However, the low in vivo reactivity of the generated cyclooctyne effectors towards azides and their covalent and non-covalent interactions with serum proteins impeded a sufficient bond formation between the azido-conjugated mAbs and the 177Lu-probes in this study.42,45 Soon after that, a report by Kim and co-workers demonstrated that SPAAC indeed holds promises for application in tumour pretargeting. In contrast to the study of Rossin and Robillard, the authors did not use mAb conjugates to pretarget the tumour but mesoporous silica nanoparticles that passively accumulate and retain in tumours through the enhanced permeability and retention (EPR) effect. These nanoparticles were PEGylated as well as functionalised with dibenzocyclooctyne (DBCO) and subsequently intravenously administered into tumour-bearing mice. After a pretargeting interval of 24 h, the radiolabelled effectors, 18F-labelled azides, were injected. Following the postinjection interval, PET-CT imaging indicated an accumulation of radioactivity at the tumour side in mice pretargeted with the nanoparticles, whereas much less radioactivity was deposited in the tumours of mice without nanoparticle pretreatment.42,45 This later study provides a good indication that SPAAC could, indeed, be used for tumour pretargeting provided an appropriate cyclooctyne moiety with favourable reaction kinetics is chosen and a suitable protocol is applied.
4.3 Inverse electron demand Diels–Alder reaction (IEDDA)
In 2008, the bioorthogonality of inverse electron demand Diels–Alder reaction (IEDDA) between trans-cyclooctene (TCO) and tetrazine (Tz) forming pyridazines (Fig. 9C) was disclosed for the first time.43 The reaction proceeds with a fast rate without the need of a catalyst, in water, cell media, or in cell lysate and was found to have a high functional group tolerance. In fact, IEDDA was shown to be the most promising among the three bioorthogonal reactions investigated in tumour pretargeting, especially in vivo, to date. Despite promising results of IEDDA-mediated pretargeting in cultured cells, its successful translation into animal requires the IEDDA to satisfy more demanding criteria: (i) very fast reaction kinetics at low reagent concentrations, (ii) high stability of TCO and Tz moiety in vivo, (iii) prolong circulation of TCO tagged mAbs, (iv) faster accessibility to the tumour tissue and (v) rapid clearance of the circulating Tz-radiolabelled effector molecule from the body.The in vivo application of IEDDA for pretargeted radiodiagnostic imaging was first demonstrated in a landmark study by Robillard and co-workers in 2010. Therein the authors described the preparation of TCO-conjugated mAbs (CC49) directed against the pan-adenoma TAG-72 antigen as target specific component. 111In-labelled DOTA-Tz probes represent the small, fast clearing effector component of this pretargeting approach. The CC49–TCO conjugates were injected into mice bearing colon cancer xenografts and a pretargeting interval of 1 day was set to allow for their localisation to the malignant tissue. Upon injection of the radiolabelled Tz derivative, the two complementary bioorthogonal reactants rapidly reacted in vivo and SPECT/CT imaging of live mice 3 h postinjection revealed a pronounced localisation of radioactivity in the tumour and in the urinary bladder with limited uptake in blood and non-targeted tissues, such as the liver.45
Over the last few years, significant modifications have been introduced to this strategy in order to achieve improved T/NT ratio. For example, TCO and Tz components have been modified to enhance the rate of ligation, a chaser (clearing reagents) has been introduced before administration of the radiolabelled effectors to clear the circulating antibody–TCO conjugates from the blood, etc. For details on these advancements up to the year 2014, we direct the readers to consult the excellent review articles published by Knight and Cornelissen as well as Rossin and Robillard.42,45
Since the last review article in this field, there have been a few more studies published on this rapidly growing topic. The group of Jason S. Lewis at the Memorial Sloan Kettering Cancer Center in New York developed a pretargeted PET imaging strategy based on IEDDA. Members of this laboratory have recently developed two novel 64Cu-labelled Tz ligands, 64Cu-Tz-PEG7-NOTA and 64Cu-Tz-SarAr. Among these two, the later possesses excellent pharmacokinetic properties with rapid renal clearance. In combination with a TCO-modified conjugate of the colorectal cancer-targeting huA33 mAb, 64Cu-Tz-SarAr was shown to efficiently delineate malignant tissues in murine xenografts with a high tumour to background contrast in pretargeted PET imaging and biodistribution experiments. Importantly, the dosimetry calculations performed in this study suggested that the total effective radiation dose is significantly lower compared to doses deposited by imaging using directly radiolabelled mAbs.46
The same group reported recently on the development of a novel 18F-based pretargeted PET imaging system. Therein the authors described the production of immunoconjugates composed of TCO moieties and mAbs (5B1) specific for the carbohydrate antigen 19.9 (CA19.9), a biomarker for pancreatic ductal adenocarcinoma. As complementary bioorthogonal species to 5B1-TCO, aluminium-[18F]fluoride-NOTA-labelled Tz ligands were synthesised and injected into CA19.9-expressing murine xenografts 72 h after the administration of 5B1-TCO. Tumoural uptake of the radiolabelled effector was immediately evident already 1 h postinjection and tumour-to-background activity concentration ratios improved over time.47
Recently, different nanoparticles have been proposed to replace tumour antigen specific mAbs as target specific component to target a broad range of different tumour entities.48 Such nanoparticulate materials with in vivo hydrodynamic diameters above the glomerular filtration threshold and prolonged blood circulation passively accumulate in tumours through the EPR effect.49 This process depends on their leaky vasculature and a poorly functional lymph system and results in increased permeability to nanomaterials.
