Adsorption of gold subnano-structures on a magnetite(111) surface and their interaction with CO
Abstract
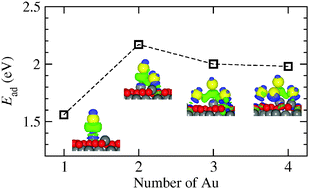
Gold deposited on iron oxide surfaces can catalyze the oxidation of carbon monoxide. The adsorption of gold subnano-structures on the Fe-rich termination of the magnetite(111) surface has been investigated using density functional theory. The structural, energetic, and electronic properties of gold/magnetite systems have been examined for vertical and flattened configurations of adsorbed Aun (n = 1–4) species. Single gold adatoms strongly bonded to the iron atoms of the Fe3O4(111) surface appear to be negatively charged, and consequently increase the work function. For a more stable class of larger, flattened Aun structures the adsorption binding energy per adatom is substantially increased. The structures exhibit a net positive charge, with the Au atoms binding with the oxide having distinctly cationic character. A charge transfer from the larger gold structures to the substrate is consistent with the lowering of the work function. The bonding of a CO molecule to a Au monomer on the Fe3O4(111) surface has been found nearly as strong as that to the iron site of the bare Fe-terminated surface. However, CO bonding to larger, oxide supported Aun structures is distinctly stronger than that to the bare oxide surface. Upon CO adsorption all Aun structures are cationic and CO shows a tendency to bind to the most cationic atom of the Aun cluster.

 Please wait while we load your content...
Please wait while we load your content...