Exploring the kinetic and thermodynamic aspects of four-electron electrochemical reactions: electrocatalysis of oxygen evolution by metal oxides and biological systems†
Abstract
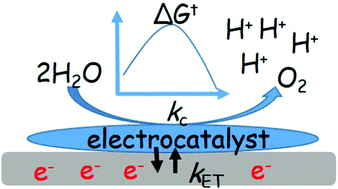
Finding fundamental and general mechanisms for electrochemical reactions, such as the oxygen evolution reaction (OER) from water and reduction of CO2, plays vital roles in developing the desired electrocatalysts for facilitating solar fuel production. Recently, density functional theory (DFT) calculations have shown that there is a universal scaling relation of adsorption energy between key intermediate species, HO(ad) and HOO(ad), on the surface of metal oxides as OER electrocatalysts. In this paper, a kinetic and thermodynamic model for the four-electron electrochemical reaction based on previous OER mechanisms proposed by DFT calculations is developed to further investigate the electrocatalytic properties over a wide range of metal oxides and photosystem II. The OER activity of metal oxides (i.e. electrocatalytic current) calculated from the DFT-calculated equilibrium potentials with kinetic properties, such as the rate constants for interfacial electron transfer and catalytic turnover, can lead to a volcano-shaped trend that agrees with the results observed in experiments. In addition, the kinetic aspects of the impact on the electrocatalysts are evaluated. Finally, comparing the results of metal oxides and photosystem II, and fitting experimental voltammograms give further insights into kinetic and thermodynamic roles. Here, the general guidelines for designing OER electrocatalysts with unified kinetic and thermodynamic properties are presented.

 Please wait while we load your content...
Please wait while we load your content...