A new IEDDA-based pretargeting approach using nanoparticles was very recently introduced by Lin and co-workers. Instead of using TCO-modified immunoconjugates, the TCO reactive moiety was encapsulated into supramolecular nanoparticles with a uniform size of ∼100 nm. Small and fast clearing effector possessing the complementary bioorthogonal motif Tz-DOTA was synthesised and radiolabelled with 64Cu. Their in vivo pretargeting experiment started with intravenous injection of the TCO-functionalised nanoparticles into xenografted mice. After a pretargeting interval of 24 h, radiolabelled Tz-DOTA was administered and various PET images were taken at different time points (Fig. 10). Strong PET signals were observed at the tumour site with highest tumour to liver signal ratio after 24 h postinjection. Notwithstanding this impressive performance, also radioactivity levels in the liver were observed, most likely due to trapping of the nanoparticles by the mononuclear phagocyte system (MPS) or transchelation of 64Cu from DOTA in vivo.48 Although the EPR effect is a common phenomenon in rodent models using subcutaneous and orthotopic xenografts, its occurrence in human primary and metastatic lesions may be less widespread and less pronounced. As nanomaterials bypassing the renal filtration process will, however, inevitably end up in the organs of the MPS (spleen and liver),49 also these organs will be exposed to radiation upon injection of the radiolabelled effector molecules as also observed by Lin and co-workers.48 This crucial aspect has to be kept in mind especially for therapeutic applications of this pretargeting system.
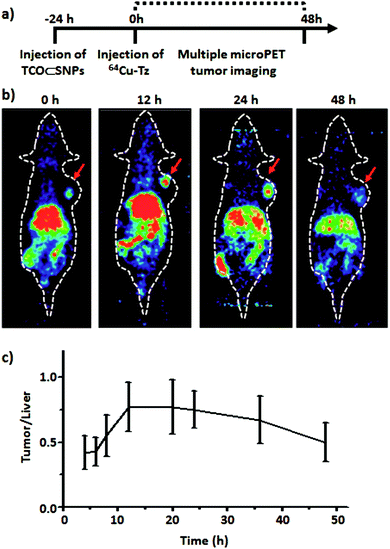 | ||
| Fig. 10 (a) Timeline of the injection protocol employed for the pretargeted study. (b) MicroPET images of the pretargeted study at various time points. (c) Ratio of PET tumour signal to liver signal of xenografted mice from the preliminary pretargeted study (n = 4). Adapted with permission from ref. 48 copyright American Chemical Society. | ||
In addition to the detection of tumour-bound fluorescent or radioactive effector molecules, the pretargeting concept has recently been extended to ultrasound imaging. In order to demonstrate the capture of gas-filled micron-sized bubbles by means of IEDDA, the group of John F. Valliant developed Tz-functionalised microbubbles and modified vascular endothelial growth factor receptor 2 (VEGFR2) specific mAbs with TCO. In their in vivo study, VEGFR2-positive murine xenografts were pretargeted using the TCO-conjugated mAbs and 24 h later the Tz-functionalised microbubbles were injected intravenously. Ultrasound images acquired 4 min thereafter showed high retention of the microbubbles in vascularised regions of the tumours with significant contrast enhancement compared to mice without TCO–mAb pretreatment.50
5. Conclusion and outlook
Preclinical and clinical studies have clearly indicated that tumour pretargeting can overcome important limitations of conventional directly radiolabelled mAbs. Since its introduction in the mid-1980s, various pretargeting strategies have been developed and progressed steadily over the years.The system based on bs mAbs and the avidin/streptavidin–biotin components represents without any doubt the most advanced tumour pretargeting approach for diagnosis as well as for therapy in the clinical settings, to date. However, this system suffers from several drawbacks including induction of immunogenicity and, therefore, future research should be directed to tackle this important issue. Emerging pretargeting systems based on hybridisation of synthetic complementary oligonucleotides or bioorthogonal chemistry in vivo have the potential to circumvent many of the limitations associated with the avidin/streptavidin–biotin system. These approaches are still in the preclinical stage, but possess immense potential. Regarding bioorthogonal chemistry, IDEEA appears to be the most suitable of all reactions tested so far for pretargeting due to easy synthetic access of the required substrates, in vivo stability of the TCO and Tz moieties and relatively fast reaction kinetics. However, the kinetics of IDEEA still need further improvement to become truly applicable in the clinic. This could be achieved, for example, by introducing suitable modifications either on the TCO or on the Tz moieties. Since the pretargeting approach is a rapidly growing field, new bioorthogonal reactions with even faster reaction kinetics and better in vivo compatibility will certainly be discovered in the near future.
