Ball-milled sulfur-doped graphene materials contain metallic impurities originating from ball-milling apparatus: their influence on the catalytic properties†
Abstract
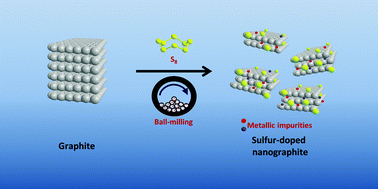
Graphene materials have found applications in a wide range of devices over the past decade. In order to meet the demand for graphene materials, various synthesis methods are constantly being improved or invented. Ball-milling of graphite to obtain graphene materials is one of the many versatile methods to easily obtain bulk quantities. In this work, we show that the graphene materials produced by ball-milling are spontaneously contaminated with metallic impurities originating from the grinding bowls and balls. Ball-milled sulfur-doped graphene materials obtained from two types of ball-milling apparatus, specifically made up of stainless steel and zirconium dioxide, were investigated. Zirconium dioxide-based ball-milled sulfur-doped graphene materials contain a drastically lower amount of metallic impurities than stainless steel-based ball-milled sulfur-doped graphene materials. The presence of metallic impurities is demonstrated by their catalytic effects toward the electrochemical catalysis of hydrazine and cumene hydroperoxide. The general impression toward ball-milling of graphite as a versatile method for the bulk production of ‘metal-free’ graphene materials without the need for post-processing and the selection of ball-milling tools should be cautioned. These findings would have wide-reaching implications for graphene research.

 Please wait while we load your content...
Please wait while we load your content...