Entropy prediction for H2 adsorption in metal–organic frameworks†
Abstract
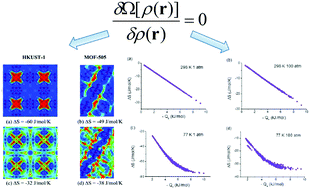
Entropy is an important thermodynamic property and serves as a bridge connecting equilibrium and non-equilibrium systems, which provides a basic understanding of various practical phenomena. In this study, classical density functional theory was introduced to efficiently predict entropy. The theory was applied to a high-throughput prediction of entropy and excess entropy for H2 adsorption in metal–organic frameworks. It seems that the entropy screening and uptake screening are generally equivalent at high temperature. Based on the entropy screening, the best hydrogen storage materials have been identified. The correlations between entropy and thermodynamic properties, such as uptake, isosteric heat and adsorption degree, were examined and are explained. The results imply that among the tested thermodynamic properties, the correlation between entropy and isosteric heat is the strongest.

 Please wait while we load your content...
Please wait while we load your content...