As the special strength of tumour pretargeting is the substantial reduction of healthy tissue exposure, it is in particular useful for therapeutic purposes. At this point, it is worth emphasizing that conventional treatments such as chemotherapy, external beam radiation and surgery still play a key role in cancer management. As tumour pretargeting is suitable for detecting tumours and metastases of minimal or microscopic size, it can be indeed applied to treat and eradicate residual disease and metastatic lesions. Furthermore, the pretargeting strategy can have a significant impact on the treatment of malignant tissues that are difficult to remove by surgery or surrounded by sensitive tissues susceptible to damage. For therapeutic purposes, it is however of utmost importance to deposit as much radiation as possible precisely in the tumour without harming bone marrow, liver, spleen and kidney. Since the blood clearance of the radionuclide-carrying small effector molecules occurs sufficiently rapid, bone marrow exposure and haematological toxicity is less a decisive factor. And as long as the renal clearable radiolabelled effectors are not retained in the kidneys or bladder but eliminated in the urine, renal doses are acceptable.10 However, not only the pharmacokinetic behaviour of the radiolabelled effector molecules determines the effectiveness of the pretargeting strategy, but also the in vivo stability of the radiometal–chelate complexes. For that reason, considerable efforts should be devoted to the development of very stable radiometal complexes for conjugation to the fast clearing effector molecules that permit simultaneously imaging, dosimetry and treatment of tumours.
Although initial clinical studies of pretargeted RIT have been very encouraging, its full potentials are yet to be proven using large clinical trials in the near future, if thereby administered doses are sufficient to result in a significant improvement in responses. It should be noted that pretargeting is a relatively complex procedure that might need exhaustive optimisation for its successful translation into clinic. The combination of pretargeted RIT with other treatment modalities such as conventional external beam radiation or chemotherapy might be reasonable. In particular, the treatment with radiosensitising or anti-angiogenic agents can produce synergistic effects when combined with pretargeted RIT.
With regard to oncotropic vector molecules, tumour antigen specific mAbs represent an essential platform to precisely detect and treat tumour malignancies due to their excellent target specificity and affinity.4 To preserve this unmatched performance, their chemical modification necessary to create the desired mAb conjugates must not affect their immunoreactivity and affinity and should result in a homogenous population of bioconjugates. However, conventional bioconjugation such as random coupling to lysine or cysteine residues result in complex mixtures of mAb conjugates with possibly divergent pharmacokinetic behaviour, stability and immunoreactivity. In contrast, controlled, site-specific bioconjugation strategies including chemoenzymatic methods have the potential to yield more homogeneous products and should be pursued consequently for the production of next-generation mAb conjugates.
Another serious problem of the pretargeting approach, which will need to be dealt with in the future, is the partial cellular internalisation of mAbs upon specific interaction with their respective cell surface-located receptors. Antigen–mAb pairs known to internalise readily to a large extent are hence suboptimal for pretargeting as the prelocalised mAb conjugates must remain accessible to form the radioimmunoconjugates with the radiolabelled effector molecules.11 In consequence, a whatsoever modification of mAb conjugates preventing their receptor-mediated internalisation would represent a key milestone for tumour pretargeting.
Finally, pretargeting approaches based on mAbs are limited to detect and treat populations of cancerous cells with unique or highly abundant surface markers that are located on the cell surface of at least some, ideally on the majority of tumour cells and, most importantly, that are readily accessible by circulating agents. A substantial advantage of delivering therapeutic radionuclides, especially β-particles, is their long range in tissues (up to 12 mm). This allows for the irradiation of tumour cells including cancer stem cells located adjacent to cells to which the radioimmunoconjugates are attached. Consequently, also tumour cell subtypes lacking the addressed antigens are exposed to radiation.6 Targeting of a single type of cell surface receptors may, however, allow some cancer cells to survive by downregulation or mutation of the addressed antigen and may permit renewed tumour growth and clinical relapse. Pretargeted radioimmunotherapy should thus be applied in combination with other anticancer therapies and should ideally hit a set of different tumour-associated antigens.
In summary, there is much space to optimise the main parameters and improve the effectiveness of the pretargeting approach. Only if significant benefits for patients can be clearly illustrated in clinical studies, will this technology be translated into practise and impact in the clinics.
Acknowledgements
We thank Dr Maik Schubert for providing a substantial part of the topic's bibliography. This work was financially supported by the Swiss National Science Foundation (Professorships No. PP00P2_133568 and PP00P2_157545 to G. G.), the University of Zurich (G. G.), the Stiftung für wissenschaftliche Forschung of the University of Zurich (G. G.), the Helmholtz Virtual Institute NanoTracking (Agreement no. VH-VI-421) as well as the research initiative Technologie und Medizin – Multimodale Bildgebung zur Aufklärung des in-vivo-Verhaltens von polymeren Biomaterialien of the Helmholtz-Portfoliothema.References
- C. F. Ramogida and C. Orvig, Chem. Commun., 2013, 49, 4720–4739 RSC.
- M. D'Huyvetter, C. Xavier, V. Caveliers, T. Lahoutte, S. Muyldermans and N. Devoogdt, Expert Opin. Drug Delivery, 2014, 11, 1939–1954 CrossRef PubMed.
- R. M. Sharkey and D. M. Goldenberg, Immunotherapy, 2011, 3, 349–370 CrossRef PubMed and references therein.
- J. Barbet, M. Bardies, M. Bourgeois, J. F. Chatal, M. Cherel, F. Davodeau, A. Faivre-Chauvet, J. F. Gestin and F. Kraeber-Bodere, Methods Mol. Biol., 2012, 907, 681–697 CAS.
- S. M. Larson, J. A. Carrasquillo, N. K. Cheung and O. W. Press, Nat. Rev. Cancer, 2015, 15, 347–360 CrossRef CAS PubMed.
- J. P. Pouget, I. Navarro-Teulon, M. Bardies, N. Chouin, G. Cartron, A. Pelegrin and D. Azria, Nat. Rev. Clin. Oncol., 2011, 8, 720–734 CrossRef CAS PubMed.
- R. M. Sharkey, C. H. Chang, E. A. Rossi, W. J. McBride and D. M. Goldenberg, Tumour Biol., 2012, 33, 591–600 CrossRef CAS PubMed and references therein.
- D. M. Goldenberg and R. M. Sharkey, Expert Opin. Biol. Ther., 2012, 12, 1173–1190 CrossRef CAS PubMed and references therein.
- D. T. Reardan, C. F. Meares, D. A. Goodwin, M. McTigue, G. S. David, M. R. Stone, J. P. Leung, R. M. Bartholomew and J. M. Frincke, Nature, 1985, 316, 265–268 CrossRef CAS PubMed.
- F. Kraeber-Bodéré, C. Rousseau, C. Bodet-Milin, E. Frampas, A. Faivre-Chauvet, A. Rauscher, R. M. Sharkey, D. M. Goldenberg, J. F. Chatal and J. Barbet, Front. Pharmacol., 2015, 6, 54 Search PubMed.
- D. M. Goldenberg, C. H. Chang, E. A. Rossi, J. W. McBride and R. M. Sharkey, Theranostics, 2012, 2, 523–540 CrossRef CAS PubMed.
- G. Liu and D. J. Hnatowich, Bioconjugate Chem., 2008, 19, 2095–2104 CrossRef CAS PubMed.
- E. Frampas, C. Rousseau, C. Bodet-Milin, J. Barbet, J. F. Chatal and F. Kraeber-Bodere, Front. Oncol., 2013, 3, 159 CAS and references therein.
- D. R. Stickney, L. D. Anderson, J. B. Slater, C. N. Ahlem, G. A. Kirk, S. A. Schweighardt and J. M. Frincke, Cancer Res., 1991, 51, 6650–6655 CAS.
- C. Rousseau, F. Kraeber-Bodere, J. Barbet and J. F. Chatal, Eur. J. Nucl. Med. Mol. Imaging, 2013, 40, 1373–1376 CrossRef PubMed.
- E. A. Rossi, D. M. Goldenberg and C. H. Chang, Bioconjugate Chem., 2012, 23, 309–323 CrossRef CAS PubMed.
- R. Schoffelen, O. C. Boerman, D. M. Goldenberg, R. M. Sharkey, C. M. van Herpen, G. M. Franssen, W. J. McBride, C. H. Chang, E. A. Rossi, W. T. van der Graaf and W. J. Oyen, Br. J. Cancer, 2013, 109, 934–942 CrossRef CAS PubMed.
- R. Schoffelen, W. Woliner-van der Weg, E. P. Visser, D. M. Goldenberg, R. M. Sharkey, W. J. McBride, C. H. Chang, E. A. Rossi, W. T. van der Graaf, W. J. Oyen and O. C. Boerman, Eur. J. Nucl. Med. Mol. Imaging, 2014, 41, 1593–1602 CAS.
- C. Bodet-Milin, L. Ferrer, A. Rauscher, D. Masson, L. Rbah-Vidal, A. Faivre-Chauvet, E. Cerato, C. Rousseau, J. Hureaux, O. Couturier, P. Y. Salaun, D. M. Goldenberg, R. M. Sharkey, F. Kraeber-Bodere and J. Barbet, Front. Med., 2015, 2, 84 Search PubMed.
- C. Bodet-Milin, A. Faivre-Chauvet, T. Carlier, A. Rauscher, M. Bourgeois, E. Cerato, V. Rohmer, O. Couturier, D. Drui, D. M. Goldenberg, R. M. Sharkey, J. Barbet and F. Kraeber-Bodere, J. Nucl. Med., 2016 DOI:10.2967/jnumed.116.172221.
- D. J. Hnatowich, F. Virzi and M. Rusckowski, J. Nucl. Med., 1987, 28, 1294–1302 CAS.
- R. M. Sharkey, H. Karacay, T. M. Cardillo, C. H. Chang, W. J. McBride, E. A. Rossi, I. D. Horak and D. M. Goldenberg, Clin. Cancer Res., 2005, 11, 7109s–7121s CrossRef CAS PubMed and references therein.
- H. P. Lesch, M. U. Kaikkonen, J. T. Pikkarainen and S. Ylä-Herttuala, Expert Opin. Drug Delivery, 2010, 7, 551–564 CrossRef CAS PubMed and references therein.
- H. S. Stoldt, F. Aftab, M. Chinol, G. Paganelli, F. Luca, A. Testori and J. G. Geraghty, Eur. J. Cancer, 1997, 33, 186–192 CrossRef CAS PubMed and references therein.
- D. M. Goldenberg, R. M. Sharkey, G. Paganelli, J. Barbet and J. F. Chatal, J. Clin. Oncol., 2006, 24, 823–834 CrossRef CAS PubMed.
- D. M. Goldenberg, C. H. Chang, R. M. Sharkey, E. A. Rossi, H. Karacay, W. McBride, H. J. Hansen, J. F. Chatal and J. Barbet, Eur. J. Nucl. Med. Mol. Imaging, 2003, 30, 777–780 CrossRef PubMed.
- G. Paganelli and M. Chinol, Eur. J. Nucl. Med. Mol. Imaging, 2003, 30, 773–776 CrossRef PubMed.
- G. Liu, K. Mang'era, N. Liu, S. Gupta, M. Rusckowski and D. J. Hnatowich, J. Nucl. Med., 2002, 43, 384–391 CAS.
- G. Liu, D. Cheng, S. Dou, X. Chen, M. Liang, P. H. Pretorius, M. Rusckowski and D. J. Hnatowich, Mol. Imaging Biol., 2009, 11, 303–307 CrossRef PubMed.
- G. Liu, S. Dou, G. Mardirossian, J. He, S. Zhang, X. Liu, M. Rusckowski and D. J. Hnatowich, Clin. Cancer Res., 2006, 12, 4958–4964 CrossRef CAS PubMed.
- G. Liu, S. Dou, S. Baker, A. Akalin, D. Cheng, L. Chen, M. Rusckowski and D. J. Hnatowich, Cancer Biol. Ther., 2010, 10, 767–774 CrossRef CAS PubMed.
- G. Liu, S. Dou, Y. Liu, Y. Wang, M. Rusckowski and D. J. Hnatowich, Bioconjugate Chem., 2011, 22, 2539–2545 CrossRef CAS PubMed.
- G. Gasser, in Peptide Nucleic Acids: Methods and Protocols, ed. P. E. Nielsen and D. H. Appella, Humana Press, 2014, vol. 1050, pp. 55–72 Search PubMed.
- G. Gasser, A. M. Sosniak and N. Metzler-Nolte, Dalton Trans., 2011, 40, 7061–7076 RSC.
- H. Stephan, C. Foerster and G. Gasser, in Peptide Nucleic Acids: Methods and Protocols, ed. P. E. Nielsen and D. H. Appella, Humana Press, 2014, vol. 1050, pp. 37–54 Search PubMed.
- G. Mardirossian, K. Lei, M. Rusckowski, F. Chang, T. Qu, M. Egholm and D. J. Hnatowich, J. Nucl. Med., 1997, 38, 907–913 CAS.
- A. Leonidova, C. Foerster, K. Zarschler, M. Schubert, H. J. Pietzsch, J. Steinbach, R. Bergmann, N. Metzler-Nolte, H. Stephan and G. Gasser, Chem. Sci., 2015, 6, 5601–5616 RSC.
- H. Urata, E. Ogura, K. Shinohara, Y. Ueda and M. Akagi, Nucleic Acids Res., 1992, 20, 3325–3332 CrossRef CAS PubMed.
- J. Schlesinger, R. Bergmann, S. Klussmann and F. Wuest, Lett. Drug Des. Discovery, 2006, 3, 330–335 CrossRef CAS.
- J. Schlesinger, I. Koezle, R. Bergmann, S. Tamburini, C. Bolzati, F. Tisato, B. Noll, S. Klussmann, S. Vonhoff, F. Wuest, H. J. Pietzsch and J. Steinbach, Bioconjugate Chem., 2008, 19, 928–939 CrossRef CAS PubMed.
- C. Foerster, M. Schubert, R. Bergmann, S. Vonhoff, S. Klussmann, M. Walther, J. Pietzsch, H.-J. Pietzsch and J. Steinbach, in Technetium and Other Radiometals in Chemistry and Medicine, ed. U. Mazzi, W. C. Eckelman and W. A. Volkert, SGEditoriali, Padova, Italy, 2010, pp. 357–362 Search PubMed.
- R. Rossin and M. S. Robillard, Curr. Opin. Chem. Biol., 2014, 21, 161–169 CrossRef CAS PubMed.
- E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974–6998 CrossRef CAS PubMed and references therein.
- L. Carroll, H. L. Evans, E. O. Aboagye and A. C. Spivey, Org. Biomol. Chem., 2013, 11, 5772–5781 CAS.
- J. C. Knight and B. Cornelissen, Am. J. Nucl. Med. Mol. Imaging, 2014, 4, 96–113 CAS.
- B. M. Zeglis, C. Brand, D. Abdel-Atti, K. E. Carnazza, B. E. Cook, S. Carlin, T. Reiner and J. S. Lewis, Mol. Pharmaceutics, 2015, 12, 3575–3587 CrossRef CAS PubMed.
- J. P. Meyer, J. L. Houghton, P. Kozlowski, D. Abdel-Atti, T. Reiner, N. V. Pillarsetty, W. W. Scholz, B. M. Zeglis and J. S. Lewis, Bioconjugate Chem., 2016, 27, 298–301 CrossRef CAS PubMed.
- S. Hou, J. S. Choi, M. A. Garcia, Y. Xing, K. J. Chen, Y. M. Chen, Z. K. Jiang, T. Ro, L. Wu, D. B. Stout, J. S. Tomlinson, H. Wang, K. Chen, H. R. Tseng and W. Y. Lin, ACS Nano, 2016, 10, 1417–1424 CrossRef CAS PubMed.
- K. Zarschler, L. Rocks, N. Licciardello, L. Boselli, E. Polo, K. P. Garcia, L. De Cola, H. Stephan and K. A. Dawson, Nanomedicine, 2016, 12, 1663–1701 CAS and references therein.
- A. Zlitni, N. Janzen, F. S. Foster and J. F. Valliant, Angew. Chem., Int. Ed., 2014, 53, 6459–6463 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |